Abstract
Background
The relationship between pulmonary vein (PV) arrhythmogenicity and its anatomy has been reported. However, that of the superior vena cava (SVC) has not been well discussed. Arrhythmogenic response induced by pacing stimulation at SVC might help with identifying SVC arrhythmogenicity. The purpose of this study was to investigate the relationship between the anatomical dilatation of SVC and the arrhythmogenic response induced by pacing at SVC.
Methods
Forty-three patients who underwent atrial fibrillation (AF) ablation were enrolled in this study. After PV isolation, scan pacing (up to triple extra stimulation following intrinsic sinus beats) was performed at SVC. The arrhythmogenic response was defined as following: (1) repetitive atrial responses, (2) non-sustained, and (3) sustained AF/ atrial tachycardia. To assess the dilatation of SVC, we measured the cross-sectional area of the SVC (SVC-area) using multi-planar reconstruction CT imaging.
Results
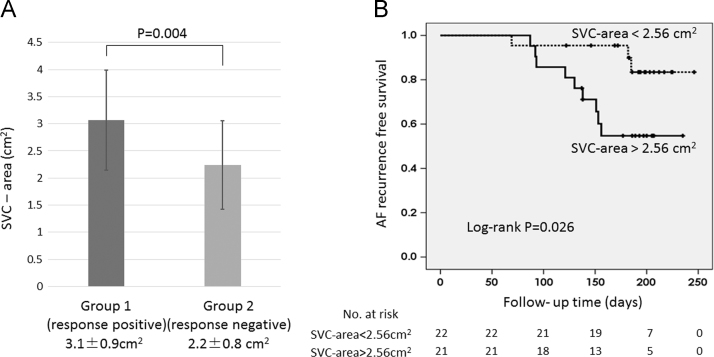
Arrhythmogenic responses were documented in 24 patients (Group 1). No arrhythmogenic responses were documented in the remaining 19 patients (Group 2). The SVC-area was significantly larger in Group 1 than Group 2 (3.1±0.9 vs. 2.2±0.8 cm2, P=0.004). A multivariate analysis revealed only SVC-area was associated with arrhythmogenic responses (odds ratio=2.87, CI 1.05–7.82, P=0.04). Furthermore, AF recurrence rate was significantly higher in patients with SVC-area>2.56 cm2 than those with SVC-area <2.56 cm2 (9 [42.9%] of 21 vs. 3 [13.6%] of 22, P=0.026).
Conclusion
Dilatation of SVC was associated with an arrhythmogenic response, and the AF recurrence rate was significantly higher in patients with large SVC-area. Adjunctive catheter intervention for the SVC might be indicated in patients with a dilated SVC and an arrhythmogenic response.
Abbreviations: AF, atrial fibrillation; AT, atrial tachycardia; CT, computed tomography; PV, pulmonary vein; SVC, superior vena cava
Keywords: Superior vena cava, Scan pacing, Arrhythmogenic response, Atrial fibrillation, Catheter ablation
1. Introduction
Pulmonary veins (PVs) have been reported to be the major site of ectopic foci initiating paroxysmal atrial fibrillation (AF) [1]. Pulmonary vein isolation (PVI) is an established therapy eliminating those foci [2], [3]. Non-PV ectopic foci from the superior vena cava (SVC) or coronary sinus have also been reported [4], [5]. Thus, an SVC isolation (SVCI) is considered to improve the rhythm outcome in patients with those foci [6]. However, the following procedural complications have also been documented after and during an SVCI: sinus node injury, right phrenic nerve injury, and SVC stenosis [7], [8], [9]. Therefore, the assessment of the arrhythmogenicity of the SVC would be necessary before the procedure. The relationship between PV arrhythmogenicity and its anatomy has been reported [10], [11], [12]. However, the relationship between the SVC arrhythmogenicity and its anatomy has not been well discussed [13], [14]. We hypothesized that dilatation of the SVC could be associated with SVC arrhythmogenicity. To avoid overestimating arrhythmogenicity at the SVC, we sought to assess the arrhythmogenic response induced by a simple extra stimulus pacing (scan pacing) at the SVC as a surrogate index of the arrhythmogenicity. And then, we also analyzed the relationship between the arrhythmogenic response and the anatomical dilatation of the SVC assessed by computed tomography (CT) images.
2. Material and methods
2.1. Patient selection
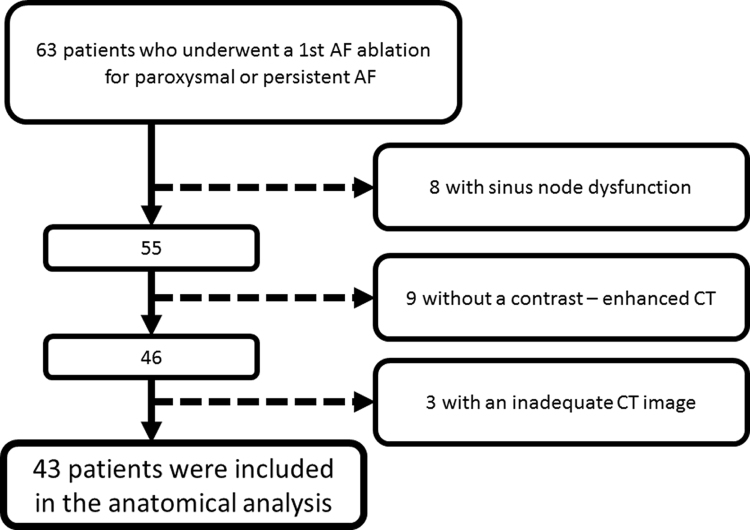
A total of 63 consecutive patients underwent their 1st catheter ablation for paroxysmal or persistent AF at Kobe University hospital from January to August 2015. Patients were excluded from this study due to the following criteria: (1) patients with long pauses after scan pacing due to sinus node dysfunction, (2) patients without a prior contrast-enhanced CT due to the chronic kidney disease or contrast allergies, (3) patients with inadequate CT imaging due to artifact from the implanted pacemaker lead. Of those, 8 with sinus node dysfunction, 9 without a prior contrast-enhancement CT, and 3 with pacemakers were excluded, resulting in a final cohort of 43 patients (Fig. 1).
Fig. 1.
Patient flow charts. Study flow diagram depicting the selection criteria for the patients included in the analysis. AF=atrial fibrillation, CT=computed tomography.
Verbal and written informed consent was obtained from all patients prior to the procedure. The study protocol, including the data collection and record keeping, was approved by the institutional ethical committee of our hospital. (The approval number: 1901, and the approval date: 08. April. 2016).
2.2. Ablation procedure
Prior to the procedure, all patients gave their written informed consent. Transesophageal echocardiography was performed to exclude any thrombus formation. The patients were studied under deep dexmedetomidine or propofol sedation while breathing spontaneously. Standard electrode catheters were placed in the right ventricular apex and coronary sinus, after which a single transseptal puncture was performed.
After integration of a 3-dimensional (3D) model of the anatomy of the left atrium (LA) and PVs obtained from preinterventional CT, mapping and ablation were performed using the CARTO3 (Biosense Webster Inc., Diamond Bar, CA, USA) or NavX (St. Jude Medical, Inc., St. Paul, MN) system as a guide. Prior to the ablation, a circular mapping catheter (Lasso, Biosense Webster, Diamond Bar, CA; Optima, St. Jude Medical Inc., St. Paul, MN, USA), ablation catheter, and ultrasound catheter (SOUNDSTAR, Biosense Webster, Diamond Bar, CA)–reconstructed LA and PV anatomy were aligned with the CT image [15], [16].
RF alternating current was delivered in a unipolar mode between the irrigated-tip electrode of the ablation catheter and an external back-plate electrode. The initial RF generator setting consisted of an upper catheter tip temperature of 43 °C, maximal RF power of 20–30 W, and irrigation flow rate of 17 or 13 mL/min using the CARTO3 and NavX systems, respectively. All patients underwent an extensive encircling pulmonary vein isolation [17]. RF applications were performed in a point-by-point manner, for 20–30 s at each point. The encircling ablation line was created approximately 0.5–1 cm from the PV ostia. As for the Cryoballoon ablation, ablation was performed with 3 min deliveries using a 28 mm Arctic Front catheter (Medtronic CryoCath, Minneapolis, MN) with a 14 French Flex Cath Steerable Sheath (Medtronic CryoCath, Minneapolis, MN) [3], [18]. The procedural endpoint was electrophysiologically proven bidirectional block of the PV-encircling ablation lines, confirmed with a circular mapping catheter. After proving bidirectional block of the PVs, scan pacing from the SVC was performed to assess the arrhythmogenicity of the SVC. Ablation of the cavotricuspid isthmus (CTI) was performed only if typical right atrial flutter was either documented previously or induced by pacing stimulation at the end of the procedure.
2.3. Detailed protocols of the SVC scan pacing
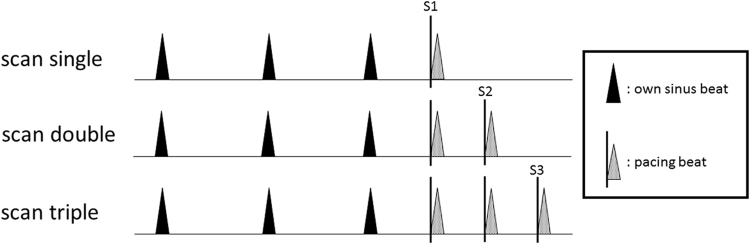
After the PVI, a ring catheter or decapolar catheter (EP star, Japan Lifeline Inc., Japan) was placed into the SVC and scan pacing was performed from paired electrodes of the ring catheter or distal electrode of the decapolar catheter where a maximal SVC potential could be recorded. The detailed protocol of the scan pacing was as follows: (1) a single extrastimulus (S1) following 3 intrinsic sinus beats with a coupling interval of 400 ms decreasing down to the atrial effective refractory period (ERP), (2) double extrastimuli (S1S2) with a coupling interval of 400 ms decreasing down to the ERP, (3) and triple extrastimuli (S1S2S3) (Fig. 2).
Fig. 2.
“The scan single” protocol in the scan pacing indicate a single extrastimulus following intrinsic sinus beats with a coupling interval of 400 ms decreasing down to the atrial ERP. “The scan double” and “The scan triple” indicate double and triple extrastimuli following intrinsic beats, respectively. The black and shaded arrowheads indicate the intrinsic sinus beats and extrastimuli, respectively. S1, S2 and S3 indicate the first, second, and third extrastimuli, respectively.
2.4. Assessment of the arrhythmogenic response induced by SVC scan pacing
To assess the arrhythmogenic response induced by SVC scan pacing, induced arrhythmias were defined as the following three pacing responses: (1) sustained AF/AT >3 min in duration, (2) self-terminating non-sustained AF/AT (NSAF/AT) <3 min in duration and (3) reproducible repetitive atrial responses (RARs) of a maximum of three beats. Sustained AF/AT, NSAF/AT, and RARs were categorized as “response positive” (Group 1). RARs without reproducibility, as well as no pacing response, were categorized as “response negative” (Group 2). Furthermore, the electrical parameters including the effective refractory period (ERP) and amplitude at the pacing site in the SVC were compared between two groups.
2.5. Measurement of the cross-sectional area of the SVC
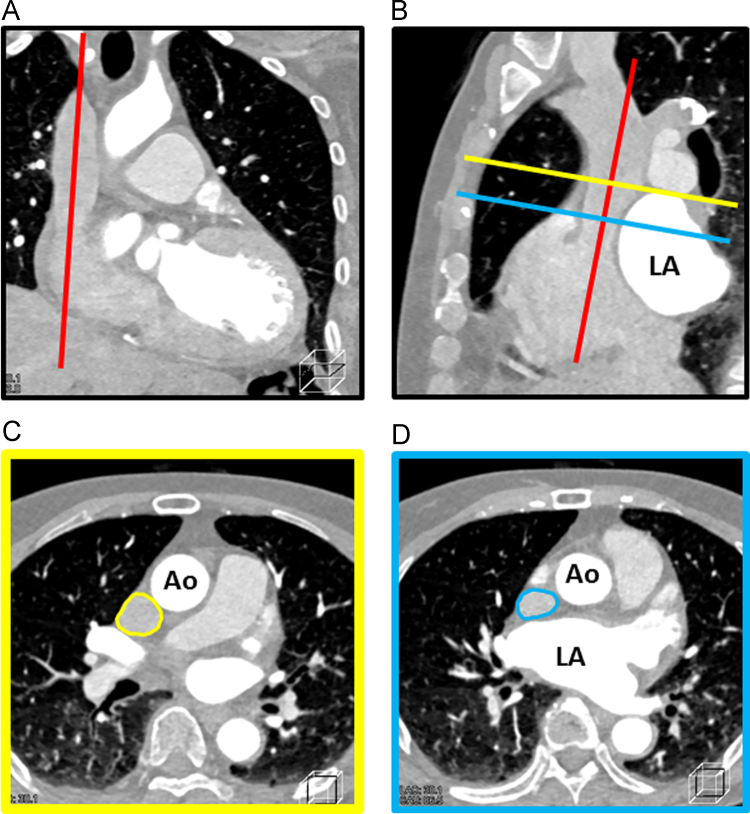
A contrast-enhanced CT was performed within 7 days before the procedure to assess the anatomy of the LA and 4 PVs. We retrospectively measured the cross-sectional area of the SVC (SVC-area) using a multi-planar reconstruction (MPR) CT image with a 3D workstation (Ziostation version 2.1.7.1, Ziosoft Inc, Tokyo, Japan). Considering the compression of the SVC by the dilated LA, the SVC-area was measured at a level over the roof of the LA. The detail methods of the measurement were as follows: (1) In both the coronal and sagittal views, the longitudinal axis of the SVC was manually confirmed (Fig. 3A and B). (2) The orthogonal plane was adjusted at the LA roof level in the sagittal view (Fig. 3B). (3) The SVC-area at the LA roof level was measured in the axial view (Fig. 3C). Of note, Fig. 3D shows the SVC compressed by the dilated LA anterior wall. These anatomical data were corrected to a body surface area (BSA) of 1.73 m2. The resultant acquisition phase was determined by the morphology of the mitral valve (diastole, if opened).
Fig. 3.
Measurement method of the SVC-area using a multi-planar reconstruction (MPR) CT image. A: The coronal view. The red line indicates the longitudinal axis of the SVC. B: The sagittal view. The red line indicates the longitudinal axis of the SVC. The yellow and blue lines indicate the LA roof and anterior wall level, respectively. C: The axial view at the LA roof level. The yellow circle indicates the SVC. D: The axial view at the LA anterior wall level. The blue circle indicates the SVC. Of note, The SVC was compressed by the dilated LA. SVC-area=cross-sectional area of the SVC, SVC=superior vena cava, LA=left atrium, Ao=ascending aorta.
2.6. Measurement of the right atrial (RA) and left atrial (LA) sizes and LA volume
The longitudinal and transverse diameters of the RA and LA were measured by the CT. The LA volume was measured by a 3D reconstructed CT (3DCT). In contrast to the LA volume measurement, the measurement of the RA volume was difficult due to the complex morphology of the RA appendage (RAA). Therefore, the longitudinal and transverse diameters of the RA were measured in the reconstructed 4ch-view by the MPR and the product of those parameters was defined as a surrogate parameter of the RA size, i.e., the RA index. According to the same formula, the LA index was also calculated as a surrogate parameter of the LA size.
2.7. CT imaging protocol
All patients underwent non-electrocardiography-gated contrast-enhanced CT using commercially available third-generation dual-source CT system (SOMATOM Force, Siemens Healthcare, Forchheim, Germany) using high-pitch double spiral scan with the following parameters: 2×196×0.60 mmmm slice collimation, tube voltage 70 kVp, rotation time 250 ms, temporal resolution 70–90 ms, and helical pitch 2.5–3.2. Iodinate contrast medium was given at a rate of 4.0 mL/s ×5 s followed by 20 mL of saline infusion. All images were acquired during a deep expiratory breath-hold.
2.8. Postablation follow-up
Patients were seen in routine clinical follow-up at 4 weeks, 3 months, 6 months, and 1 year, at which time they were queried for symptoms of arrhythmia and a 12-lead ECG was obtained. Holter monitoring (24 h) was performed as necessary. Episodes in the first 3 months post AF ablation were not considered as recurrence of arrhythmia.
2.9. Statistical analysis
The continuous variables are expressed as the mean value ±SD. The data from the two patient groups with and without arrhythmogenicity of the SVC were compared using a Welch׳s t-test for normally distributed continuous variables and Mann–Whitney U test for non-normally distributed continuous variables. A Fisher׳s exact test was used to evaluate the differences in the categorical variables. Multivariate logistic regression models were used to estimate the hazard ratios and 95% confidence intervals for the “response positive” to the SVC scan pacing. The variables in the multivariable logistic hazards regression model included the SVC-area and an RA index with a p<0.10 using a univariate logistic hazard regression analysis. Receiver operating characteristic curves were used to determine the SVC-area that provided the best sensitivity and specificity for the elimination of the arrhythmogenic activity in the SVC after the PVI. A 2-tailed p value of <0.05 defined statistical significant. All statistical analyses were performed with SPSS, Release 20.0 software (SPSS, Chicago, IL, USA).
3. Results
3.1. Patient characteristics
The patient characteristics are displayed in Table 1. A total of 43 patients who underwent a 1st AF ablation were enrolled in this study. Persistent atrial fibrillation was present in 12 (28%) patients. Their mean age, LA diameter, and left ventricular ejection fraction (LVEF) were 63±9 years, 40±6 mm, and 62±9%, respectively. A CTI ablation was performed in 12 (28%) patients. PVI was performed with a conventional radiofrequency ablation catheter and cryoballoon in 25 (58%) and 18 (42%) patients, respectively. No significant differences in terms of the baseline variables (age, sex, body mass index [BMI], type of AF, left ventricular ejection fraction [LVEF], LA diameter, use of anti-arrhythmic drugs [AADs], beta-blockers, angiotensin converting enzyme inhibitors [ACEIs], and angiotensin II receptor blockers [ARBs]) were found between the patients with or without an SVC scan pacing response. Furthermore, the procedural parameters did not differ between the two groups.
Table 1.
Patient characteristics and anatomical parameters grouped by the SVC pacing response.
| All patients (n=43) | Group 1 (response positive) (n=24) | Group 2 (response negative) (n=19) | P value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age (years) | 63±9 | 62±8 | 62±9 | P=0.75 |
| Male, n (%) | 34 (79) | 20/24 (83) | 14/19 (74) | P=0.35 |
| BMI (kg/m2) | 24±3 | 23±3 | 24±4 | P=0.31 |
| Persistent AF, n (%) | 12 (28) | 8/24 (33) | 4/19 (21) | P=0.37 |
| LVEF (%) | 62±9 | 61±10 | 64±7 | P=0.26 |
| LA Diameter (echocardiography) (mm) | 40±6 | 41±5 | 39±7 | P=0.20 |
| AAD use before ablation (class I or III), n (%) | 18 (42) | 9/24 (38) | 9/19 (47) | P=0.52 |
| Beta-blocker, n (%) | 24 (56) | 15/24 (63) | 9/19 (47) | P=0.32 |
| ACEI/ARB, n (%) | 15 (35) | 8/24 (33) | 7/19 (37) | P=0.81 |
| Cryoballoon ablation, n (%) | 18 (42) | 13/24 (54) | 7/19 (37) | P=0.26 |
| CTI ablation, n (%) | 12 (28) | 7/24 (29) | 5/19 (26) | P=0.84 |
| Anatomical parameters | ||||
| SVC-area (cm2) | 3.1±0.9 | 2.2±0.8 | P=0.004 | |
| RA index | 24.2±5.5 | 20.8±5.9 | P=0.056 | |
| LA index | 20.7±7.7 | 20.0±7.5 | P=0.73 | |
| LA volume (cm3) | 120.2±39.5 | 101.5±33.4 | P=0.11 | |
| Phase of CT (systolic), n (%) | 9/24 (38%) | 11/19 (58%) | P=0.18 | |
| Electrical parameters | ||||
| ERP-single (ms) | 299±58 | 312±54 | P=0.46 | |
| ERP-double (ms) | 223±62 | 267±53 | P=0.018 | |
| ERP-triple (ms) | 216±58 | 246±47 | P=0.079 |
BMI=body mass index, AF=atrial fibrillation, LVEF=left ventricular ejection fraction, LA=left atrium, AAD=anti arrhythmic drug.
ACEI=angiotensin converting enzyme inhibitor, ARB=angiotensin II receptor blocker, CTI=cavotricuspid isthmus.
SVC-area=the cross-sectional area of the superior vena cava, RA=right atrium, LA=left atrium, CT=computed tomography.
ERP=effective refractory period.
3.2. Arrhythmogenic response induced by SVC scan pacing
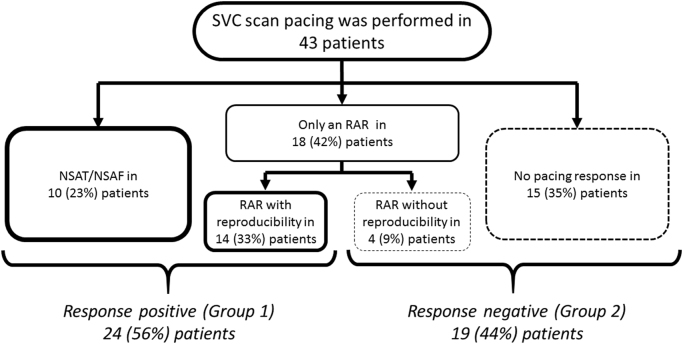
No sustained AF/AT was induced in these patients. NSAF/AT and RARs were induced in 10 (23%) and 18 (42%) of the 43 patients, respectively. Reproducible RARs were documented in 14 (33%) patients. Overall, 24 (56%) of the 43 patients were classified as “response positive” (Group 1), and the remaining 19 (44%) as “response negative” (Group 2). The detailed results of the SVC scan pacing response are shown in Fig. 4.
Fig. 4.
The arrhythmogenic responses induced by the SVC scan pacing. Overall, 24 (56%) of 43 patients were classified as “response positive” (Group 1), and the remaining 19 (44%) as “response negative” (Group 2). SVC=superior vena cava, NSAT/AF=non-sustained atrial tachycardia/ atrial fibrillation, RAR=repetitive atrial response.
3.3. Anatomical parameters grouped by the SVC scan pacing response
Table 1 also shows the anatomical parameters of two groups. The SVC-area was significantly larger in Group 1 than Group 2 (3.1±0.9 vs. 2.2±0.8 cm2, P=0.004) (Fig. 5A). The RA index was likely greater in Group 1 than Group 2, but the difference was not significant (24.2±5.5 vs. 20.8±7.5 cm2, P=0.056). Of interest, both the LA index and LA volume did not differ between the two groups (LA index: 20.7±7.7 vs. 20.0±7.5 cm2, P=0.73; LA volume: 120.2±39.5 vs. 101.5±33.4 cm3, P=0.11). The acquisition phase (systolic) of the CT did not differ between the two groups (9 [38%] vs. 11 [58%], P=0.18).
Fig. 5.
A: The SVC-area grouped by the SVC scan pacing response. The SVC-area was significantly larger in Group 1 (response positive) than Group 2 (response negative) (3.1±0.9 vs. 2.2±0.8 cm2, P=0.004). B: The Kaplan–Meier analysis for the AF-free survival showed a significantly lower AF recurrence in patients with SVC-area <2.56 cm2 than those with SVC-area>2.56 cm2 (log-rank P=0.026). SVC-area=the cross-sectional area of the SVC, SVC=superior vena cava. AF=atrial fibrillation.
3.4. Electrical parameters grouped by the SVC scan pacing response
Table 1 showed the electrical parameters including the ERP of the SVC sleeve and the amplitude at the pacing site in the SVC in the two groups. We express the ERP of scan single (the last own sinus beat-S1) as “ERP-single”, the ERP of scan double (S1-S2) as “ERP-double”, scan triple (S2-S3) as “ERP-triple”, respectively. ERP-single, double, triple were shorter in Group 1 (response positive) compared to Group 2 (response negative). Especially, ERP-double was significantly shorter in Group 1 (223±62 ms vs. 267±53 ms, P=0.018). The amplitude at the pacing site in the SVC were comparative between the two groups (0.48±0.36 mV in Group 1 vs. 0.54±0.45 mV in Group 2, P=0.634). Spontaneous premature atrial contraction from SVC was documented in 1 (4.2%) of 24 patients in Group 1 and 1 (5.3%) of 19 patients in Group 2, respectively.
3.5. Predictors of an arrhythmogenic response
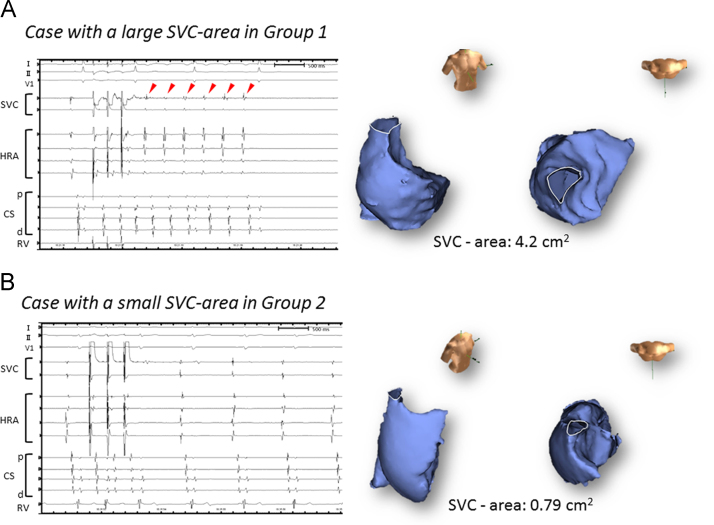
A univariate logistic regression analysis identified two factors related to an arrhythmogenic response to SVC scan pacing: the SVC-area and RA index (SVC-area: OR: 3.20, CI: 1.31–7.79, P=0.01; RA index: OR: 1.12, CI: 0.99–1.26, P=0.063) (Table 2). The age, type of AF, LA index, and LA volume were not associated with an arrhythmogenic response. According to the multivariate logistic regression analysis, the most predictive model of the arrhythmogenic response induced by SVC scan pacing consisted of the SVC-area (OR: 2.87, CI: 1.05–7.82, P=0.039). Fig. 6 shows two representative cases with a large or small SVC. A receiver operating characteristic curve analysis yielded an optimal cutoff value for the SVC-area of 2.59 cm2.
Table 2.
Predictors of an arrhythmogenic response to the SVC scan pacing.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | CI | P value | Odds ratio | CI | P value | |
| Age (years) | 0.99 | 0.92–1.06 | 0.74 | |||
| Sex | 0.56 | 0.13–2.46 | 0.44 | |||
| BMI (kg/m2) | 0.91 | 0.75–1.09 | 0.31 | |||
| Persistent AF, N (%) | 0.53 | 0.13–2.15 | 0.38 | |||
| LVEF (%) | 0.95 | 0.88–1.03 | 0.24 | |||
| LAD (mm) | 1.07 | 0.96–1.19 | 0.20 | |||
| AAD (class I or III) | 1.5 | 0.44–5.09 | 0.42 | |||
| Beta-blocker | 0.54 | 0.16–1.83 | 0.32 | |||
| ACI/ARB | 1.17 | 0.33–4.12 | 0.81 | |||
| Applied Cryoballoon ablation | 0.49 | 0.14–1.69 | 0.26 | |||
| Applied CTI ablation | 0.87 | 0.23–3.34 | 0.84 | |||
| SVC-area (cm2) | 3.2 | 1.31–7.79 | 0.01 | 2.87 | 1.05–7.82 | 0.039 |
| RA index (cm2) | 1.12 | 0.99–1.26 | 0.06 | 1.03 | 0.89–1.19 | 0.67 |
| LA index (cm2) | 1.01 | 0.93–1.10 | 0.75 | |||
| LA volume (cm3) | 1.02 | 1.00–1.03 | 0.11 | |||
BMI=body mass index, AF=atrial fibrillation, LVEF=left ventricular ejection fraction, LAD=left atrial diameter, AAD=anti arrhythmic drug.
ACEI=angiotensin converting enzyme inhibitor, ARB=angiotensin II receptor blocker, CTI=cavotricuspid isthmus.
SVC-area=the cross-sectional area of the superior vena cava, RA=right atrium, LA=left atrium.
Fig. 6.
Two representative cases. A: A patient with a large SVC in Group 1. Reproducible 6 beats RAR was induced by the SVC scan pacing (left panel). The red arrowheads indicate the RARs. Of note, the signal in the high right atrium (HRA) was followed by an SVC potential. B: A patient with a small SVC in Group 2. The SVC and RA were reconstructed three-dimensionally using the NavX system (right panel). SVC=superior vena cava, RAR=repetitive atrial response, HRA=high right atrium, RA=right atrium, CS=coronary sinus, p=proximal site, d=distal site, RV=right ventricle.
3.6. The clinical outcome
During 174±42.5 days of follow-up, the AF recurrence rate was similar between Group 1 and Group 2 (Group 1: 7[29%] of 24; Group 2: 5 [26%] of 19). The optimal cutoff value of the SVC-area predicting the AF recurrence was 2.56 cm2, which was similar to one predicting the pacing response. The sensitivity, specificity, and positive and negative predictive values with an SVC-area of 2.56 cm2 for inducing an arrhythmogenic response were 67%, 74%, 76%, and 64%, respectively. AF recurrence rate was significantly higher in patients with SVC-area>2.56 cm2 than those with SVC-area <2.56 cm2 (9 [42.9%] of 21 vs. 3 [13.6%] of 22, P=0.026) (Fig. 5B). Of interest, no AF recurrence was documented in Group 1 after SVC isolation in addition to PV-LA re-isolation in the 2nd AF ablation.
4. Discussion
4.1. Main findings
The study presented here demonstrated a relationship between an arrhythmogenic response to SVC scan pacing and anatomical dilatation of the SVC. An arrhythmogenic response had a 2.9 times increase in the probability for every 1 cm2 increase in the SVC-area. The arrhythmogenic response was not associated to dilation of the LA. Furthermore, the recurrence rate of AT/AF was significantly higher in patients with SVC-area>2.56 cm2.
4.2. SVC scan pacing stimulation
A previous study reported that AF could be induced in all patients by aggressive pacing such as burst or ramp pacing [19]. Therefore, the AF inducibility by burst pacing following a PVI was not associated with AF recurrences [20]. On the other hand, Richter et al. reported that the AF inducibility by a comparably non-aggressive stimulation protocol was associated with the AF recurrences [21]. Therefore, the more simple stimulation protocol was considered more specific for predicting the arrhythmogenicity than burst or ramp pacing. To avoid overestimating the arrhythmogenicity, we performed the scan pacing as a very simple induction protocol in this study.
4.3. Anatomical dilatation and arrhythmogenicity of SVC as a trigger of AF
Yamane et al. reported the relationship between the dilatation of the PVs and the arrhythmogenicity of the PVs. The diameters of the arrhythmogenic PVs were significantly larger than those of the non-arrhythmogenic PVs, which might imply a possible role of PV dilatation in the arrhythmogenicity [10]. They speculated that dilatation of the PVs might stretch the atrial myocardium of the PVs, which could result in changing the electrophysiological characteristics of the myocardial sleeves of the PVs. Tsao et al. reported the relationship between the PV size assessed by magnetic resonance imaging and the arrhythmogenicity. More anatomical dilatation was observed in both the right and left superior PVs than in the inferior PVs. Furthermore, 26 (72%) of 36 arrhythmogenic foci were located in the right superior or left superior PVs. However, they reported that only 28% patients had arrhythmogenic foci from the ‘largest’ PV. This indicated that a dilated PV with a certain greater value of the PV size could have the possibility to induce an arrhythmogenic trigger. The calculated cross-sectional area of the right and left superior PVs was measured as a maximum of 2.54 cm2 [11]. Notably, the threshold of the SVC-area with an arrhythmogenic response was 2.56 cm2 in our study. This finding was completely consistent with their report. Furthermore, in our study, the recurrence rate of AF was higher in patients with SVC-area>2.56 cm2. Therefore, SVC scan pacing might assess the true arrhythmogenicity of the SVC as an AF trigger due to the stretched myocardium of the SVC.
4.4. SVC scan pacing and the arrhythmogenicity of SVC as a perpetuator of AF
As for the arrhythmogenicity of the SVC, Miyazaki et al. reported the role of an arrhythmogenic SVC in AF. An arrhythmogenic SVC was documented in 5.2% patients who underwent AF ablation. In one-third of the patients after the SVCI, the AF terminated or converted to AT, and/or an SVC tachycardia was observed. This indicated that the SVC might play a role in AF not only as a trigger but also as a perpetuator [22]. Kang et al. reported that additional liner ablation from SVC to the RA after PVI improved the clinical outcome of catheter ablation [23]. This result indicated the effectiveness of the modification to SVC as a perpetuator rather than a trigger of AF. Of interest, the earliest activation site of the NSAT which was induced by the scan pacing was located between the SVC and high RA (HRA). Additional liner ablation from SVC to the RA might eliminate the arrhythmogenic substrate around SVC. In this study, we could demonstrate that SVC scan pacing response could reflect the larger SVC-area to maintain AF. In selective patients with an extremely large SVC-area, the cycle length of the SVC was the same as that of the HRA, and the activation map of the SVC fulfilled the cycle length, which indicated that the mechanism was re-entry. Notably, The ERP of the SVC sleeve was significantly shorter in Group 1 (response positive). The shorter ERP of SVC sleeve could facilitate the AF substrate as a perpetuator including re-entry mechanism. We speculated that the arrhythmogenic mechanism gradually changed from an AF trigger to an AF substrate in the myocardial sleeve of the SVC, which might correspond to the progression of the SVC dilation.
4.5. The relations of “inducibility” and “anatomical property” and “arrhythmogenicity” of SVC
Nakamura et al. reported that long SVC sleeve was associated with the inducibility of arrhythmogenic response by pacing stimulation at SVC [24]. Higuchi et al. reported that long SVC sleeve and large amplitude of SVC potential were associated with the arrhythmogenicity of SVC [25]. They showed the association between the “inducibility” and the “anatomical property” or between the “anatomical property” and the “arrhythmogenicity”. We also could demonstrate clearly that the “inducibility (arrhythmogenic response)” was strongly associated with the “anatomical property (SVC dilatation)”, furthermore, the “anatomical property” was also associated with the “arrhythmogenicity (AF recurrence)”. However, we could not demonstrate the association between the “inducibility” and the “arrhythmogenicity”. The SVC scan pacing was considered specific rather than sensitive for predicting SVC foci. Considering the AF recurrence as SVC foci, we should confirm the SVC firing by ISP infusion or aggressive pacing protocol and no PV-LA re-conduction. The impact of PV-LA re-conduction for predicting the AF recurrence was considered greater than that of other electrical findings including SVC Scan pacing. Unfortunately, PV-LA re-conduction was demonstrated in all patients with AF recurrence in the 2nd session. Therefore, we could not strengthen the impact of SVC scan pacing for predicting AF recurrence as SVC foci.
However, we thought our findings were valuable because not RA/LA but only SVC dilatation was associated to AF recurrence. In these days, the PVI could be performed more certainly by the Cryo ablation or contact force technology and so on. So we need the more exact method to reveal the non-PV foci for “beyond PVI”. We believe the SVC scan pacing might be an important hint to reveal the SVC arrhythmogenicity as a trigger or perpetuator of AF.
4.6. Clinical implications
The 3DCT was a useful tool for measuring the cross-sectional area of the SVC three-dimensionally. AF ablation integrated with 3DCT was routinely performed in the majority of the centers. Thus, we could assess the SVC arrhythmogenicity easily and determine the necessity of an SVCI prior to the AF ablation.
4.7. Limitations
First, a CT acquisition was performed without ECG-navigation and the CT image was retrospectively analyzed. The acquisition phase of the CT might have differed among the patients. However, there was no statistically significant difference in the CT acquisition phase between the patients with or without a pacing response. We would like to believe that the difference in the CT acquisition phase was acceptable. Second, the pacing output during the SVC scan pacing varied among the patients due to the cardiac or respiratory motion and phrenic nerve capture, which might influence the ERP assessment and AF inducibility. Third, the length of SVC sleeve were not measured and the amplitude of the SVC sleeve may be underestimated due to the incomplete mapping of the SVC. Therefore, a further study should be done to clarify this critical issue.
5. Conclusion
The dilatation of the SVC was associated with the arrhythmogenic response to the SVC scan pacing. Furthermore, the AF recurrence rate was significantly higher in patients with large SVC-area. Adjunctive catheter intervention for the SVC could be considered in patients with a dilated SVC.
Conflict of interest
Section of Arrhythmia is financially supported by Medtronic Japan and St. Jude Medical Japan.
Acknowledgments
We would like to thank Mr. John Martin for his linguistic assistance.
References
- 1.Haissaguerre M., Jais P., Shah D.C. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Liu X., Dong J., Mavrakis H.E. Achievement of pulmonary vein isolation in patients undergoing circumferential pulmonary vein ablation: a randomized comparison between two different isolation approaches. J Cardiovasc Electrophysiol. 2006;17:1263–1270. doi: 10.1111/j.1540-8167.2006.00621.x. [DOI] [PubMed] [Google Scholar]
- 3.Packer D.L., Kowal R.C., Wheelan K.R. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–1723. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 4.Lin W.S., Tai C.T., Hsieh M.H. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–3183. doi: 10.1161/01.CIR.0000074206.52056.2D. [DOI] [PubMed] [Google Scholar]
- 5.Shah D., Haissaguerre M., Jais P., Hocini M. Nonpulmonary vein foci: do they exist? Pacing Clin Electrophysiol. 2003;26:1631–1635. doi: 10.1046/j.1460-9592.2003.t01-1-00243.x. [DOI] [PubMed] [Google Scholar]
- 6.Corrado A., Bonso A., Madalosso M. Impact of systematic isolation of superior vena cava in addition to pulmonary vein antrum isolation on the outcome of paroxysmal, persistent, and permanent atrial fibrillation ablation: results from a randomized study. J Cardiovasc Electrophysiol. 2010;21:1–5. doi: 10.1111/j.1540-8167.2009.01577.x. [DOI] [PubMed] [Google Scholar]
- 7.Bai R., Patel D., Di Biase L. Phrenic nerve injury after catheter ablation: should we worry about this complication? J Cardiovasc Electrophysiol. 2006;17:944–948. doi: 10.1111/j.1540-8167.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen G., Dong J.Z., Liu X.P. Sinus node injury as a result of superior vena cava isolation during catheter ablation for atrial fibrillation and atrial flutter. Pacing Clin Electrophysiol. 2011;34:163–170. doi: 10.1111/j.1540-8159.2010.02903.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuhne M., Schaer B., Osswald S., Sticherling C. Superior vena cava stenosis after radiofrequency catheter ablation for electrical isolation of the superior vena cava. Pacing Clin Electrophysiol. 2010;33:e36–e38. doi: 10.1111/j.1540-8159.2009.02588.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamane T., Shah D.C., Jais P. Dilatation as a marker of pulmonary veins initiating atrial fibrillation. J Interv Card Electrophysiol. 2002;6:245–249. doi: 10.1023/a:1019561820830. [DOI] [PubMed] [Google Scholar]
- 11.Tsao H.M., Yu W.C., Cheng H.C. Pulmonary vein dilation in patients with atrial fibrillation: detection by magnetic resonance imaging. J Cardiovasc Electrophysiol. 2001;12:809–813. doi: 10.1046/j.1540-8167.2001.00809.x. [DOI] [PubMed] [Google Scholar]
- 12.Kiuchi K., Yoshida A., Takei A. Topographic variability of the left atrium and pulmonary veins assessed by 3D-CT predicts the recurrence of atrial fibrillation after catheter ablation. J Arrhythmia. 2015;31:286–292. doi: 10.1016/j.joa.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X.H., Liu X., Sun Y.M. Pulmonary vein isolation combined with superior vena cava isolation for atrial fibrillation ablation: a prospective randomized study. Europace. 2008;10:600–605. doi: 10.1093/europace/eun077. [DOI] [PubMed] [Google Scholar]
- 14.Arruda M., Mlcochova H., Prasad S.K. Electrical isolation of the superior vena cava: an adjunctive strategy to pulmonary vein antrum isolation improving the outcome of AF ablation. J Cardiovasc Electrophysiol. 2007;18:1261–1266. doi: 10.1111/j.1540-8167.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 15.Singh S.M., Heist E.K., Donaldson D.M. Image integration using intracardiac ultrasound to guide catheter ablation of atrial fibrillation. Heart Rhythm. 2008;5:1548–1555. doi: 10.1016/j.hrthm.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Kiuchi K., Kircher S., Watanabe N. Quantitative analysis of isolation area and rhythm outcome in patients with paroxysmal atrial fibrillation after circumferential pulmonary vein antrum isolation using the pace-and-ablate technique. Circ Arrhythm Electrophysiol. 2012;5:667–675. doi: 10.1161/CIRCEP.111.969923. [DOI] [PubMed] [Google Scholar]
- 17.Takigawa M., Takahashi A., Kuwahara T. Long-term follow-up after catheter ablation of paroxysmal atrial fibrillation: the incidence of recurrence and progression of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:267–273. doi: 10.1161/CIRCEP.113.000471. [DOI] [PubMed] [Google Scholar]
- 18.Andrade J.G., Khairy P., Macle L. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) Trial. Circ Arrhythm Electrophysiol. 2014;7:69–75. doi: 10.1161/CIRCEP.113.000586. [DOI] [PubMed] [Google Scholar]
- 19.Ramanna H., De Bakker J.M., Hauer R.N. Mechanism of propensity to atrial fibrillation in patients undergoing isthmus ablation for typical atrial flutter. J Cardiovasc Electrophysiol. 2005;16:167–172. doi: 10.1046/j.1540-8167.2005.40085.x. [DOI] [PubMed] [Google Scholar]
- 20.Leong-Sit P., Robinson M., Zado E.S. Inducibility of atrial fibrillation and flutter following pulmonary vein ablation. J Cardiovasc Electrophysiol. 2013;24:617–623. doi: 10.1111/jce.12088. [DOI] [PubMed] [Google Scholar]
- 21.Richter B., Gwechenberger M., Filzmoser P. Is inducibility of atrial fibrillation after radio frequency ablation really a relevant prognostic factor? Eur Heart J. 2006;27:2553–2559. doi: 10.1093/eurheartj/ehl307. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki S., Takigawa M., Kusa S. Role of arrhythmogenic superior vena cava on atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:380–386. doi: 10.1111/jce.12342. [DOI] [PubMed] [Google Scholar]
- 23.Kang K.W., Pak H.N., Park J. Additional linear ablation from the superior vena cava to right atrial septum after pulmonary vein isolation improves the clinical outcome in patients with paroxysmal atrial fibrillation: prospective randomized study. Europace. 2014;16:1738–1745. doi: 10.1093/europace/euu226. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T., Hachiya H., Yagishita A. The relationship between the profiles of SVC and sustainability of SVC fibrillation induced by provocative electrical stimulation. Pacing Clin Electrophysiol. 2016;39:352–360. doi: 10.1111/pace.12814. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi K., Yamauchi Y., Hirao K. Superior vena cava as initiator of atrial fibrillation: factors related to its arrhythmogenicity. Heart Rhythm. 2010;7:1186–1191. doi: 10.1016/j.hrthm.2010.05.017. [DOI] [PubMed] [Google Scholar]