Abstract
Background
Ablation targeting complex fractionated atrial electrograms (CFAEs) or high dominant frequency (DF) sites is generally effective for persistent atrial fibrillation (AF). CFAEs and/or high DF sites may exist in low-voltage regions, which theoretically represent abnormal substrates. However, whether CFAEs or high DF sites reflect low voltage substrates during sinus rhythm (SR) is unknown.
Methods
Sixteen patients with AF (8 with paroxysmal AF; 8, persistent AF) underwent high-density mapping of the left atrium (LA) with a 3-dimensional electroanatomic mapping system before ablation. The LA was divided into 7 segments and the mean bipolar voltage recorded during AF and SR, CFAEs (cycle lengths of 50–120 ms), and DF sites were assessed in each segment with either a duo-decapolar ring catheter (n=10) or a 64-pole basket catheter (n=6). Low-voltage areas were defined as those of <0.5 mV during AF and <1.0 mV during SR.
Results
Regional mean voltage recorded from the basket catheter showed good correlation between AF and SR (r=0.60, p<0.01); however, the % low-voltage area in the LA recorded from the ring catheter showed weak correlation (r=0.34, p=0.05). Mean voltage was lower during AF than during SR (1.0 mV [IQR, 0.5–1.4] vs. 2.6 mV [IQR, 1.8–3.6], p<0.01). The regional and overall % low-voltage area of the LA was greater during AF than during SR (20% vs. 11%, p=0.05). CFAEs and high DF sites (>8 Hz) did not correlate with % low-voltage sites during SR; however, CFAEs sites were located in high-voltage regions during AF and high DF sites were located in low voltage regions during AF.
Conclusions
CFAEs and high DF areas during AF do not reflect damaged atrial myocardium as shown by the SR voltage. However, CFAEs and high DF sites may demonstrate different electrophysiologic properties because of different voltage amplitude during AF.
Keywords: Atrial fibrillation, Complex fractionated atrial electrogram, Dominant frequency, Sinus rhythm
1. Introduction
Catheter-based pulmonary vein isolation (PVI) has become a widely accepted means of treating symptomatic drug-refractory atrial fibrillation (AF) [1]. However, for terminating persistent AF (PerAF), extensive ablation, including ablation at sites of complex fractionated atrial electrograms (CFAEs) and high dominant-frequency (DF) and/or multiple linear ablations may also be necessary [2], [3], [4], [5]. CFAEs and/or high DF sites have been shown to be effective targets for AF termination, which suggests the importance of these sites in the maintenance of AF [2], [5], [6], [7], [8]. CFAEs and high DF sites theoretically represent abnormal substrates; however, sinus rhythm (SR) voltage recorded at the CFAE sites has been shown to be normal [9]. It has also been shown that most CFAE and high DF sites identified during AF do not correspond with high DF sites or low voltage areas identified during SR [10]. In the present study, we compared left atrial (LA) CFAEs and high DF sites identified during AF and LA bipolar voltage recorded during AF and SR by comparing LA bipolar electrograms obtained from both high-density mobile-catheter mapping and fixed-position basket-catheter mapping.
2. Material and methods
2.1. Study patients
This study involved 16 consecutive patients (14 men; mean age 58±11 years) scheduled for their first catheter ablation of AF. Eight had paroxysmal AF (PAF), defined as AF lasting <7 days; 8, persistent AF (PerAF), defined as AF lasting ≥7 days. Patients with cardiomyopathy, valvular heart disease, or congenital heart disease were excluded from the study. Adequate oral anticoagulation therapy was administered for at least 1 month before the ablation procedure, and all antiarrhythmic drugs were discontinued for at least 5 half-lives before the procedure. Transesophageal and transthoracic echocardiography were performed upon admission, and the following baseline echocardiographic data was obtained: maximum LA volume by the prolate-ellipsoid method and left ventricular ejection fraction by the Teichholz method. The study protocol was approved by the Institutional Review Board of Nihon University and Itabashi Hospital (December 7, 2012; RK-121109-5) and all patients provided written informed consent for their participation.
2.2. Electrophysiologic study
Electrophysiologic evaluation was performed in all patients under conscious sedation using dexmedetomidine, propofol, and fentanyl, as described previously [10], [11]. After vascular access was obtained, a single transseptal puncture was performed and intravenous heparin was administered to maintain an activated clotting time of more than 300 s. After two long sheaths (1 SL0 sheath and 1 Agilis sheath; St. Jude Medical, Inc., St. Paul, MN) were inserted into the left atrium via a transseptal puncture, the 3-dimensional (3D) geometry of the left atrium and 4 pulmonary veins (PVs) was reconstructed with the use of an EnSite NavX Classic system (St. Jude Medical, Inc.) and a 20-pole circular mapping catheter with 4-4-4-mm interelectrode spacing (AFocus II catheter, St. Jude Medical, Inc.). We recorded multiple bipolar signals (filter setting: 30–300 Hz) from the AFocus II catheter with the EnSite NavX system classic (St. Jude Medical, Inc.) in 10 patients. We recorded bipolar signals from a 64-pole basket catheter (Constellation, Boston Scientific, Marlborough, MA) placed in the left atrium (Fig. 1A and B) in 6 patients. If the patient was in SR, AF was induced by rapid atrial pacing from the coronary sinus ostium for recording of the CFAEs and DFs at 5 min after AF induction. If the patient was in AF, SR electrograms were recorded after cardioversion.
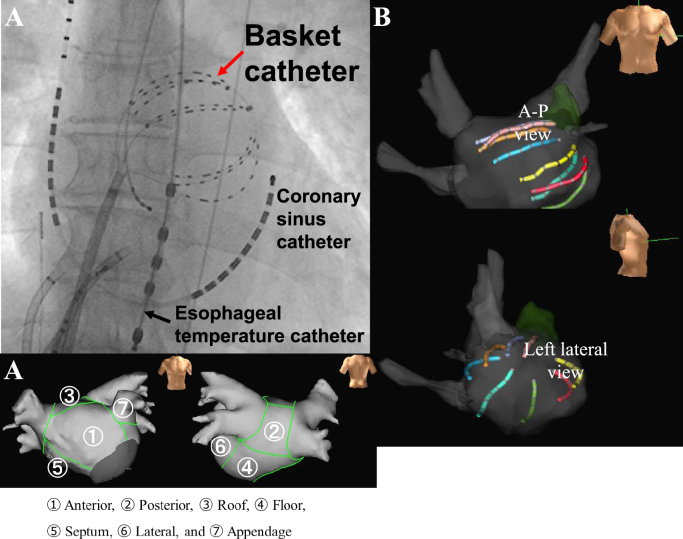
Fig. 1.
A. X-ray position of the basket catheter in the left atrium. A-P=antero-posterior. B. Basket catheter position shown on the NavX system. C. Left atrial segmentation. The left atrium was divided into 7 segments and analyzed.
2.3. Bipolar electrogram recordings
-
1)
AFocus II 20-pole dual ring catheter: Nineteen bipolar electrograms (1–2…19–20) from the 20-pole circular electrodes with 4-mm spacing were recorded simultaneously for a single beat during SR and for 5 seconds during AF, and high-density 3D electroanatomic mapping (>300 signals) of the entire left atrium was performed. Single-beat peak-to-peak bipolar voltages during SR were calculated. Peak-to-peak bipolar voltages during AF were averaged from 5-second recordings at 5 points within each LA segment. The 5 points where the recordings were obtained in each segment during SR and AF were located within 3 mm of each other on the LA 3D map.
-
2)
Constellation 64-pole basket catheter: Owing to the limit on the number of electrodes that can be recorded by the EnSite system classic version, the signals from 1 proximal electrode of each spline of the basket catheter could not be recorded. Thus, 6 bipolar pairs of 7 possible bipolar electrode pairs on each spline (total, 48 bipolar electrograms: 6 pairs×8 splines) were analyzed. With the basket catheter in a stable position, the baseline bipolar signals were recorded for a single beat during SR and were averaged over 5 s during the recording of AF from each bipolar electrode of the 48 bipolar electrograms (Fig. 1). The basket catheters used were 38 mm (n=1), 48 mm (n=4), and 60 mm (n=1) in diameter.
-
3)
Bipolar electrogram amplitudes were measured during AF and SR and low voltage was defined as <0.5 mV during AF and <1.0 mV during SR [12].
2.4. Time-domain atrial electrogram interval analysis during AF
For the analysis of atrial electrogram intervals, the NavX mapping parameters were set to CFAE-mean, an algorithm was used to determine the average time of the atrial electrogram interval (fractionation intervals; FIs) at each site, and a color map of the FIs was constructed [11], [13], [14]. The FI was considered the average time between consecutive deflections over a 5-second recording period. Local bipolar activation timing was defined as the peak negative dV/dt point. The settings included a refractory period of 40 ms, peak-to-peak sensitivity between 0.05 mV and 0.1 mV, and duration for each electrogram of <10 ms. Continuous CFAEs were defined as those with a mean FI of <50 ms and variable CFAEs as those with a mean FI of 50–120 ms.
2.5. Fast Fourier transform (FFT) analysis
For FFT analysis, the DF (the highest power frequency) was analyzed by the DF analysis software installed in the NavX mapping system (sampling rate: 1200 Hz; resolution: 0.14 Hz; low-pass filter: 20 Hz; high-pass filter: 1 Hz with a Hamming window function), as reported previously [11], [13], [14]. Five-second bipolar signals recorded during AF were used for the DF analysis. The DF was defined as the frequency, range of 3–14 Hz, with the maximum power. A high DF site was defined as a site with a frequency of >8 Hz. The regularity index was considered the area within the 0.75-Hz band around the DF divided by the area of the frequencies sampled from 3–14 Hz [15], [16]. Signals with a regularity index of <0.2 were excluded from the analysis.
2.6. LA segmentation
The left atrium was divided into 7 segments (anterior, septum, floor, posterior, roof, appendage, and mitral isthmus) (Fig. 1C) and the CFAE sites, high DF sites, and bipolar atrial electrogram amplitudes were compared for each segment.
2.7. Statistical analysis
Continuous variables are expressed as the mean±SD. Differences in variables were analyzed using the Mann-Whitney U test, and the correlation between bipolar voltages during SR and those during AF was tested by Spearman׳s rank correlation coefficient. All statistical analyses were performed with JMP 8 software (SAS Institute, Cary, NC) and p<0.05 was considered significant.
3. Results
3.1. Baseline clinical characteristics and echocardiographic values
Clinical characteristics of the patients with PAF and those with PerAF are shown in Table 1. There were no significant differences in these characteristics between the two groups. The LA dimension was significantly greater in the PerAF group than in the PAF group (42.3±8.4 mm vs. 35.2±3.3 mm, p=0.0445); however, the left ventricular ejection fraction did not differ significantly (67.3±6.2% vs. 73.2±9.4%; p=0.2024).
Table 1.
Clinical characteristics of the patients by study group.
| Paroxysmal AF (n=8) | Persistent AF (n=8) | p-value | |
|---|---|---|---|
| Age (years) | 63±7 | 57±11 | 0.2476 |
| Sex, male (%) | 6 (75) | 8 (100) | 0.1306 |
| AF duration (months) | 42 (9-61) | 30 (12-76) | 0.7513 |
| BMI (kg/m2) | 23±3 | 24±3 | 0.5955 |
| HT (%) | 4 (50) | 7 (88) | 0.1056 |
| DM (%) | 1(13) | 1 (13) | 1.0000 |
| HF (%) | 1 (13) | 2 (25) | 0.5218 |
| LAD (mm) | 35±3 | 42±8 | 0.0445 |
| LVEF (%) | 73±9 | 67±6 | 0.2024 |
AF=atrial fibrillation; BMI=body mass index; DM=diabetes mellitus; HF=heart failure; HT=hypertension; LAD=left atrial diameter; LVEF=left ventricular ejection fraction.
3.2. LA voltages recorded during SR and AF by AFocus II catheter
Representative LA voltage maps acquired during SR and AF are shown in Fig. 2 and the low-voltage point(s) in each LA segment are shown in Fig. 3. The vertical axis and horizontal axis in Fig. 3 represent the number of low voltage points from 5 adjacent bipolar electrograms recorded at ≤5 mm apart during AF (<0.5 mV) and SR (<1.0 mV) in each LA segment, respectively. The low voltage points within each LA segment during AF and SR were weakly correlated and the number of low voltage points in each LA segment was significantly greater during AF overall (left panel), PAF (right upper panel), and PerAF (right lower panel) than during SR.
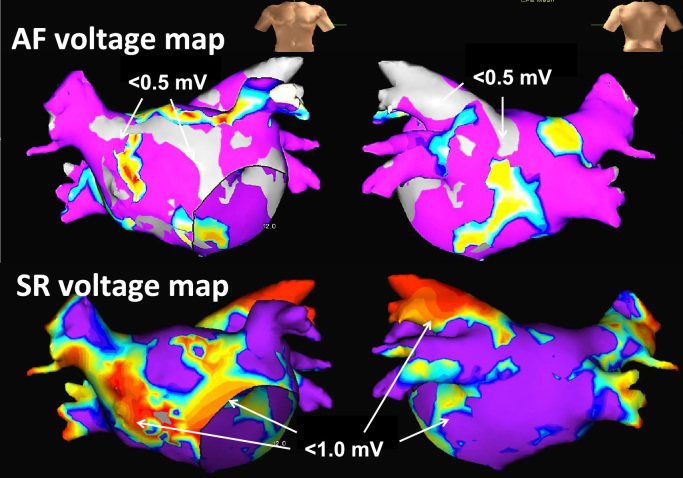
Fig. 2.
Mapping images of areas of left atrial bipolar voltage during atrial fibrillation (AF, upper panel) and sinus rhythm (SR, lower panel). Low-voltage areas during AF were defined as those <0.5 mV and are shown in grey color. Low-voltage areas during SR were defined as those <1.0 mV and are shown in non-purple colors.
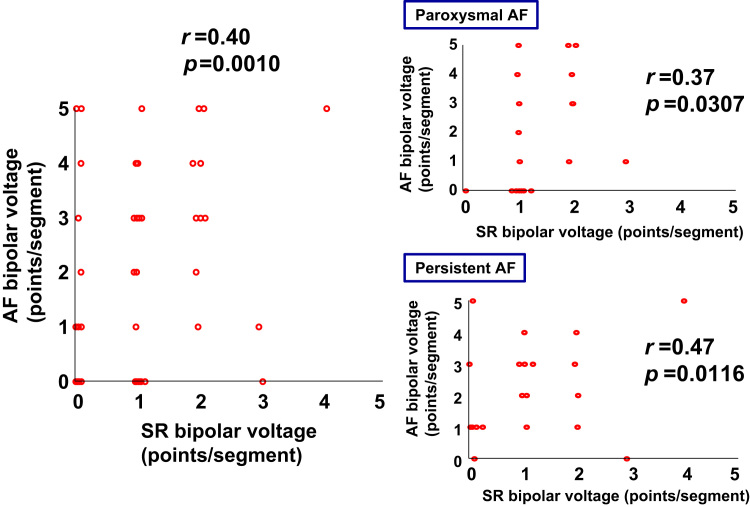
Fig. 3.
Graphs of low-voltage points in each left atrial segment. Low-voltage points from 5 similar positions in each segment were higher during atrial fibrillation (AF) than during sinus rhythm (SR); however, low-voltage points during SR and AF were significantly correlated. The left panel shows all low voltage points, whereas the two right panels show points for paroxysmal AF (upper panel) and persistent AF (lower panel).
3.3. LA voltages recorded during SR and AF by basket catheter
Voltages of the bipolar electrograms recorded from the basket catheter located at the same LA positions during AF and SR were compared for one-to-one correspondence. Positive correlation was found between LA voltage during AF and SR; however, LA voltages recorded during AF for AF overall (upper panel), PAF (lower left), and PerAF (lower right) were smaller than those recorded during SR (Fig. 4).
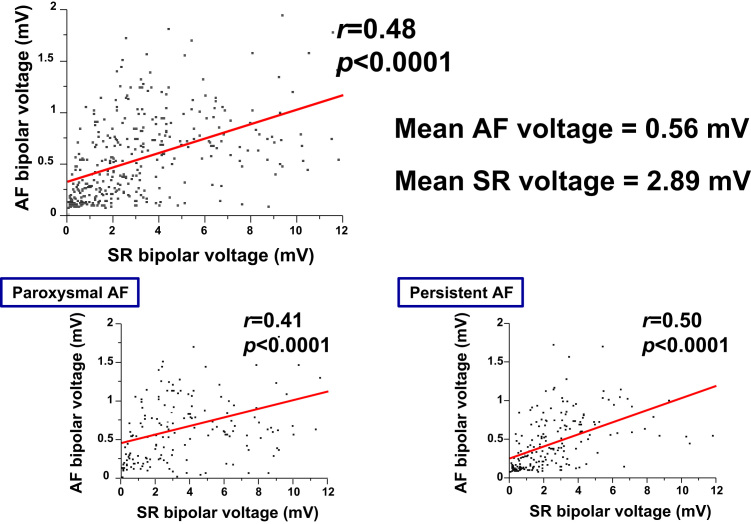
Fig. 4.
Scatter plots of low-voltage points recorded at the same position. Low-voltage points recorded from the basket catheter were higher during atrial fibrillation (AF) than during sinus rhythm (SR); however, low-voltage points during SR and AF were significantly correlated. Upper panel shows all low-voltage points, whereas the two lower panels show points for paroxysmal AF (left panel) and persistent AF (right panel).
3.4. CFAE segments and LA voltages recorded during SR by AFocus II catheter
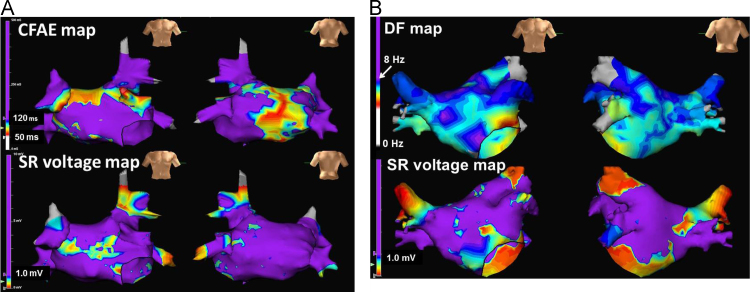
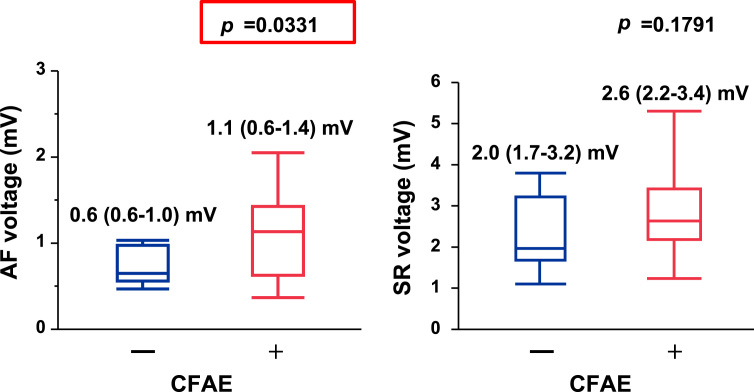
Representative LA voltage maps of CFAE-positive areas and bipolar voltage during SR are shown in Fig. 5A. CFAE-positive segments were located in the normal voltage area. The mean bipolar voltage recorded in each LA segment during AF increased in the CFAE-positive segments (Fig. 6, left panel), whereas those recorded in each segment during SR did not differ significantly between the CFAE-positive and CFAE-negative sites (Fig. 6, right panel).
Fig. 5.
A. Representative maps of complex fractionated atrial electrogram (CFAE) locations (upper panel) and left atrial voltage during sinus rhythm (SR, lower panel). The CFAE areas are shown in non-purple colors. Low-voltage areas during SR were defined as those <1.0 mV and are shown in non-purple colors. Note that there was no overlap between the CFAE areas and the low-voltage areas during SR. B. Representative maps comparing the high dominant frequency (DF) locations (upper panel) and left atrial voltage recorded during sinus rhythm (SR, lower panel). The high DF areas are shown in purple color. The low-voltage areas during SR were defined as those <1.0 mV and are shown in non-purple colors. Note that there was no overlap between the high DF areas and low voltage areas during SR.
Fig. 6.
Graphs comparing left atrial bipolar voltage recorded during atrial fibrillation (AF, left panel) and during sinus rhythm (SR, right panel) at the complex fractionated atrial electrogram (CFAE-positive and CFAE-negative) sites. Note that the CFAE-positive sites showed higher voltage during AF than the CFAE-negative sites; however, there was no difference in the SR voltage between the high DF and non-high DF sites.
3.5. High DF segments and LA voltage recorded during SR by AFocus II™ catheter
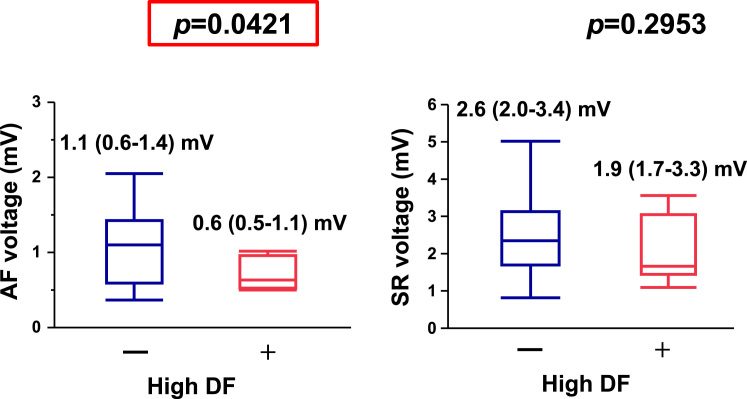
Representative maps of LA DF sites and bipolar voltage during SR are shown in Fig. 5B. The mean bipolar voltage recorded in each LA segment during AF decreased in the high DF segments (Fig. 7, left panel), whereas those recorded in each LA segment during SR did not differ significantly between the high DF and non-high DF segments (Fig. 7, right panel).
Fig. 7.
Graphs of left atrial bipolar voltage recorded during atrial fibrillation (AF, left panel) and during sinus rhythm (SR, right panel) at the high dominant frequency (DF) and non-high DF sites. Note that the high DF sites showed lower voltage during AF than the complex fractionated atrial electrogram (–) sites; however, there was no difference in the SR voltage between the high DF and non-high DF sites.
4. Discussion
4.1. Major findings
In this study, we first compared 5 bipolar electrogram voltages recorded at adjacent locations during AF and SR at each of 7 LA segments with a 20-pole catheter and showed that the number of low voltage bipolar electrograms (<0.5 mV) recorded during AF was greater than that of the low voltage bipolar electrograms (<1.0 mV) recorded during SR. The LA bipolar voltages recorded during AF and SR with a 64-pole basket catheter also showed a positive correlation between the bipolar electrograms recorded during AF and SR; however, the bipolar voltages were lower during AF than during SR. Second, we recorded the mean bipolar electrogram voltages in CFAE-positive and CFAE-negative segments during AF and SR and found that those recorded during AF at the CFAE-positive sites were higher than those at the CFAE-negative sites were. However, the bipolar electrogram voltages recorded during SR did not differ significantly between the CFAE-positive and CFAE-negative sites. Third, we recorded mean bipolar electrogram voltages in high DF and non-high DF segments during AF and SR and showed that during AF, voltages were significantly lower at the high DF sites than at the non-high DF sites; however, the bipolar electrogram voltages recorded during SR did not differ significantly between these two sites.
4.2. LA voltages during SR and AF
Previously, we showed that LA bipolar and unipolar voltages obtained during SR by a 20-pole electrode at CFAE-positive sites were significantly higher than those at CFAE-negative sites and LA bipolar and unipolar voltages at high DF sites and non-high DF sites did not differ significantly [17]. Furthermore, only 7.9% of CFAE sites overlapped with high DF sites [17]. In this study, LA bipolar electrogram voltages recorded during SR and AF were compared using a 20-pole electrode catheter and a 64-pole basket catheter. Bipolar low-voltage points obtained in each LA segment during SR and AF with the 20-pole electrode catheter showed a weak positive correlation, and the number of low-voltage points recorded during AF was greater than that recorded during SR in both patients with PerAF and those with PAF. To compare bipolar electrogram voltages from the same position, local LA bipolar electrograms were compared during SR and AF using a 64-pole basket catheter. The LA bipolar electrogram voltages recorded during SR and AF showed a weakly positive correlation, and SR voltages were higher than AF voltages in the same positions. Although we defined low voltage during AF and SR as <0.5 mV and <1.0 mV, respectively, in other studies, low voltage during AF has also been defined as <0.5 mV [12], [18] and <0.3 mV [19], while low voltage during SR has been defined as <0.5 mV [20], <1.0 mV [21], and ≤1.3 mV [22]. Therefore, a more detailed and consistent voltage-threshold setting and recording time for measurement of AF voltage may be needed to compare bipolar voltages during SR and AF, uniformly.
4.3. Voltages at CFAE and high DF sites during SR and AF
In this study, bipolar electrogram voltages recorded during AF were significantly higher at CFAE-positive sites than at CFAE-negative sites. However, bipolar electrogram voltages recorded during SR did not differ significantly between these sites. Previously, we showed that LA bipolar voltages recorded during SR at CFAE-positive sites were significantly higher than CFAE-negative sites [17]. Although the reason for the difference is unclear, the few patients in the previous study and those in this study might have influenced the results. Previous studies have shown that areas of CFAEs correspond to areas of normal atrial voltage [9], [23]; however, another study reported that CFAEs increase with age and occur in regions of low atrial voltage and slow conduction [24]. A previous report found that common causes of CFAE include wavefront collision, conduction through channels of functional block, and re-entry [25]. Therefore, the vast majority of CFAEs were probably caused by these mechanisms rather than by true “drivers” of AF and display normal voltage during SR [26]. Furthermore, using magnetic resonance imaging, Jadidi et al. showed that 90% of continuous CFAE sites occurred at LA sites of non-delayed enhancement and patchy delayed enhancement [27], and ablation of sites with fractionated activity within or at border zones of low-voltage areas is more effective than a conventional PVI-only strategy for PerAF [18]. Therefore, as suggested by Miyamoto et al. [28], in PAF and PerAF associated with a minimally damaged left atrium, the CFAE sites in patients with AF terminated by PVI alone represent healthy atrial tissue with rapid electrical activity in response to an AF driver located in a PV. In patients without AF termination, they represent more damaged tissue responsible for maintaining AF. Previous reports have also shown that the overlap of CFAE sites with high DF sites identified in both bipolar and unipolar electrograms ranged from only 7.9% to 50% [6], [17]. There are no previous reports on the relationship between DF and atrial voltage during SR and AF. In this study, SR voltage did not differ between the high DF and non-high DF sites; however, AF voltage was smaller at the high DF sites. Therefore, high DF sites may be located in areas of damaged atrium, whereas CFAE sites may be located in areas of relatively healthy atrium. Previous reports on the relationship between CFAEs and high DF sites have shown that CFAE sites are found adjacent to and partially surrounding high DF sites [29]. In their retrospective study, Verma et al. [6] reported that AF termination occurred at sites of overlapping CFAEs and DF, when the DF was above the mean value recorded.
4.4. Study limitations
The main limitation of our analysis is its small sample size. Further, we used the automated algorithm of the NavX system to define FI and DF. We cannot exclude the possibility that using a different automated algorithm with a different mapping system or a mapping catheter with different inter-electrode spacing might have yielded different results. The ablation strategy used in all of the patients was extended encircling PVI only and ablation of CFAEs, sites of high DF and LA linear ablation were not performed. Therefore, it is uncertain whether CFAEs and sites of high DF calculated from the NavX software contribute to AF maintenance. Owing to the few patients, we did not investigate the relationship between the low voltage areas recorded during SR and AF and the areas of CFAEs and high DF and the freedom from AF/atrial tachycardia during follow-up. The LA voltage recording positions in each segment during SR and AF by AFocus II catheter were different. Therefore, comparison of the LA voltage during SR and AF by AFocus II catheter might be limited.
5. Conclusions
Left atrial voltages recorded during SR and AF showed a positive but weak correlation. CFAEs and high DF sites did not reflect the regions of low voltage substrates seen during SR. The voltages recorded during AF were higher at CFAE sites; however, they were lower at high DF sites.
Conflict of Interest
All authors declare no conflict of interest related to this study.
Contributor Information
Naoko Sasaki, Email: naonaonanana@hotmail.co.jp.
Ichiro Watanabe, Email: Watanabe.ichirou@nihon-u.ac.jp.
Yasuo Okumura, Email: yasuwo128@yahoo.co.jp.
Koichi Nagashima, Email: cocakochan@gmail.com.
Rikitake Kogawa, Email: ricky1003feb2nd@yahoo.co.jp.
Kazumasa Sonoda, Email: ksono@yahoo.com.
Kazuki Iso, Email: heartily.dr.ka2-0603@hotmail.co.jp.
Keiko Takahashi, Email: keiktaka0327@yahoo.co.jp.
Masaru Arai, Email: arai.masashi@nihon-u.ac.jp.
Ryuta Watanabe, Email: watanabe.ryuta@nihon-u.ac.jp.
Sayaka Kurokawa, Email: Kurokawa.sayaka@nihon-u.ac.jp.
Kimie Ohkubo, Email: okubo.kimie@nihon-u.ac.jp.
Toshiko Nakai, Email: Nakai.toshiko@nihon-u.ac.jp.
Atsushi Hirayama, Email: hirayama.atsushi@nihon-u.ac.jp.
Mizuki Nikaido, Email: Mizuki_Nikaidou@mb7.nkc.co.jp.
References
- 1.Wazni O., Wilkoff B., Saliba W. Catheter ablation for atrial fibrillation. N Engl J Med. 2011;365:2296–2304. doi: 10.1056/NEJMct1109977. [DOI] [PubMed] [Google Scholar]
- 2.Nademanee K., McKenzie J., Kosar E. A new approach for catheter ablation of atrial fibrillation: mapping of electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 3.Haïssaguerre M., Sanders P., Hocini M. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 4.O׳Neill M.D., Jaïs P., Takahashi Y. The stepwise ablation approach for chronic atrial fibrillation--evidence for a cumulative effect. J Interv Card Electrophysiol. 2006;16:153–167. doi: 10.1007/s10840-006-9045-1. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt C., Estner H., Hecher B. Radiofrequency ablation of complex fractionated atrial electrograms (CFAE): preferential sites of acute termination and regularization in paroxysmal and persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:1039–1046. doi: 10.1111/j.1540-8167.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 6.Verma A., Lakkireddy D., Wulffhart Z. Relationship between complex fractionated electrograms (CFE) and dominant frequency (DF) sites and prospective assessment of adding DF-guided ablation to pulmonary vein isolation in persistent atrial fibrillation (AF) J Cardiovasc Electrophysiol. 2011;22:1309–1316. doi: 10.1111/j.1540-8167.2011.02128.x. [DOI] [PubMed] [Google Scholar]
- 7.Kumagai K., Sakamoto T., Nakamura K. Combined dominant frequency and complex fractionated atrial electrogram ablation after circumferential pulmonary vein isolation of atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:975–983. doi: 10.1111/jce.12166. [DOI] [PubMed] [Google Scholar]
- 8.Kumagai K., Nakano M., Kutsuzawa D. The efficacy of ablation based on the combined use of the dominant frequency and complex fractionated atrial electrograms for non-paroxysmal atrial fibrillation. J Cardiol. 2016;67:540–550. doi: 10.1016/j.jjcc.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Teh A.W., Kistler P.M., Lee G. The relationship between complex fractionated electrograms and atrial low voltage zones during atrial fibrillation and paced rhythm. Europace. 2011;13:1709–1716. doi: 10.1093/europace/eur197. [DOI] [PubMed] [Google Scholar]
- 10.Kofune M., Okumura Y., Watanabe I. Comparative distribution of complex fractionated atrial electrograms, high dominant frequency (HDF) sites during atrial fibrillation and HDF sites during sinus rhythm. J Interv Card Electrophysiol. 2013;36:297–306. doi: 10.1007/s10840-012-9748-4. [DOI] [PubMed] [Google Scholar]
- 11.Okumura Y., Watanabe I., Kofune M. Characteristics and distribution of complex fractionated atrial electrograms and the dominant frequency during atrial fibrillation: relationship to the response and outcome of circumferential pulmonary vein isolation. J Interv Card Electrophysiol. 2012;34:267–275. doi: 10.1007/s10840-011-9637-2. [DOI] [PubMed] [Google Scholar]
- 12.Jadidi A., Lehrmann H., Park C. Targeted ablation of atrial fibrillation low voltage regions is associated with high rate of AF freedom despite low RF delivery – a novel substrate-based ablation approach for persistent atrial fibrillation. Heart Rhythm. 2014;11:S272. [Google Scholar]
- 13.Nagashima K., Okumura Y., Watanabe I. Does location of epicardial adipose tissue correspond to endocardial high dominant frequency or complex fractionated atrial electrogram sites during atrial fibrillation? Circ Arrhythm Electrophysiol. 2012;5:676–683. doi: 10.1161/CIRCEP.112.971200. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y.J., Tai C.T., Kao T. Spatiotemporal organization of the left atrial substrate after circumferential pulmonary vein isolation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:233–241. doi: 10.1161/CIRCEP.108.812024. [DOI] [PubMed] [Google Scholar]
- 15.Atienza F., Almendral J., Jalife J. Real-time dominant frequency mapping ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y.J., Tsao H.M., Chang S.L. Role of high dominant frequency sites in nonparoxysmal atrial fibrillation patients: insights from high-density frequency and fractionation mapping. Heart Rhythm. 2010;7:1255–1262. doi: 10.1016/j.hrthm.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Kogawa R., Watanabe I., Okumuya Y. Dominant frequencies and fractionation intervals: a comparison of bipolar and unipolar electrogram-derived values. J Nihon Univ Med Assoc. 2016 [in press] [Google Scholar]
- 18.Jadidi A.S., Lehrmann H., Keyl C. Ablation of persistent atrial fibrillation targeting low voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.002962. [in press] [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki T., Takenaka S., Murase T. Efficacy of line ablation based on low voltage areas in patients with cardioversion resistent atrial fibrillation. Circ J. 2016;80:I-2310. [Google Scholar]
- 20.Rolf S., Kircher S., Arya A. Tailored atrial substrate modification based on low voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:825–833. doi: 10.1161/CIRCEP.113.001251. [DOI] [PubMed] [Google Scholar]
- 21.Fukui A., Yamaguchi T., Nagamoto Y. Low voltage zone (LVZ) harbors rotors: an analysis with a novel (quasi-) real time phase mapping system called “ExTRa Mapping”. Circ J. 2015;79:I-741. [Google Scholar]
- 22.Lin Y., Yang B., Garcia F.C. Comparison of left atrial electrophysiologic abnormalities during sinus rhythm with different type of atrial fibrillation. J Interv Card Electrophysiol. 2014;39:57–67. doi: 10.1007/s10840-013-9838-y. [DOI] [PubMed] [Google Scholar]
- 23.Viles-Gonzalea J.F., Gomes J.A., Miller M.A. Areas with complex fractionated atrial electrograms recorded after pulmonary vein isolation represent normal voltage and conduction velocity in sinus rhythm. Europace. 2013;15:339–346. doi: 10.1093/europace/eus321. [DOI] [PubMed] [Google Scholar]
- 24.Roberts-Thompson K.C., Kistler P.M., Sanders P. Fractionated atrial electrograms during sinus rhythm: relationship to age, voltage and conduction velocity. Heart Rhythm. 2009;6:587–591. doi: 10.1016/j.hrthm.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Gerstenfeld E.P., Lavi N., Bazan V. Mechanism of complex fractionated electrograms recorded during atrial fibrillation in a canine model. Pacing Clin Electrophysiol. 2011;34:844–857. doi: 10.1111/j.1540-8159.2011.03071.x. [DOI] [PubMed] [Google Scholar]
- 26.Jadidi A.S., Duncan E., Miyazaki S. Functional nature of electrogram fractionation demonstrated by left atrial high-density mapping. Circ Arrhythm Electrophysiol. 2012;5:32–42. doi: 10.1161/CIRCEP.111.964197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jadidi A.S., Cochet H., Shah A. Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation: combined magnetic resonance imaging and high-density mapping. J Am Coll Cardiol. 2013;62:802–812. doi: 10.1016/j.jacc.2013.03.081. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto K., Tsuchiya T., Nagamoto Y. Characterization of bipolar electrograms during sinus rhythm for complex fractionated atrial electrograms recorded in patients with paroxysmal and persistent atrial fibrillation. Europace. 2010;12:494–501. doi: 10.1093/europace/euq033. [DOI] [PubMed] [Google Scholar]
- 29.Lee G., Roberts-Thompson K., Madry A. Relationship among complex signals, short cycle length activity, and dominant frequency in patients with long-lasting persistent AF: a high-density epicardial mapping study in humans. Heart Rhythm. 2011;8:1714–1719. doi: 10.1016/j.hrthm.2011.05.021. [DOI] [PubMed] [Google Scholar]