Abstract
Background
Hypertrophic cardiomyopathy (HCM) is characterized by myocyte hypertrophy, disarray, fibrosis, and increased risk for ventricular arrhythmias. Increased QT dispersion has been reported in patients with HCM, but the underlying mechanisms have not been completely elucidated. In this study, we examined the relationship between diffuse interstitial fibrosis, replacement fibrosis, QTc dispersion and ventricular arrhythmias in patients with HCM. We hypothesized that fibrosis would slow impulse propagation and increase dispersion of ventricular repolarization, resulting in increased QTc dispersion on surface electrocardiogram (ECG) and ventricular arrhythmias.
Methods
ECG and cardiac magnetic resonance (CMR) image analyses were performed retrospectively in 112 patients with a clinical diagnosis of HCM. Replacement fibrosis was assessed by measuring late gadolinium (Gd) enhancement (LGE), using a semi-automated threshold technique. Diffuse interstitial fibrosis was assessed by measuring T1 relaxation times after Gd administration, using the Look–Locker sequence. QTc dispersion was measured digitally in the septal/anterior (V1–V4), inferior (II, III, and aVF), and lateral (I, aVL, V5, and V6) lead groups on surface ECG.
Results
All patients had evidence of asymmetric septal hypertrophy. LGE was evident in 70 (63%) patients; the median T1 relaxation time was 411±38 ms. An inverse correlation was observed between T1 relaxation time and QTc dispersion in leads V1–V4 (p<0.001). Patients with HCM who developed sustained ventricular tachycardia had slightly higher probability of increased QTc dispersion in leads V1–V4 (odds ratio, 1.011 [1.004–1.0178, p=0.003). We found no correlation between presence and percentage of LGE and QTc dispersion.
Conclusion
Diffuse interstitial fibrosis is associated with increased dispersion of ventricular repolarization in leads, reflecting electrical activity in the hypertrophied septum. Interstitial fibrosis combined with ion channel/gap junction remodeling in the septum could lead to inhomogeneity of ventricular refractoriness, resulting in increased QTc dispersion in leads V1–V4.
Keywords: Hypertrophic cardiomyopathy, Corrected QT dispersion, Late gadolinium enhancement, T1 relaxation time
1. Introduction
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiovascular disease characterized pathologically by myocyte hypertrophy, myocyte disarray, fibrosis, and arteriolar remodeling [1]. HCM is also the most common cause of sudden cardiac death in young individuals [2]. Increased QT dispersion is considered a risk factor for sudden death in HCM [3], [4], [5], [6]. The possible mechanisms underlying increased QT dispersion in HCM are ion channel [7] and gap junction remodeling [8], leading to increased action potential duration, decreased conduction velocity, and increased spatiotemporal dispersion of repolarization [9], which would manifest as QRS prolongation and increased QT dispersion on surface electrocardiogram (ECG).
An important feature of HCM is inhomogeneity of the distribution of left ventricular (LV) hypertrophy, which has been shown to predispose to increased QT dispersion [10]. Another possible contributor to increased QT dispersion that has not been investigated is fibrosis. Fibrosis could slow impulse propagation and enhance dispersion of repolarization induced by hypertrophy.
Gadolinium (Gd)-enhanced cardiac magnetic resonance (CMR) imaging permits non-invasive assessment of replacement fibrosis [11] and diffuse interstitial fibrosis [12], [13], [14], which are commonly seen in patients with HCM [15]. Gadolinium rapidly diffuses out of capillaries into the cardiac interstitium, but is unable to cross intact cell membranes. A greater volume of distribution combined with slower kinetics of Gd efflux from areas of interstitial and replacement fibrosis leads to higher amounts of Gd per unit volume in areas of fibrosis compared with the normal myocardium, which is detected by CMR imaging.
In this study, we examined the relationship between fibrosis, dispersion of ventricular repolarization, and ventricular arrhythmias in patients with HCM. QTc dispersion on surface ECGs was used to assess dispersion of ventricular repolarization [16]. Diffuse interstitial fibrosis [17] and replacement fibrosis [14] (Fig. 1) were assessed by measuring post-contrast T1 relaxation time and late Gd enhancement (LGE), respectively, using CMR imaging.
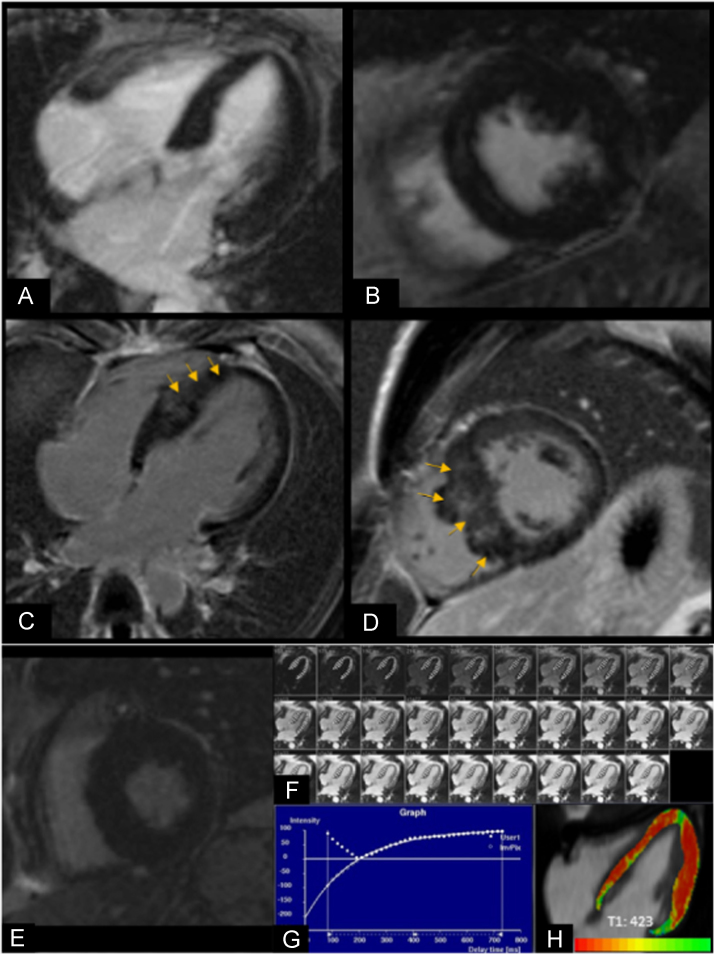
Fig. 1.
A, B. CMR images of a patient with HCM without LGE: (A) horizontal long-axis and (B) short-axis view of the nulled myocardium. C, D. CMR images of a patient with HCM with LGE within the septal wall of the LV (orange arrows) in the (C) horizontal long-axis and (D) short-axis view. E–H. T1 relaxation time calculation in a patient with HCM (E) Short-axis image shows nulled myocardium without LGE. (F) Horizontal long-axis image illustrates the method used for calculating T1 relaxation time where the endocardial and epicardial contours were manually drawn in every slice of the TI scout image. (G) Graph depicts how T1 times were calculated using pixel by pixel fit performed to a three-parameter model. (H) Horizontal long-axis image shows areas of interstitial fibrosis depicted in red–orange; mean T1 time was 423 ms.
2. Methods
The study was approved by the Johns Hopkins Institutional Review Board (IRB# NA_00079621, last approval date 01-07-2013). Consecutive, unrelated adult patients who were seen in the Johns Hopkins HCM Clinic between 2009 and 2012 were retrospectively studied if they fulfilled the standard diagnostic criteria for HCM, namely left ventricular hypertrophy (maximum wall thickness ≥15 mm) and/or septal-to-posterior free wall ratio > 1.3 by echocardiography, in the absence of other causes such as hypertension and/or valvular disease.
All patients had undergone contrast-enhanced CMR imaging and echocardiography within 12 months after ECG. Patients were excluded if they had a history of myocardial infarction, alcohol septal ablation, myectomy, ventricular pacing, left bundle branch block, poor ECG traces, and/or incomplete CMR data. None of the patients included in the study had an implantable cardioverter defibrillator (ICD) implanted before CMR imaging.
The mean follow-up period was 12 months. ICD discharges and ventricular tachycardia (VT) events were recorded by reviewing Holter, exercise ECG tracings, ICD interrogation reports, and clinic visit notes. Sustained VT was considered as VT with a rate of >100 beats per minute and duration of >30 s or VT that resulted in an ICD shock or anti-tachycardia pacing. Appropriate ICD therapies were all confirmed by an electrophysiologist and resulted from ventricular tachyarrhythmias, not arrhythmias, such as atrial flutter or fibrillation associated with a rapid ventricular response or device/lead malfunction.
2.1. Cardiac magnetic resonance imaging
2.1.1. Image acquisition
All CMR imaging was performed on a 1.5 T (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany) system. LGE images were obtained by using 2D FLASH T1-weighed gradient-echo in short-axis view 10 min after administration of 0.2-mmol/kg gadopentate dimeglumine. Myocardial T1 mapping was acquired with gradient echo multiphase-IR (TI scout) images from the Look-Locker (LL) sequence, using a single image plane in four-chamber view.
Please see Supplemental Data Section for detailed methods for CMR and Echocardiography image acquisition and image analysis.
2.2. Electrocardiographic analyses
Standard 12-lead ECGs were obtained with patients in the supine position and recorded at a paper speed of 25 mm/s. Heart rate, PR interval, QRS duration, QT interval and QTc (using Bazett׳s formula) were measured automatically at acquisition and were confirmed using Cardio Caliper software®. A prolonged QTc interval was defined as ≥450 ms and ≥470 ms in men and women, respectively, in the absence of bundle branch block or intraventricular conduction delay. QT intervals were measured from the QRS onset to the end of the T wave, defined as the intersecting point of a tangent line on the terminal T wave and T-P baseline. QTc dispersion was defined as the difference between the maximum and minimum QTc [16] in the following groups of leads that reflect electrical activity in septal/anterior (V1, V2, V3, and V4), inferior (II, III, and aVF), and lateral (I, aVL, V5, and V6) heart walls. Leads with T waves whose height/depth was <1.5 mm were excluded from the analysis. Lead grouping was performed based on previous studies correlating ECG with coronary artery territories in patients presenting with acute myocardial infarction [18], [19].
2.3. Statistical analysis
After calculating the QTc dispersion in each patient, the collective leads, representing selected myocardial regions, were characterized as a median value (Table 1) because distribution of values was non-Gaussian. The medians were used to describe continuous variables, unless stated otherwise. The Wilcoxon-rank sum test was applied for independent samples not showing normal distribution. T1 relaxation time was plotted against QTc dispersion by using the Spearman correlation coefficient. Spearman correlation was also used for comparing LV mass values with T1 relaxation times and QTc dispersion. When performing multiple correlations, the Bonferroni correction was applied to adjust the level of statistical significance of correlation coefficients and therefore reduce type I error. The threshold levels of significance for correlation coefficients were adjusted for multiple comparisons in a set of k correlation coefficients (k=1, 5, 10, 20, 50, 100) using the Bonferroni correction.
Table 1.
Demographic and clinical characteristics of the HCM cohort (n=112).
| Age (years) | 49±15 |
|---|---|
| Male | 73 (65) |
| NYHA Class I | 45 (40) |
| NYHA Class II | 37 (33) |
| NYHA Class III | 14 (12.5) |
| Symptoms | |
| Angina | 36 (32) |
| Dyspnea | 47 (42) |
| Presyncope, syncope | 15 (13) |
| Palpitations | 14 (12.5) |
| Dizziness | 19 (17) |
| Arrhythmia (NSVT, VT) | 30 (27) |
| Medications | |
| Beta-blockers | 73 (65) |
| CCB | 19 (17) |
| ACEi /ARB | 17 (15) |
| ICD | 37 (33) |
Values in parentheses indicate percentages. NYHA: New York Heart Association; CCB: calcium-channel blockers, ACEi: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blocker. Arrhythmia: non-sustained ventricular tachycardia (NSVT) and sustained ventricular tachycardia (VT).
3. Results
We studied 112 patients (mean age 49±15 years); 73 (65%) were male. Majority of the patients were classified as New York Heart Association (NYHA) class I or II (Table 1). Angina and pre-syncope were present in 36 (32%) and 15 (13%) patients, respectively; a significant proportion of patients (27%) had ventricular arrhythmias, consisting of non-sustained VT (n=17) and sustained VT (n=13).
3.1. CMR, Echocardiogram, and ECG analyses
All patients had evidence of asymmetric septal hypertrophy. LGE by CMR was evident in 70 (63%) patients and was most frequently localized within the septum and/or anterior right ventricular insertion point. Because LGE has been reported to be a risk factor for ventricular arrhythmias in HCM, patients with HCM were categorized into two groups based on the presence/absence of LGE by CMR (Table 2).
Table 2.
Imaging characteristics of patients with HCM with and without LGE.
| No LGE (n=42) | LGE (n=70) | p Value | |
|---|---|---|---|
| ECHO | |||
| Rest LVOTG (mmHg) | 11±8 | 10±8 | 0.50 |
| Exercise LVOTG (mmHg) | 35±32 | 39±36 | 0.50 |
| IVS_DT (cm) | 1.9±0.4 | 2.2±0.5 | 0.001 |
| CMR | |||
| LV mass index | 80±26 | 89±31 | <0.001 |
| LVEF (%) | 67±8 | 65±7 | 0.047 |
ECHO: echocardiography; LVOTG: left ventricular outflow tract gradient; IVS_DT: inter-ventricular septum diastolic wall thickness; CMR: cardiac magnetic resonance imaging; LV: left ventricle; LVEF: left ventricular ejection fraction.
The peak left ventricular outflow tract gradients at rest and following exercise were similar in the two groups. The LV mass index was significantly higher (p<0.001) in patients with HCM with evidence of LGE. Median T1 relaxation time was 411±38 ms for the entire population. We found no difference in T1 relaxation times between patients with HCM with and without LGE (Table 3).
Table 3.
QTc dispersion and T1 relaxation times in patients with HCM with and without LGE.
| QTc dispersion | All | LGE | No LGE | p Value |
|---|---|---|---|---|
| II, III, aVF | 22±20 ms | 19±20 ms | 23±14 ms | 0.30 |
| I, aVL, V5, V6 | 27±15 ms | 27±13 ms | 29±20 ms | 0.97 |
| V1, V2, V3, V4 | 24±21 ms | 23±21 ms | 27±19 ms | 0.77 |
| T1 relaxation time | 411±38 ms | 406±37 ms | 417±425 ms | 0.21 |
All continuous variables are medians represented with interquartile deviations. The p Value was calculated by using a two-sample Wilcoxon rank-sum test.
QRS duration was similar in patients with HCM with/without LGE (LGE positive: 96±9 ms; LGE negative: 97±11 ms; p=0.6). No significant difference was found in QTc dispersion between patients with HCM with/without LGE (Table 3).
3.2. Association between CMR and ECG variables
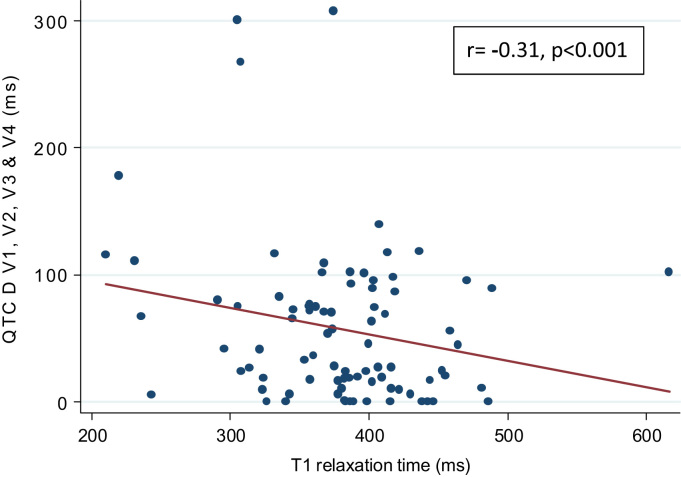
We observed a statistically significant inverse correlation between T1 relaxation times and QTc dispersion in the V1–V4 lead group (Table 4, Fig. 2). LV mass and presence/percentage of LGE were not correlated with QTc dispersion.
Table 4.
Correlation between T1 relaxation time and QTc dispersion.
| QTc dispersion Lead groups | Rho Spearman (n=99) | p Value | Bonferroni correction |
|---|---|---|---|
| II, III, aVF | −0.22 | 0.03 | 0.21 |
| I, aVL, V5, V6 | −0.16 | 0.12 | 0.84 |
| V1, V2, V3, V4 | −0.31 | <0.001 | <0.001 |
Fig. 2.
Modest inverse correlation is present between T1 relaxation time and QTc dispersion in leads V1–V4 (r = −0.31; p<0.001).
3.3. Arrhythmias
Patients with HCM with sustained VT had a slightly higher probability of increased QTc dispersion in the V1–V4 lead group (odds ratio, 1.011 [1.004–1.0178; p=0.003). QTc dispersion was not significantly different in patients with non-sustained VT or syncope (Table 5). We observed no correlation between T1 relaxation time and ventricular arrhythmias.
Table 5.
Association between ventricular arrhythmia and QTc dispersion in the V1–V4 lead group.
| OR | p Value | 95% CI | |
|---|---|---|---|
| Sustained VT | |||
| QTc dispersion V1–V4 | 1.011 | 0.003 | 1.004–1.018 |
| Non-sustained VT | |||
| QTc dispersion V1–V4 | 0.998 | 0.604 | 0.99–1.006 |
| Syncope | |||
| QTc dispersion V1–V4 | 1.003 | 0.232 | 0.998–1.008 |
4. Discussion
4.1. Diffuse interstitial fibrosis, but not replacement fibrosis, is associated with dispersion of ventricular repolarization in leads V1–V4
The main result of our study is a statistically significant correlation between interstitial fibrosis and QTc dispersion in leads V1–V4, which reflect electrical activity in the septum/anterior wall. Because all patients with HCM in our study had asymmetric septal hypertrophy, we attribute this result to structural and electrical (ion channel and gap junction) [9] remodeling in the hypertrophied septum/anterior wall, resulting in increased transmural dispersion of repolarization. At the cellular level, a high degree of cell–cell coupling dampens transmural repolarization heterogeneities normally present due to differences in electrophysiology between epicardial, endocardial, and mid-myocardial myocytes. Interstitial fibrosis would be expected to amplify the transmural dispersion of repolarization induced by hypertrophy by reducing electrotonic interactions between cardiac myocytes in the septum/anterior wall. Lack of association between interstitial fibrosis, which is diffuse in HCM, and QTc dispersion in the inferior and lateral walls, may be attributed to lack of overt ventricular hypertrophy and electrical remodeling in these regions. Our results, which suggest greater degree of electrical and structural remodeling in the interventricular septum, compared with other regions, are concordant with a previous invasive electrophysiological study that compared electrograms and local stimulus-to-V intervals in the septum and lateral wall in nine patients with HCM who demonstrated asymmetric septal hypertrophy. Using 3D electroanatomic mapping, Schumacher et al. observed a high prevalence of fractionated, split, and low amplitude potentials associated with conduction slowing in the interventricular septum, but not in the lateral wall [20], indicating markedly higher prevalence of electrophysiologic abnormalities and heterogeneity in the septum in patients with HCM.
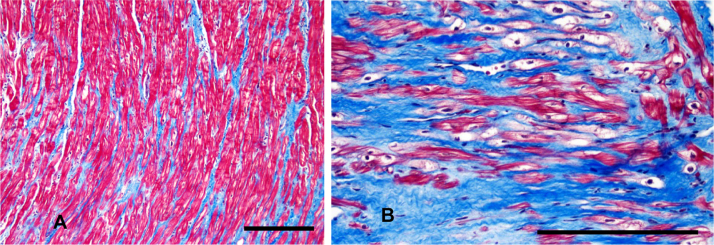
CMR imaging and pathologic analysis of the septal myocardium [21] in patients undergoing surgical myectomy reveal a high prevalence of interstitial and replacement fibrosis in the interventricular septum (Fig. 3A and B). Based on these results, we expected a correlation between replacement fibrosis (LGE) and QTc dispersion, but we were surprised to find no association between LGE and QTc dispersion. This result may be explained by LGE being a less sensitive fibrosis indicator than T1 relaxation time [15].
Fig. 3.
Septal tissue from a patient with HCM (from the JHU-HCM Registry) who underwent surgical septal myectomy. Masson Trichrome stain reveals evidence of (A) interstitial fibrosis and (B) replacement fibrosis in blue color. Calibration bars represent 200 µm.
4.2. Fibrosis imaging by CMR
CMR imaging is the gold standard imaging technique to assess cardiac anatomy, function, fibrosis, edema, and inflammation [22] in a variety of cardiac diseases. Replacement fibrosis regions have higher Gd concentrations compared with normal myocardium due to increased distribution volume and delayed Gd clearance. Differences in tissue concentrations of Gd between areas of replacement fibrosis and normal myocardium results in the appearance of replacement fibrosis as focal white areas, and normal myocardium as black, using the inversion-recovery sequence (Fig. 1A–D). LGE has recently been shown to represent both interstitial and replacement fibrosis when measured at 4 SD and 5 SD above the mean signal intensity of the normal myocardium [15]. Moravsky et al. found that a threshold of 10 SD was required to discriminate replacement fibrosis from interstitial fibrosis [15]. A threshold of 6 SD was used in this study; hence, LGE probably reflects both replacement and interstitial fibroses.
Reduced post-contrast T1 relaxation times have been shown to correlate with diffuse interstitial fibrosis [17]. This technique quantifies the T1 relaxation time of a tissue by using image-based signal intensities. Here, a specific inversion recovery time is used to determine the recovery rate of longitudinal magnetization. Increased Gd concentration in diffuse interstitial fibrosis speeds magnetization recovery, resulting in the shortening of T1 relaxation time. This technique overcomes the limitation of standard LGE techniques where diffuse fibrosis is not detectable because of the requirement to null the signal in a myocardial reference region, which is likely abnormal. Reduced T1 relaxation times have been shown to correlate with serum biomarkers of fibrosis in genotype-positive patients with HCM who lack LVH or evidence of LGE, indicating that it is an early and sensitive indicator of structural remodeling/fibrosis in HCM. Notably, small pathologic studies in asymptomatic children and young adults with HCM who died suddenly [23] reveal marked increase and disorganization of interstitial and perivascular collagen content, but no evidence of replacement fibrosis, suggesting the importance of including an assessment of interstitial fibrosis in risk stratification for ventricular arrhythmias in HCM.
4.3. QTc dispersion reflects dispersion of ventricular repolarization
Cardiac myocytes are well coupled electrically by gap junctions in normal hearts, resulting in rapid propagation of the electrical impulse and repolarization. Experimental models reveal that cardiac pathologies induce heterogeneous changes in myocyte electrophysiology, which primarily affect the repolarization phase of the action potential, leading to increased transmural dispersion of ventricular repolarization [24]. Transmural dispersion of repolarization is quantified by the difference between the longest and shortest action potentials in the heart. QTc dispersion has been proposed as an index of heterogeneity of ventricular repolarization — this is supported by the link between dispersion of ventricular recovery times (measured using monophasic action potentials) and genesis of arrhythmias [24], [25], [26].
QTc dispersion has been measured in several lead groups [27]. Whether QTc dispersion truly represents dispersion of ventricular repolarization or is a mere reflection of different projections of cardiac electrical activity onto varying lead axes has been debated [28], [29]. Coumel et al. have opined that QTc dispersion measurement in the unipolar leads, V1–V6 (as opposed to limb leads), is most reflective of local activity in the myocardium. A statistically significant inverse correlation was only observed between T1 relaxation times and QTc dispersion in the V1–V4 lead group in our study, which strengthens the significance of our results. Increased QTc dispersion has also been reported in patients with hypertensive heart disease, dilated cardiomyopathy, and cardiac amyloidosis [30], [31], [32].
4.4. Lack of correlation between QTc dispersion and LV hypertrophy
We found no association between LV mass and QTc dispersion in patients with HCM in contrast to previous studies in hypertensive patients with LV hypertrophy [33]. A possible explanation for this result is as follows: unlike hypertension, where increased afterload predictably leads to hypertrophy and fibrosis, the molecular mechanisms underlying fibrosis and hypertrophy in HCM are probably separate. This is exemplified in patients with HCM with mutations in the gene encoding cardiac troponin T (TNNT2) who often have mild LV hypertrophy [34] on clinical imaging, evidence of interstitial fibrosis and/or myocyte disarray [35], and high incidence of sudden cardiac death. Hence, QTc dispersion would be expected to correlate with interstitial fibrosis, but may not correlate with degree of hypertrophy in HCM.
4.5. Clinical Implications
The link between increased QTc dispersion and ventricular arrhythmias has been previously demonstrated in patients with HCM [3], long QT syndrome [16], and myocardial infarction [36], [37]. In our study of 112 patients with HCM, patients with sustained VT (but not non-sustained VT) had slightly greater odds of higher QTc dispersion. A possible explanation for this result is that QTc dispersion could predispose to development of reentrant arrhythmias, manifesting as sustained VT; in contrast, triggered activity due to abnormalities in Ca2+ handling could underlie non-sustained VT.
No association was present between T1 relaxation times and ventricular arrhythmias. These results could be attributed to the small numbers of patients and short follow-up in our study. Another possible explanation is that fibrosis is pro-arrhythmic only in the presence of dispersion of ventricular repolarization.
4.6. Limitations
Myocyte disarray, which is commonly seen in HCM, can also affect T1 relaxation times and cannot be distinguished from interstitial fibrosis by CMR. All T1 relaxation time calculations were performed retrospectively. Although a previously validated correction factor was used, contrast dose and timing of imaging post-injection were variable, which could lead to slight variations in T1-weighted scout images. We did not correct for the signal-to-noise ratio; therefore, an incorrect selection of the inversion time could have resulted in poor myocardial suppression, increasing the signal-to-noise ratio with possible overestimation of LGE quantification. None of the patients included in this study underwent surgical septal myectomy or endomyocardial biopsy. Hence, we were unable to correlate CMR results with histopathology in this study.
5. Conclusion
This is the first non-invasive study that suggests a link between interstitial fibrosis and dispersion of ventricular repolarization in patients with HCM. The association between interstitial fibrosis and QTc dispersion was most evident in leads reflecting electrical activity in the septum/anterior wall, the maximally hypertrophied segments. Our results suggest that interstitial fibrosis and ion channel/gap junction remodeling in the septum could lead to inhomogeneity of ventricular refractoriness, resulting in increased QTc dispersion in leads V1–V4. Prospective studies incorporating QTc dispersion and CMR assessment of interstitial fibrosis (T1 relaxation time) in addition to replacement fibrosis (LGE) may be useful to improve risk stratification for ventricular arrhythmias in patients with HCM.
Conflict of interest
All authors declare no conflict of interest related to this study.
Acknowledgments
This study was supported by a grant from the John Taylor Babbit Foundation. We are grateful to Dr. Marc Halushka for providing histopathology images and Dr. Nestor Enrique Vasquez for assistance with manuscript preparation.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.joa.2016.10.005.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Hansen M.W., Merchant N. MRI of hypertrophic cardiomyopathy: part I, MRI appearances. Am J Roentgenol. 2007;189:1335–1343. doi: 10.2214/AJR.07.2286. [DOI] [PubMed] [Google Scholar]
- 2.Maron B.J. Sudden death in hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009;2:368–380. doi: 10.1007/s12265-009-9147-0. [DOI] [PubMed] [Google Scholar]
- 3.Buja G., Miorelli M., Turrini P. Comparison of QT dispersion in hypertrophic cardiomyopathy between patients with and without ventricular arrhythmias and sudden death. Am J Cardiol. 1993;72:973–976. doi: 10.1016/0002-9149(93)91118-2. [DOI] [PubMed] [Google Scholar]
- 4.Miorelli M., Buja G., Melacini P. QT-interval variability in hypertrophic cardiomyopathy patients with cardiac arrest. Int J Cardiol. 1994;45:121–127. doi: 10.1016/0167-5273(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 5.Yetman A.T., Hamilton R.M., Benson L.N. Long-term outcome and prognostic determinants in children with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1998;32:1943–1950. doi: 10.1016/s0735-1097(98)00493-8. [DOI] [PubMed] [Google Scholar]
- 6.Yi G., Elliot P., McKenna W.J. QT dispersion and risk factors for sudden cardiac death in patients with hypertrophic cardiomyopathy. Am J Cardiol. 1998;82:1514–1519. doi: 10.1016/s0002-9149(98)00696-1. [DOI] [PubMed] [Google Scholar]
- 7.Hiramatsu M., Furukawa T., Sawanobori T. Ion channel remodeling in cardiac hypertrophy is prevented by blood pressure reduction without affecting heart weight increase in rats with abdominal aortic banding. J Cardiovasc Pharmacol. 2002;39:866–874. doi: 10.1097/00005344-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Emdad L., Uzzaman M., Takagishi Y. Gap junction remodeling in hypertrophied left ventricles of aortic-banded rats: prevention by angiotensin II type 1 receptor blockade. J Mol Cell Cardiol. 2001;33:219–231. doi: 10.1006/jmcc.2000.1293. [DOI] [PubMed] [Google Scholar]
- 9.Guo D., Yu M., Liu Q. Ventricular hypertrophy amplifies transmural dispersion of repolarization by preferentially increasing the late sodium current in endocardium. J Electrocardiol. 2014;47:642–648. doi: 10.1016/j.jelectrocard.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Sakata K., Shimizu M., Ino H. QT dispersion and left ventricular morphology in patients with hypertrophic cardiomyopathy. Heart. 2003;89:882–886. doi: 10.1136/heart.89.8.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubinshtein R., Glockner J.F., Ommen S.R. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51–58. doi: 10.1161/CIRCHEARTFAILURE.109.854026. [DOI] [PubMed] [Google Scholar]
- 12.Fang L., Beale A., Ellims A.H. Associations between fibrocytes and postcontrast myocardial T1 times in hypertrophic cardiomyopathy. J Am Heart Assoc. 2013;2:5. doi: 10.1161/JAHA.113.000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puntmann V.O., Voigt T., Chen Z. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:475–484. doi: 10.1016/j.jcmg.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 14.O׳Hanlon R., Grasso A., Roughton M. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Moravsky G., Ofek E., Rakowski H. Myocardial fibrosis in hypertrophic cardiomyopathy: accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging. 2013;6:587–596. doi: 10.1016/j.jcmg.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Malik M., Batchvarov V.N. Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol. 2000;36:1749–1766. doi: 10.1016/s0735-1097(00)00962-1. [DOI] [PubMed] [Google Scholar]
- 17.Sibley C.T., Noureldin R.A., Gai N. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265:724–732. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs R.M., Achuff S.C., Grunwald L. Electrocardiographic localization of coronary artery narrowings: studies during myocardial ischemia and infarction in patients with one-vessel disease. Circulation. 1982;66:1168–1176. doi: 10.1161/01.cir.66.6.1168. [DOI] [PubMed] [Google Scholar]
- 19.Blanke H., Cohen M., Schlueter G.U. Electrocardiographic coronary arteriographic correlations during acute myocardial infarction. Am J Cardiol. 1984;54:249–255. doi: 10.1016/0002-9149(84)90176-0. [DOI] [PubMed] [Google Scholar]
- 20.Schumacher B., Gietzen F.H., Neuser H. Electrophysiological characteristics of septal hypertrophy in patients with hypertrophic obstructive cardiomyopathy and moderate to severe symptoms. Circulation. 2005;112(14):2096–2101. doi: 10.1161/CIRCULATIONAHA.104.515643. [DOI] [PubMed] [Google Scholar]
- 21.Kwon D.H., NGM Smedira, Rodriguez E.R. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol. 2009;54:242–249. doi: 10.1016/j.jacc.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Ho C.Y., Abbasi S.A., Neilan T.G. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6:415–422. doi: 10.1161/CIRCIMAGING.112.000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirani J., Pick R., Roberts W.C., Maron B.J. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol. 2000;35:36–44. doi: 10.1016/s0735-1097(99)00492-1. [DOI] [PubMed] [Google Scholar]
- 24.Killeen M.J., Sabir I.N., Grace A.A. Dispersions of repolarization and ventricular arrhythmogenesis: lessons from animal models. Prog Biophys Mol Biol. 2008;98:219–229. doi: 10.1016/j.pbiomolbio.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Kuo C.S., Amlie J.P., Munakata K. Dispersion of monophasic action potential durations and activation times during atrial pacing, ventricular pacing, and ventricular premature stimulation in canine ventricles. Cardiovasc Res. 1983;17:152–161. doi: 10.1093/cvr/17.3.152. [DOI] [PubMed] [Google Scholar]
- 26.Kuo C.S., Munakata K., Reddy C.P. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation. 1983;67:1356–1367. doi: 10.1161/01.cir.67.6.1356. [DOI] [PubMed] [Google Scholar]
- 27.Macfarlane P.W., McLaughlin S.C., Rodger J.C. Influence of lead selection and population on automated measurement of QT dispersion. Circulation. 1998;98:2160–2167. doi: 10.1161/01.cir.98.20.2160. [DOI] [PubMed] [Google Scholar]
- 28.Mayet J., Kanagaratnam P., Shahi M. QT dispersion in athletic left ventricular hypertrophy. Am Heart J. 1999;137:678–681. doi: 10.1016/s0002-8703(99)70222-x. [DOI] [PubMed] [Google Scholar]
- 29.Coumel P., Maison-Blanche P., Badilini F. Dispersion of ventricular repolarization: reality? Illusion? Significance? Circulation. 1998;97:2491–2493. doi: 10.1161/01.cir.97.25.2491. [DOI] [PubMed] [Google Scholar]
- 30.Parthenakis F.I., Vardas P.E., Ralidis L. QT interval in cardiac amyloidosis. Clin Cardiol. 1996;19:51–54. doi: 10.1002/clc.4960190110. [DOI] [PubMed] [Google Scholar]
- 31.Clarkson P.B., Naas A.A., McMahon A., MacLeod C. QT dispersion in essential hypertension. Q J Med. 1995;88:327–332. [PubMed] [Google Scholar]
- 32.Alonso J.L., Martinez P., Vallverdu M. Dynamics of ventricular repolarization in patients with dilated cardiomyopathy versus healthy subjects. Ann Noninvasive Electrocardiol. 2005;10:121–128. doi: 10.1111/j.1542-474X.2005.05583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oikarinen L., Nieminen M.S., Viitasalo M. Relation of QT interval and QT dispersion to echocardiographic left ventricular hypertrophy and geometric pattern in hypertensive patients. The LIFE study. The Losartan Intervention For Endpoint Reduction. J Hypertens. 2001;19:1883–1891. doi: 10.1097/00004872-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Pasquale F., Syrris P., Kaski J.P. Long-term outcomes in hypertrophic cardiomyopathy caused by mutations in the cardiac troponin T gene. Circ Cardiovasc Genet. 2012;5:10–17. doi: 10.1161/CIRCGENETICS.111.959973. [DOI] [PubMed] [Google Scholar]
- 35.Varnava A.M., Elliott P.M., Baboonian C. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001;104:1380–1384. doi: 10.1161/hc3701.095952. [DOI] [PubMed] [Google Scholar]
- 36.Chang M.G., Zhang Y., Chang C.Y. Spiral waves and reentry dynamics in an in vitro model of the healed infarct border zone. Circ Res. 2009;105:1062–1071. doi: 10.1161/CIRCRESAHA.108.176248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higham P.D., Furniss S.S., Campbell R.W. QT dispersion and components of the QT interval in ischaemia and infarction. Br Heart J. 1995;73:32–36. doi: 10.1136/hrt.73.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material