Abstract
Background
Atrial fibrillation (AF) is a common complication after cardiac surgery. Ranolazine is a Food and Drug Administration approved anti-ischemic drug, which also has anti-arrhythmic properties. Recent studies have demonstrated the benefit of ranolazine in preventing post-operative AF (POAF) in patients undergoing cardiac surgery. Hence, we performed a meta-analysis of published studies comparing ranolazine plus standard therapy versus standard therapy for POAF prevention in patients undergoing cardiac surgery.
Methods
We performed a comprehensive search of Medline, Google Scholar, PubMed, abstracts from annual scientific sessions, and Cochrane library database for studies that assessed the effectiveness of ranolazine plus standard therapy by comparing it with standard therapy alone in preventing POAF in patients undergoing cardiac surgery. From all the studies, data on POAF events among groups were collected, and the random-effects (DerSimonian and Laird) method was used for meta-analysis.
Results
Four studies with 663 patients were included in the final analysis, with 300 and 363 patients in the ranolazine plus standard therapy and standard therapy groups, respectively. The types of cardiac surgeries were coronary artery bypass grafting (CABG), valve surgery or combination of CABG, and valve surgeries. After pooled analysis, ranolazine plus standard therapy was associated with a significant reduction in POAF events compared to standard therapy alone (risk ratio=0.44 [0.25, 0.78], p-value=0.005). There was no difference in adverse events between the two therapies. However, in one study, more patients in the ranolazine group had transient symptomatic hypotension after the surgery.
Conclusions
Ranolazine may prove beneficial in POAF prevention following cardiac surgeries. Although the pooled treatment effect is quite impressive with a reduction of more than 50% of risk of developing POAF, small number of studies and variation in ranolazine dose regimen in each study make our results inconclusive, but worthy of further investigation. That is why this result has to be interpreted as only hypothesis generating, rather than conclusion drawing.
Keywords: Atrial fibrillation, Cardiac surgery, Metaanalysis, Ranolazine, Post-operative atrial fibrillation
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, and it commonly occurs after cardiac surgery. AF is often associated with stroke, congestive heart failure, and myocardial infarction, all of which contribute to the increase in the length of hospital stay, higher medical costs, and increase in morbidity and mortality [1], [2], [3], [4], [5], [6]. Approximately 20%–50% of patients experience post-operative atrial fibrillation (POAF) after cardiac surgery [1], [6]. Based on the American and European task forces, AF prevention is one of the essential goals after any cardiac procedure [4], [7], [8]. POAF prevention has been a therapeutic challenge so far, and a number of medications have been studied, such as beta-blockers, amiodarone, colchicine, and calcium channel blockers [4], [5], [9], [10]. Recent meta-analysis showed that beta-blockers reduces POAF incidence rate from 31% to 16.3% compared with controls, whereas amiodarone decreased the incidence of POAF to 19.4% compared with a 33.3% incidence rate in the control group [5], [6]. Ranolazine is a Food and Drug Administration-approved anti-anginal drug (AAD), which also blocks abnormal late sodium channels and rapidly activates delayed rectifier potassium channels, which leads to attenuation of sodium–calcium currents and excessive electrical activity in atrial tissue. Thus, reduced after depolarization reserve suppresses AF [6], [11], [12]. Moreover, the mechanism of ranolazine to increase the refractory period after repolarization [5], [13] could decrease AF after cardiac surgery. Ranolazine has been studied to prevent POAF; however, ranolazine is not required to prevent POAF, based on formal guidelines. Recent studies demonstrated promising results of ranolazine plus standard therapy compared to standard therapy in preventing POAF in patients undergoing cardiac surgery. Effectiveness of ranolazine in preventing POAF has been studied in a few randomized control trials, and the data suggests that ranolazine may have a role in preventing POAF without causing a significant increase in postoperative complications or mortality.
2. Materials and methods
2.1. Search strategy and study selection
We evaluated all the relevant studies published before December 2015. We included all the studies where ranolazine plus standard therapy was used and compared with the standard therapy for prevention of AF following cardiac surgeries. The studies were searched from Medline, Google Scholar, PubMed, Cochrane library database, annual scientific sessions of American Heart Association, American College of Cardiology, Heart Rhythm Society, and European Society of Cardiology. Two independent reviewers performed the search electronically or manually. Disagreements were resolved through discussion to reach final decisions. All the animal, editorial, and review studies were excluded. Data on the type of cardiac surgery, ranolazine dosage, duration of therapy, type of comparison group, type of cardiac surgery, and AF incidence rate following cardiac surgery were collected.
2.2. Selected published clinical studies
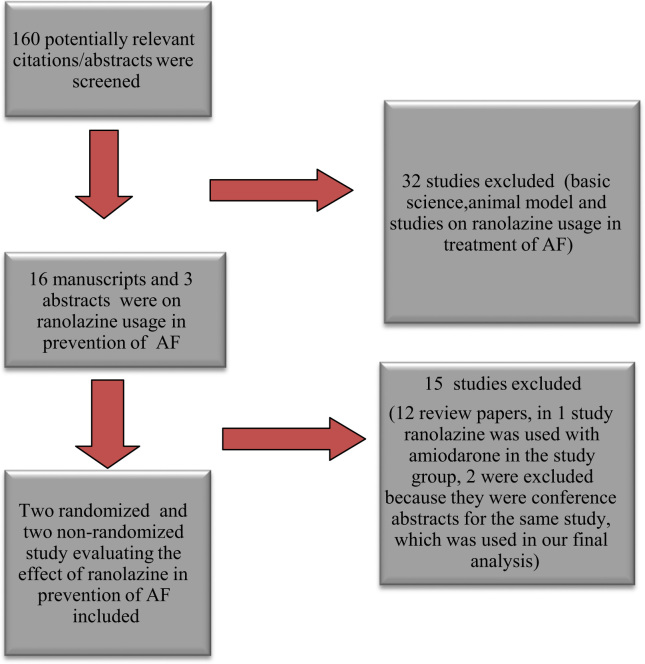
We reviewed 116 manuscript publications and 44 conference abstracts (Fig. 1). Out of those, 19 studies assessed the effect of ranolazine on AF after cardiac surgery. We excluded 12 studies because they were review articles, and 1 article was excluded because ranolazine was used in combination with amiodarone in the study group instead of ranolazine alone. Two studies were excluded because they were abstract presentations at conferences for the same study, which was included in our final analysis. Finally, four studies (three manuscript publications and one abstract publication from the American Heart Association׳s Scientific Session) [14], [15], [16], [17] were included in the final analysis.
Fig. 1.
Selection process of studies included in the systematic review. (AF=atrial fibrillation) Search criteria: ("ranolazine"[MeSH Terms] OR "ranolazine"[All Fields]) AND ("atrial fibrillation"[MeSH Terms] OR ("atrial"[All Fields] AND "fibrillation"[All Fields]) OR "atrial fibrillation"[All Fields]).
2.3. Statistical analysis
We performed a meta-analysis by including four clinical studies to provide an overall estimate of the effect of ranolazine therapy in preventing post-operative AF in patients undergoing cardiac surgery. The presence of heterogeneity among these studies was evaluated with Cochrane Q χ2 test, and inconsistency was assessed with I2 test that describes the percentage of the variability in effect estimates that is due to heterogeneity. Publication bias was assessed and displayed as a funnel plot of precision. Furthermore, we performed Egger׳s test and Begg and Mazumdar׳s rank correlation test to assess publication bias. Statistical level of significance for the summary treatment effect estimate was analyzed by random effect method [18]. Overall p-value of less than 0.05 was considered as statistically significant except for heterogeneity and publication bias testing where a two-tailed p-value of less than 0.1 considered as statistically significant. The meta-analysis was performed by the Review Manager 5.3 (The Cochrane Collaboration, 2011).
3. Results
3.1. Study characteristics
Table 1, Table 2 summarize the study and population characteristics, respectively.
Table 1.
Characteristics of the included studies in the meta-analysis.
| Study Characteristics | Miles et al. [15] | Tagarakis et al. [14] | Hammond et al. [16] | Bekheit et al. [17] |
|---|---|---|---|---|
| Number of patients | 393 | 102 | 205 | 54 |
| Design | Single-center, non-randomized retrospective cohort study | Prospective, single-center, single-blinded, randomized study | Single-center, retrospective cohort study | Single-center, double-blinded, randomized trial |
| Follow up | 30 days | 10 days | 7 days | 14 days |
| Type of Cardiac Procedures | CABG | CABG | CABG, valve and combination surgeries | CABG and/or aortic valve replacement surgeries |
| Mode of AF diagnosis | Continuous electrocardiographic monitoring | Continuous electrocardiographic monitoring | Continuous electrocardiographic monitoring | Holter monitoring |
| Intervention | Amiodarone 400 mg/day 7 days preoperatively and until the 10–14th postoperative day. Ranolazine 1000 mg one day before or on the day of surgery and 1000 mg twice daily until the 10–14th postoperative day | Randomized in 1:2 to receive either standard therapy or ranolazine 375 mg twice daily 3 days prior to surgery until day of discharge | Ranolazine group received ranolazine 1000 mg on the morning of surgery and 1000 mg twice daily until the 7th postoperative day. Non-ranolazine group received standard therapy | Randomized to receive either placebo or ranolazine 1000 mg twice daily 48 hours prior to surgery to 14th postoperative day |
| Primary endpoint | Atrial fibrillation | Atrial fibrillation or other arrhythmias | Atrial fibrillation | Atrial fibrillation |
CABG: coronary artery bypass grafting.
Table 2.
Baseline characteristics of study patients.
| Variables | Miles et al. [15] |
Tagarakis et al. [14] |
Hammond et al.* [16] |
Bekheit et al. [17] |
||||
|---|---|---|---|---|---|---|---|---|
| Ranolazine | Control | Ranolazine | Control | Ranolazine | Control | Ranolazine | Control | |
| (n=182) | (n=211) | (n=34) | (n=68) | (n=57) | (n=57) | (n=27) | (n=27) | |
| Age, years | 66.7±9.3 | 64.9±10.9 | 69±7 | 67±8 | 60.3±11.1 | 59.6±11.5 | 64.3±11.4 | |
| Male | 127(70) | 162(77) | 24 (71) | 45 (66) | 38(67) | 38(67) | 44(81) | |
| Hypertension | 158(87) | 182(86) | – | – | 45(79) | 48(84) | 48(89) | |
| Diabetes | 71(39) | 76(36) | – | – | 20(35) | 20(35) | 22(41) | |
| History of AF | 8 (4) | 16(8) | – | – | 1(2) | 1(2) | – | |
| LVEF (%) | 57.7±9.8 | 54.7±12.7 | 52.6±8.6 | 53.8±9.4 | – | – | 46.4±14.6 | |
Values are reported as mean ± SD or n (%). AF, atrial fibrillation; LVEF, left ventricular ejection fraction; CABG, coronary artery bypass grafting * propensity score-matched analysis was used for the study.
Tagarakis et al. assessed the associations between ranolazine and POAF in a prospective, single-center, single-blinded, randomized study of 102 patients (34 patients in the ranolazine group, 68 patients in the standard therapy group) scheduled for elective on-pump coronary artery bypass grafting (CABG). The ranolazine group received 375 mg ranolazine orally twice daily, which was started 3 days before the planned surgery and continued until the discharge day. The control group received standard care which consists of aspirin, atorvastatin, metoprolol and perindopril. Although patients in the ranolazine group were older and required longer aortic cross clamp time, only 3 (8.8%) patients from the ranolazine group demonstrated AF compared to 21(30.8%) patients in the control group (p-value < 0.001).
Miles et al. compared effectiveness and safety of ranolazine with amiodarone in prevention of POAF after CABG in a single-center, non-randomized retrospective cohort study involving 393 patients. Of these 393 patients, 211 were administered amiodarone 400 mg/day for 7 days before elective CABG, and all patients were maintained on amiodarone 400 mg/day for 10 to 14 days postoperatively. In the ranolazine group, there were 182 patients who received ranolazine 1500 mg one day before CABG or on the day of CABG in an emergent situation. Ranolazine was continued at 1000 mg twice daily for 10–14 days after the surgery. No significant difference was found in the baseline characteristics of both groups, except 3% lower ejection fraction and slightly high incidence of class IV heart failure in the amiodarone group of patients. POAF occurred in 56(26.5%) patients in the amiodarone group compared with 32(17.5%) patients in the ranolazine group (p-value=0.035). No significant difference was found in adverse events across the groups.
Hammond et al. performed a single-center, retrospective cohort study to evaluate the incidence of POAF and the role of ranolazine in 205 patients who underwent CABG, valve, or combination surgeries. A total of 136 patients in the non-ranolazine group received standard beta-blocker therapy, and 69 patients were administered 1000 mg ranolazine before the surgical procedure and continued on the same dose twice daily for 7 days or until discharge in postoperative period. Because of non-randomized nature of the study propensity score matching was adopted and in the final analysis 57 pair of patients were matched on propensity scores that were estimated by using age, sex, ethnicity, comorbidities, type of surgery, urgency of surgery, preoperative medications and type of insurance. By propensity score match analysis POAF incidence occurred in 6 (10.5%) patients in ranolazine group and 26(45.7%) patients in the control group (p-value<0.001).
Bekheit et al. performed a single-center, prospective, double-blinded, randomized trial involving 54 patients in order to assess the role of ranolazine for primary prevention of POAF in patient undergoing CABG and/or aortic valve replacement surgery. Twenty seven patients were randomly selected to receive ranolazine 1000 mg twice daily for 2 weeks and same number of patient received placebo for similar duration. Incidence rate of POAF was 5(19%) versus 8(30%) in ranolazine and control group respectively (p-value=0.53).
3.2. Efficacy outcome
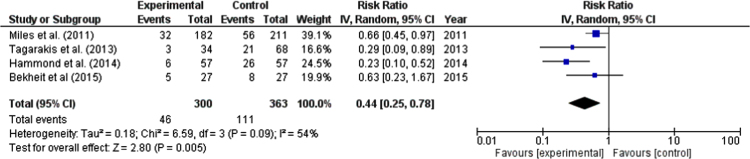
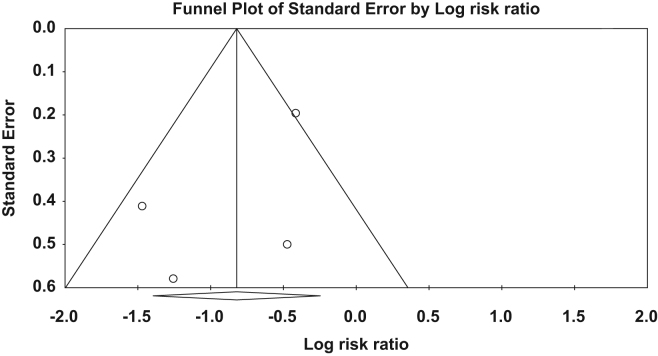
Overall, 663 patients (300 ranolazine, 363 control group) were included in the analysis. After a pooled analysis, ranolazine was significantly associated with 56% reduction in AF events compared to the control group (risk ratio: 0.44, 95% confidence interval: (0.25, 0.78), p-value=0.005) (Fig. 2). There was moderate amount of heterogeneity (I2: 54.0%); however, it was not statistically significant. There was no publication bias on visual estimation (Fig. 3). Also, there was no evidence of publication bias by Egger׳s test (p-value=0.31) or Begg and Mazumdar׳s rank correlation test (p-value=0.50).
Fig. 2.
Forest plot showing the risk ratio (RR) of atrial fibrillation associated with ranolazine use in each study and the overall RR. Square boxes denote RR; horizontal lines represent 95% confidence interval (random effects model was used to calculate pooled estimate).
Fig. 3.
Funnel plot of the standard error by logarithm risk ratio. There is no presence of publication bias on visual estimation [Also, there was no evidence of publication bias by Egger׳s test (p-value=0.31) or Begg and Mazumdar׳s rank correlation test (p-value=0.50).]
3.3. Safety outcome
Because of variable safety outcome among studies and small number of patients with adverse outcome, pooled estimate was not calculated. In general, there was no difference in adverse events between two groups. Only in the study by Hammond et al., more patients in ranolazine group had transient symptomatic hypotension after the surgery. In that study more patients in the ranolazine group had significant hypotension within 3 days after the surgery, and ranolazine was discontinued for symptomatic hypotension in one patient; however, hypotension did not persist at 1 week after cardiac surgery. No difference was found in the intensive care unit length of stay, 30 days readmission, or mortality between the two groups.
In the study by Miles et al., small number of patients in each group developed renal failure, which required dialysis; however, it was not different between the groups. In addition, no significant difference was found in the 30-day readmission or mortality and prolonged ventilation between the two groups. One patient had thromboembolic complication in the ranolazine group.
In the study by Tagrakis et al., no adverse outcomes were observed in either group. None of the patients required ionotropic support or moderate blood transfusion. Two patients died in each group; however the cause of death was as a result of a non-cardiac condition.
In the study by Bekheit et al., QT duration was longer in the ranolazine group; besides that, no significant difference was found in the 30-day readmission and length of hospital stay between the two groups.
4. Discussion
A number of clinical studies have confirmed the beneficial effect of ranolazine in either prevention or treatment of AF. The first strong evidence was provided by MERLIN-TIMI 36 trial [the Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST-Elevation Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction] [19], which showed that ranolazine may reduce the incidence rate of paroxysmal AF in patients with non-ST elevated acute coronary syndrome, and it also reduced overall AF burden. Few more studies showed the benefit of ranolazine in pharmacological cardioversion. Fragakis et al. [20] concluded that ranolazine–amiodarone combination showed a higher rate of pharmacological cardioversion compared to amiodarone alone, suggesting a potential synergistic effect of ranolazine when added to amiodarone. In another study on pharmacological cardioversion conducted by Murdock et al. [21], patients with paroxysmal AF converted to sinus rhythm within only 6 h of ranolazine administration. The HARMONY trial [22] evaluated the safety and efficacy of ranolazine–dronedarone combination in the treatment of patients having paroxysmal AF. In that trial, a significant AF reduction was observed by synergistic effect of ranolazine plus dronedarone, with a good safety profile. Another groundbreaking RAFFAELLO clinical trial [Ranolazine in Atrial Fibrillation Following an ELectricaL CardiOversion] [23] assessed the safety and efficacy of ranolazine in the prevention of AF recurrence after successful electrical cardioversion and to ascertain the most appropriate dose of ranolazine. The RAFFAELLO trial was a prospective, multicenter, randomized, double-blind, placebo-controlled parallel group phase II dose-ranging clinical study and concluded that ranolazine on 500 mg and 750 mg significantly reduce recurrence after successful electrical cardioversion. Although several studies have shown the effect of ranolazine in the prevention or treatment of arrhythmia, most of them were designed differently except for the studies on the prevention of AF post-cardiac surgery. Thus, we decided to perform the meta-analysis on Efficacy of Ranolazine in Preventing Atrial Fibrillation following cardiac surgery, and to the best of our knowledge, this is the first study that evaluated the effectiveness of ranolazine in preventing POAF occurrence after cardiac surgery through a meta-analysis. The finding from our study shows that the addition of ranolazine to the standard therapy reduces POAF nearly 55% compared to standard therapy alone. POAF is the most common tachyarrhythmia and frequently occurring complication following cardiac surgery. POAF can lead to severe thromboembolic complications, such as stroke. It reduces the quality of life and increases the hospitalization period. Furthermore, early POAF is the predictor of late recurrence, and hence, preventing POAF incidence is important. AF after cardiac surgery remains a challenge, and the results from currently available treatment options are unsatisfactory. Amiodarone is the most potent AAD and often used along with standard therapy to prevent AF after cardiac surgery; however, it is frequently associated with hepatic, pulmonary, and thyroid adverse events. Therefore, it is imperative to find a treatment plan to prevent the POAF. Ranolazine, an anti-ischemic medication with novel inhibitory action on late inward sodium channels within cardiomyocytes, demonstrates promising potential in AF prevention. Several recent studies have shown the benefit of ranolazine in POAF prevention in patients undergoing cardiac surgery. Moreover, a recently published review article from Saad et al. [24] thoroughly discussed the potential of ranolazine in prevention of not only atrial arrhythmias but also ventricular arrhythmias. Our findings will help in designing a randomized control trial to evaluate the efficacy, safety, dose regimen and cost-effectiveness analysis of ranolazine in AF management. Despite this promising finding, our study has several limitations. First, only four studies were included in the analysis, and the overall sample size was small. In addition, a minor difference was found in the study design among the included studies. Out of four studies, two studies were non-randomized retrospective studies. Moreover, the ranolazine dose was different in one study, and the duration of ranolazine therapy was different in each study. In the study by Miles et al. [15], a major limitation was the retrospective study design and comorbidities, such as heart failure, were more in the amiodarone group, which could have influenced the result. Moreover, it was the only study of the four wherein ranolazine was compared with amiodarone, as amiodarone was the standard therapy at that hospital. Hammond et al. study evaluated the patients by propensity matching, which could have reduced the bias, but it was also a retrospective design. The study by Tagarakis et al. was the first randomized trial comparing ranolazine to placebo in prevention of AF post cardiac surgery but the sample size was too small. In addition, low-dose ranolazine was used in that study compared with other studies. Last, the study by Bekheit et al. was a conference presentation; hence, we were unable to collect data in detail. Despite this minor discrepancy, the role of ranolazine in prevention of AF cannot be disregarded and it will help in designing future randomized clinical trials.
5. Conclusions
Ranolazine may prove beneficial in the prevention of POAF following cardiac surgeries. As such the result from this study has to be interpreted as only hypothesis generating, rather than conclusion drawing because of some limitations. Although the pooled ranolazine effect is quite impressive with a reduction of more than 50% in the risk of developing POAF, variation in ranolazine dosage and duration in each study make our results inconclusive, but worthy of further investigation.
Conflict of interest
All authors declare no conflict of interest related to this study.
Acknowledgements
The authors thank Kuldeep Yadav for assistance in article search and proof reading the manuscript.
References
- 1.Hillis L.D., Smith P.K., Anderson J.L. ACCF/AHA guideline for coronary artery bypass graft surgery. Circulation. 2011;2011(124):2610–2642. doi: 10.1161/CIR.0b013e31823b5fee. [DOI] [PubMed] [Google Scholar]
- 2.Echahidi N., Pibarot P., O’Hara G. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51(8):793–801. doi: 10.1016/j.jacc.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V., Rydén L.E., Cannom D.S. ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57(11) doi: 10.1016/j.jacc.2010.09.013. [e101–98] [DOI] [PubMed] [Google Scholar]
- 4.Mitchell L.B. CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention and treatment of atrial fibrillation after cardiac surgery. Can J Cardiol. 2011;27(1):91–97. doi: 10.1016/j.cjca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Arsenault K.A., Yusuf A.M., Crystal E. Interventions for preventing post‐operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD003611.pub3. [CD003611; PMID:23440790] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsu L.V., Lee S. Use of ranolazine in the prevention and treatment of postoperative atrial fibrillation in patients undergoing cardiac surgery. Ann Pharmacother. 2014;48:633–637. doi: 10.1177/1060028014523257. [1060028014523257] [DOI] [PubMed] [Google Scholar]
- 7.Camm A.J., Kirchhof P., Lip G.Y. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 8.Wann L.S., Curtis A.B., January C.T. ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57(2):223–242. doi: 10.1016/j.jacc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Trivedi C., Sadadia M. Colchicine in prevention of atrial fibrillation after cardiac surgery: systematic review and meta-analysis. Indian J Pharmacol. 2014;46(6):590. doi: 10.4103/0253-7613.144905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J., Wang C., Gao D. Meta‐analysis of amiodarone versus beta‐blocker as a prophylactic therapy against atrial fibrillation after cardiac surgery. Intern Med J. 2012;42(10):1078–1087. doi: 10.1111/j.1445-5994.2012.02844.x. [DOI] [PubMed] [Google Scholar]
- 11.Antzelevitch C., Burashnikov A., Sicouri S. Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011;8(8):1281–1290. doi: 10.1016/j.hrthm.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polytarchou K., Manolis A.S. Ranolazine and its antiarrhythmic actions. Cardiovasc Hematol Agents Med Chem. 2015;13(1):31–39. doi: 10.2174/187152571301150730113903. [DOI] [PubMed] [Google Scholar]
- 13.Burashnikov A., Di Diego J.M., Zygmunt A.C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116(13):1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tagarakis G., Aidonidis I., Daskalopoulou S.S. Effect of ranolazine in preventing postoperative atrial fibrillation in patients undergoing coronary revascularization surgery. Curr Vasc Pharmacol. 2013;11(6):988–991. doi: 10.2174/157016111106140128123506. [DOI] [PubMed] [Google Scholar]
- 15.Miles R.H., Passman R., Murdock D.K. Comparison of effectiveness and safety of ranolazine versus amiodarone for preventing atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2011;108(5):673–676. doi: 10.1016/j.amjcard.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Hammond D.A., Smotherman C., Jankowski C.A. Short-course of ranolazine prevents postoperative atrial fibrillation after coronary artery bypass grafting and valve surgeries. Clin Res Cardiol. 2015;104(5):410–417. doi: 10.1007/s00392-014-0796-x. [DOI] [PubMed] [Google Scholar]
- 17.Bekeith S., Meghani M., Shariff M.A. Effect of ranolazine on the incidence of atrial fibrillation after cardiac surgery. Circulation. 2015;132(Suppl 3):A13387. [Google Scholar]
- 18.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Scirica B.M., Morrow D.A., Hod H. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non–st-segment–elevation acute coronary syndrome results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non–ST-Elevation Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116(15):1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 20.Fragakis N., Koskinas K.C., Katritsis D.G. Comparison of effectiveness of ranolazine plus amiodarone versus amiodarone alone for conversion of recent-onset atrial fibrillation. Am J Cardiol. 2012;110(5):673–677. doi: 10.1016/j.amjcard.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 21.Murdock D.K., Kersten M., Kaliebe J. The use of oral ranolazine to convert new or paroxysmal atrial fibrillation: a review of experience with implications for possible" pill in the pocket" approach to atrial fibrillation. Indian Pacing Electrophysiol J. 2009;9(5):260. [PMC free article] [PubMed] [Google Scholar]
- 22.Reiffel J.A., Camm A.J., Belardinelli L., Zeng D. The HARMONY Trial: combined Ranolazine and Dronedarone in the Management of Paroxysmal Atrial Fibrillation: mechanistic and Therapeutic Synergism. Circ Arrhythm Electrophysiol. 2015;5(8):1048–1056. doi: 10.1161/CIRCEP.115.002856. [DOI] [PubMed] [Google Scholar]
- 23.De Ferrari G.M., Maier L.S., Mont L. Ranolazine in the treatment of atrial fibrillation: results of the dose-ranging RAFFAELLO (Ranolazine in Atrial Fibrillation Following an ELectricaL CardiOversion) study. Heart Rhythm. 2015;12(5):872–878. doi: 10.1016/j.hrthm.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Saad M., Mahmoud A., Elgendy I.Y. Ranolazine in cardiac arrhythmia. Clin Cardiol. 2016;39(3):170–178. doi: 10.1002/clc.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]