Abstract
Importance
Appropriate Use Criteria for coronary revascularization were developed to critically evaluate and improve patient selection for percutaneous coronary intervention (PCI). National trends in the appropriateness of PCI have not been examined.
Objective
To examine trends in PCI utilization, patient selection, and procedural appropriateness following the introduction of Appropriate Use Criteria.
Design, Setting, Participants
Multi-center, longitudinal, cross-sectional analysis of patients undergoing PCI between July 1, 2009 and December 31, 2014 at hospitals continuously participating in NCDR-CathPCI Registry over the study period.
Main Outcome Measures
Proportion of non-acute PCIs classified as inappropriate at the patient- and hospital-level using the 2012 Appropriate Use Criteria for Coronary Revascularization.
Results
A total of 2.7 million PCI procedures from 766 hospitals were included. Annual PCI volume for acute indications was consistent over the study period (2010: 377,540; 2014: 374,543), but the volume for non-acute PCIs decreased from 89,704 in 2010 to 59,375 in 2014. Among patients undergoing non-acute PCI, there were significant increases in angina severity (CCS III/IV angina, 15.8% and 38.4% in 2010 and 2014 respectively), use of anti-anginal medications prior to PCI (at least 2 anti-anginal medication, 22.3% and 35.1% in 2010 and 2014 respectively), and high-risk findings on non-invasive testing (22.2% and 33.2% in 2010 and 2014 respectively) (p<0.001 for all), but only modest increases in multivessel CAD (43.7% and 47.5% in 2010 and 2014 respectively, p<0.001). The proportion (95% CI) of non-acute PCIs classified as inappropriate decreased from 26.2% (95% CI, 25.8%–26.6%) to 13.3% (95% CI, 13.1%–13.6%) and the absolute number of inappropriate PCIs decreased from 21,781 to 7,921. Hospital-level variation in the proportion of PCIs classified as inappropriate was persistent over the study period (median 12.6%, IQR 5.9%–22.9% in 2014).
Conclusions and Relevance
Since the publication of the Appropriate Use Criteria for Coronary Revascularization in 2009, there have been significant reductions in non-acute PCI volume. The proportion of non-acute PCIs classified as inappropriate has declined though hospital-level variation in inappropriate PCI persists.
Introduction
In 2009, the American College of Cardiology, the American Heart Association, along with other professional societies released Appropriate Use Criteria for Coronary Revascularization to critically examine and improve patient selection for PCI as well as address concerns about potential overuse.1,2 Prior studies demonstrated that 1 in 6 non-acute PCIs were classified as inappropriate (new Appropriate Use Criteria documents use the term ‘rarely appropriate’), indicating that the benefits of the procedure were unlikely to outweigh the risks.3,4 Furthermore, there was substantial variation in the proportion of non-acute PCI considered inappropriate across hospitals.3,4 These findings received considerable attention in both the academic literature and media5,6, prompting numerous efforts to improve the appropriateness of PCI.
In 2011, the National Cardiovascular Data Registry’s CathPCI Registry (NCDR CathPCI) began providing hospitals information about their performance on PCI appropriateness, which were benchmarked against other participating hospitals. Simultaneously, national quality improvement campaigns such as the American Board of Internal Medicine’s Choosing Wisely Initiative, identified PCI appropriateness as a key area for intervention,7 insurers incorporated measures of PCI appropriateness into pay-for-performance programs,8 and some payers declined reimbursement for certain PCIs classified as inappropriate.9
Despite the attention the appropriateness of PCI has received, there has been no comprehensive, national examination of trends in the indications, patient characteristics, and appropriateness of PCI procedures following the introduction of the Appropriate Use Criteria. Similarly, the extent of hospital-level variation in the proportion of non-acute PCI considered inappropriate has not been systematically examined over time. To address these gaps in knowledge, we examined national trends in patient selection for PCI, changes in PCI appropriateness, and hospital variation in inappropriate PCI using the registry.
Methods
Data Source and Appropriate Use Criteria
Details of the registry have been described previously.10,11 In brief, NCDR CathPCI is the largest national registry of diagnostic cardiac catheterization and PCI with more than 1500 participating institutions. Detailed information on clinical characteristics, cardiac testing, angiographic findings, as well as in-hospital management and clinical outcomes are collected by trained staff at participating hospitals using a standardized data collection form (http://cvquality.acc.org/en/NCDR-Home/Data-Collection/What-Each-Registry-Collects.aspx). All data submissions must meet specified quality standards and randomly identified sites are monitored through annual audits. The Human Investigation Committee of the Yale University School of Medicine approved the use of a limited dataset from the registry for research without requiring informed consent.
The methodology used to develop the Appropriate Use Criteria for Coronary Revascularization has been previously described (see Box for additional details).1,12,13 The registry has developed validated algorithms mapping data collected using version 4 of the data collection form (beginning July 2009) to the Appropriate Use Criteria.3 The initial Appropriate Use Criteria for Coronary Revascularization were revised in 2012 to provide greater specificity in defining non-acute indications.12 For this analysis, we exclusively used the 2012 Appropriate Use Criteria.
An overview of the 2012 Appropriate Use Criteria for Coronary Revascularization and methodology for determination of the appropriateness of PCI
The methodology for developing the Appropriate Use Criteria for coronary revascularization, which are based upon the modified RAND methodology and reflect a synthesis of contemporary clinical trial evidence, clinical practice guidelines, and expert opinion, has been previously described14 Using a modified Delphi approach, a 17-member expert panel adjudicated the appropriateness of coronary revascularization, compared with medical therapy, for 198 distinct clinical indications, which were categorized by the clinical indication, angiographic severity, magnitude of ischemia, severity of angina symptoms and intensity of medical therapy. From the individual ratings of the technical panel members, each clinical indication was classified as appropriate, uncertain, or inappropriate. An ‘Appropriate’ rating denotes that coronary revascularization, as compared with medical therapy, would likely improve a patient’s health status (symptoms, function, or quality of life) or survival, an ‘Uncertain’ rating implies that more research and/or patient information is needed to further classify the indication, and an ‘Inappropriate’ rating suggests that the benefits of coronary revascularization are unlikely to outweigh the risks.
For additional details see: 2012 Appropriate Use Criteria for Coronary Revascularization.12
Study Population and Definitions
The study cohort included all PCIs in the NCDR registry between July 1st, 2009 and December 31st, 2014. To accurately assess trends in appropriateness, we restricted our cohort to PCIs performed at hospitals that participated continuously in the registry during the entire study period. For patients undergoing multiple PCIs in a single visit, only the first PCI was included. We excluded hospitals that performed an average of fewer than 10 non-acute PCIs in each calendar year to provide more robust estimates of hospital performance.
Each PCI in our study cohort was initially classified as acute, non-acute, or non-mappable. Acute PCI were defined as those performed in the setting of an acute coronary syndrome. Non-mappable PCIs were PCIs which could not be classified because of missing data elements (typically because non-invasive testing was not performed or not available). All other PCIs were considered non-acute. Each mappable PCI was then assigned a rating of procedural appropriateness (appropriate, uncertain, or inappropriate) based upon the 2012 Appropriate Use Criteria for Coronary Revascularization.12
Statistical Analysis
All analyses were performed at either the patient-level, using all PCIs to calculate an estimate, or at the hospital-level, aggregating each hospitals’ data to calculate a hospital specific estimate.
PCI volume and the relative proportions of acute, non-acute, and non-mappable PCIs were examined at the patient-level by year. Hospital-level variation in the proportions of PCIs for acute, non-acute, and non-mappable indications was examined across calendar year. Median hospital-level proportions with interquartile ranges (IQRs) were used to characterize the distribution and are displayed using box plots.
Baseline demographic and clinical characteristics as well as clinical presentation, background medical therapy, and results from non-invasive and angiographic studies were compared over time for all patients undergoing PCI and among those undergoing non-acute PCI. The proportions of appropriate, inappropriate, and uncertain PCIs at the patient-level were calculated for each 6-month interval and compared over time. The proportion of non-acute PCIs considered inappropriate at the hospital level was calculated by aggregating all non-acute PCIs in the calendar year and displayed using box plots.
To identify the presence of different subgroups of hospital-level change in proportion of inappropriate PCI, we performed a latent growth curve analysis.15,16 Latent class growth curve analysis, employing growth mixture modeling, serves to identify distinct patterns of change over time using each hospital’s observed trajectory of the proportion of non-acute PCIs classified as inappropriate. Hospitals with similar patterns over time are grouped together and considered to form a latent class. The use of growth mixture modeling estimates a mean growth curve for each latent class while allowing for individual variation around the growth curve within each class. We fit 4 models: 2 group, 3 group, 4 group, and 5 group. For each model we evaluated the change in the BIC and calculated the approximated Bayes factor. We also plotted the observed verse the predicted values to evaluate model fit. The average posterior probabilities were used to ensure that the model adequately distinguished between identified groups. We chose the 4 group model because it performed best on these criteria. We performed this secondary analysis among hospitals in the highest quartile of proportion of inappropriate PCI between July 2009 and December 2010 to understand the trajectories of hospitals with the greatest opportunity for improvement. For each hospital, we then examined the proportion of inappropriate non-acute PCI from January 2011 to December 2014, grouping hospitals with similar patterns over time together. Finally, we compared hospital characteristics across groups to identify hospital features associated with various patterns.
Statistical testing of trends was performed using Cochran-Armitage Test17,18 for binary variables and Jonckheere-Terpstra Test19 for categorical variables. Further to assess sensitivity of hospital-level results to the aggregation of estimates within hospitals, we confirmed all test results using weighted general linear models, weighting estimates by hospital volume. Absolute changes in PCI volume and patient characteristics were calculated using 2010 and 2014 data as the study interval began July 1, 2009. All tests for statistical significance were 2-tailed and evaluated at a significance level of 0.05 corrected for multiple comparisons using the Šidák correction.20 All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, North Carolina).
Results
There were more than 3.5 million PCIs performed at 1,561 hospitals between July 2009 and December 2014. We excluded 550,836 patients treated at 583 hospitals that did not participate continuously in the registry during the study period and an additional 273,167 cases performed at 212 facilities who performed an average of fewer than 10 non-acute PCIs in each calendar year, leaving 2,685,683 PCI procedures from 766 hospitals as the primary study cohort. Characteristics of the hospitals in the primary study cohort are shown in eTable 1.
PCI indication over time
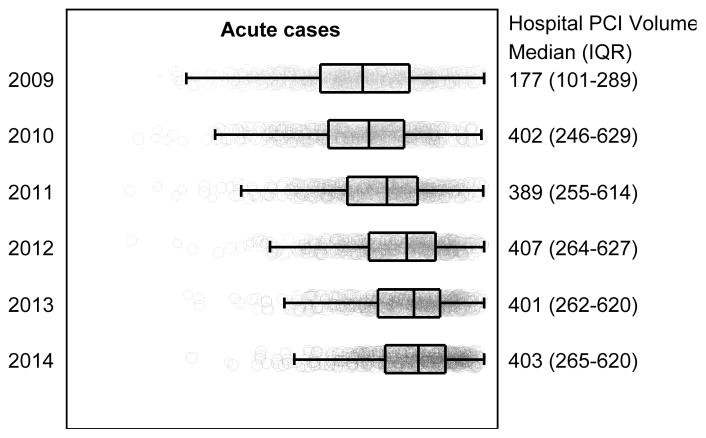
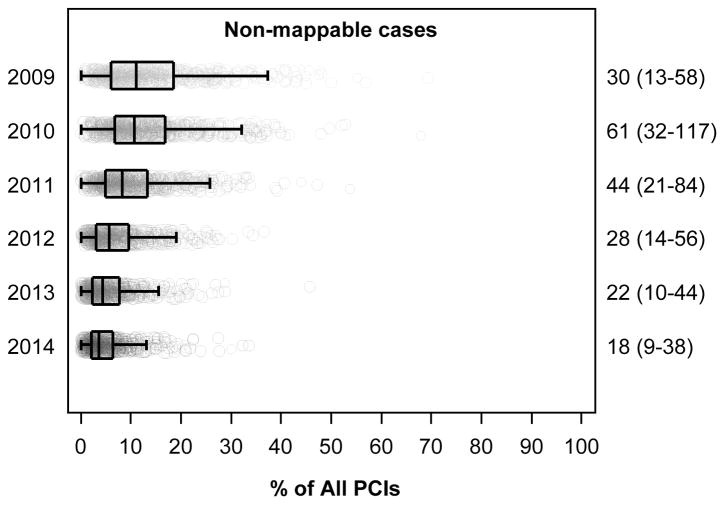
Of the PCI procedures included in our analysis, 76.3% (95% CI, 76.2%–76.3%) were for acute indications, 14.8% (95% CI, 14.8%–14.9%) for non-acute indications, and 8.9% (95% CI, 8.9%–9.0%) were non-mappable (Table 1). Annual PCI volume declined over the study period from 538,076 in 2010 to 456,507 in 2014. The volume of acute PCI was relatively stable over time (2010: 377,540; 2014: 374,543), but there were significant declines in the volume of non-acute PCI (2010: 89,704; 2014: 59,375; p<0.001) and non-mappable PCI (2010: 70,832; 2014: 22,589; p<0.001). As a consequence, the proportion (95% CI) of PCIs performed for acute indications rose from 69.1% (95% CI, 68.8%–69.3%) in 2009 to 82.0% (95% CI, 81.9%–82.2%) in 2014. The proportion of PCIs for non-acute indications declined from 16.8% (95% CI, 16.7%–17.0%) to 13.0% (95% CI, 12.9%–13.1%) while the proportion of non-mappable PCIs declined from 14.0% (95% CI, 13.9%–14.2%) in 2009 to 4.9% (95% CI, 4.9%–5.0%) in 2014. Similar findings were noted at the hospital-level (Figure 1).
Table 1.
Number and percentage (95% CI) of acute, non-acute and non-mappable PCIs from July 1, 2009to Decmeber 31, 2014.
| PCI indication/Year | Total | 2009* | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|---|
| Overall, n | 2,685,683 | 243,580 | 538,076 | 502,995 | 481,889 | 462,636 | 456,507 |
| Acute, n (95% CI) | 2,047,853 76.3% (76.2%–76.3%) |
168,366 69.1% (68.9%–69.3%) |
377,540 70.2% (70.0%–70.3%) |
373,423 74.2% (74.1%–74.4%) |
380,331 78.9% (78.8%–79.0%) |
373,650 80.8% (80.7%–80.9%) |
374,543 82.0% (81.9%–82.2%) |
| Non-acute, n (95% CI) | 397,737 14.8% (14.8%–14.9%) |
41,024 16.8% (16.7%–17.0%) |
89,704 16.7% (16.6%–16.8%) |
78,328 15.6% (15.5%–15.7%) |
66,849 13.9% (13.8%–14.0%) |
62,457 13.5% (13.4%–13.6%) |
59,375 13.0% (12.9%–13.1%) |
| Non-mappable, n (95% CI) | 240,093 8.9% (8.9%–9.0%) |
34,190 14.0% (13.9%–14.2%) |
70,832 13.2% (13.1%–13.3%) |
51,244 10.2% (10.1%–10.3%) |
34,709 7.2% (7.1%–7.3%) |
26,529 5.7% (5.7%–5.8%) |
22,589 4.9% (4.9%–5.0%) |
Includes July 1, 2009 to December 31, 2009
Abbreviations: PCI, percutaneous coronary intervention.
Figure 1. Proportion of PCI Performed for Acute, Non-acute, and Non-mappable Indications at the Hospital-level from 2009 to 2014.
Hospital-level proportion of acute, non-acute, and non-mappable indications for all PCIs performed from July 1, 2009 to December 31, 2014 at 766 hospitals participating continuously in the NCDR-CathPCI Registry over the study period. For each box-plot, the vertical line in the center of the rectangle represents the median, the left and right vertical lines of each rectangle represent the 25th and 75th percentiles respectively, and the vertical lines capping the horizontal lines extending from the rectangle represent 1.5-times the interquartile range. Each hospital is represented as a point in the box-plot, the size of the point reflects the hospital volume. Note: Results for 2009 include 6-months of data.
Baseline Characteristics
Baseline demographic and clinical characteristics as well as the presence and severity of angina symptoms, background anti-anginal medical therapy, results of non-invasive testing, and angiographic findings for the entire study cohort are included in eTable 2 and for patients undergoing non-acute PCI in Table 2.
Table 2.
Baseline Characteristics of Patients Undergoing Non-Acute PCI from July 1, 2009 to December 31, 2014.
| Total | 2009$ | 2010 | 2011 | 2012 | 2013 | 2014 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Patient Characteristics | # | % | # | % | # | % | # | % | # | % | # | % | # | % |
| N | 397,737 | 100.0 | 41,024 | 10.3 | 89,704 | 22.6 | 78,328 | 19.7 | 66,849 | 16.8 | 62,457 | 15.7 | 59,375 | 14.9 |
|
| ||||||||||||||
| Demographics | ||||||||||||||
| Age - Mean (SD) | 66.5 | 10.9 | 65.9 | 11.1 | 66.1 | 11.0 | 66.3 | 10.9 | 66.6 | 10.8 | 66.9 | 10.8 | 67.1 | 10.8 |
| Sex: Male | 275,469 | 69.3 | 27,574 | 67.2 | 60,902 | 67.9 | 53,801 | 68.7 | 46,433 | 69.5 | 44,457 | 71.2 | 42,302 | 71.3 |
| Race: White | 350,988 | 88.3 | 36,376 | 88.7 | 79,591 | 88.7 | 68,884 | 87.9 | 58,822 | 88.0 | 55,124 | 88.3 | 52,191 | 87.9 |
|
| ||||||||||||||
| Insurance | ||||||||||||||
| Private | 278,236 | 70.1 | 27,640 | 67.5 | 61,789 | 69.0 | 54,489 | 69.7 | 47,129 | 70.7 | 44,514 | 71.4 | 42,675 | 72.0 |
| Public Only | 109,827 | 27.7 | 12,432 | 30.4 | 25,723 | 28.7 | 21,734 | 27.8 | 17,909 | 26.9 | 16,417 | 26.3 | 15,612 | 26.3 |
| Non-US | 266 | 0.1 | 33 | 0.1 | 57 | 0.1 | 46 | 0.1 | 37 | 0.1 | 40 | 0.1 | 53 | 0.1 |
| None | 8,607 | 2.2 | 854 | 2.1 | 2,004 | 2.2 | 1,872 | 2.4 | 1,600 | 2.4 | 1,349 | 2.7 | 928 | 1.6 |
|
| ||||||||||||||
| Clinical Risk Factors and comorbidities | ||||||||||||||
| Current/Recent Smoker(< 1 year) | 77,355 | 19.5 | 8,528 | 21.0 | 18,437 | 20.6 | 15,522 | 19.8 | 12,822 | 19.2 | 11,352 | 18.2 | 10,694 | 18.0 |
| Hypertension | 344,698 | 86.7 | 34,932 | 85.2 | 77,378 | 86.3 | 67,532 | 86.3 | 58,262 | 87.2 | 54,656 | 87.5 | 51,938 | 87.5 |
| Dyslipidemia | 341,445 | 85.9 | 34,755 | 84.8 | 77,123 | 86.0 | 67,145 | 85.8 | 57,191 | 85.6 | 53,981 | 86.5 | 51,250 | 86.4 |
| Family history of coronary artery disease | 93,873 | 23.6 | 10,084 | 24.6 | 21,969 | 24.5 | 18,789 | 24.0 | 16,194 | 24.2 | 14,450 | 23.1 | 12,387 | 20.9 |
| Prior PCI | 173,734 | 43.7 | 17,075 | 41.6 | 38,785 | 43.2 | 34,273 | 43.8 | 29,323 | 43.9 | 27,794 | 44.5 | 26,484 | 44.6 |
| Prior CABG surgery | 57,394 | 14.4 | 5,096 | 12.4 | 11,615 | 13.0 | 10,877 | 13.9 | 9,986 | 14.9 | 10,116 | 16.2 | 9,704 | 16.3 |
| Diabetes mellitus | 156,865 | 39.5 | 15,505 | 37.8 | 34,023 | 37.9 | 30,794 | 39.3 | 26,627 | 39.8 | 25,467 | 40.8 | 24,449 | 41.2 |
|
| ||||||||||||||
| Clinical Presentation | ||||||||||||||
| Coronary artery disease presentation | ||||||||||||||
| No symptoms, no angina | 91,046 | 22.9 | 11,899 | 29.0 | 23,889 | 26.6 | 18,367 | 23.5 | 13,902 | 20.8 | 12,301 | 19.7 | 10,688 | 18.0 |
| Symptoms unlikely to be ischemic | 41,247 | 10.4 | 4,145 | 10.1 | 9,577 | 10.7 | 8,301 | 10.6 | 7,179 | 10.7 | 6,165 | 9.9 | 5,880 | 9.9 |
| Stable angina | 265,444 | 66.7 | 24,980 | 60.9 | 56,238 | 62.7 | 51,660 | 66.0 | 45,768 | 68.5 | 43,991 | 70.4 | 42,807 | 72.1 |
|
| ||||||||||||||
| Angina | ||||||||||||||
| No symptoms | 102,920 | 25.9 | 12,443 | 30.3 | 26,313 | 29.3 | 20,541 | 26.2 | 16,313 | 24.4 | 14,420 | 23.1 | 12,890 | 21.7 |
| CCS I | 44,889 | 11.3 | 6,297 | 15.4 | 12,752 | 14.2 | 10,070 | 12.9 | 6,484 | 9.7 | 4,934 | 7.9 | 4,352 | 7.3 |
| CCS II | 148,898 | 37.4 | 15,824 | 38.6 | 34,958 | 39.0 | 31,366 | 40.0 | 25,842 | 38.7 | 21,571 | 34.5 | 19,337 | 32.6 |
| CCS III | 89,909 | 22.6 | 5,575 | 13.6 | 13,442 | 15.0 | 14,454 | 18.5 | 16,299 | 24.4 | 19,412 | 31.1 | 20,727 | 34.9 |
| CCS IV | 11,121 | 2.8 | 885 | 2.2 | 2,239 | 2.5 | 1,897 | 2.4 | 1,911 | 2.9 | 2,120 | 3.4 | 2,069 | 3.5 |
|
| ||||||||||||||
| No. of antianginal medications | ||||||||||||||
| 0 | 102,655 | 25.8 | 13,811 | 33.7 | 27,076 | 30.2 | 21,306 | 27.2 | 15,719 | 23.5 | 13,222 | 21.2 | 11,521 | 19.4 |
| 1 | 187,154 | 47.1 | 19,272 | 47.0 | 42,610 | 47.5 | 37,427 | 47.8 | 31,930 | 47.8 | 28,884 | 46.3 | 27,031 | 45.5 |
| >=2 | 107,885 | 27.1 | 7,928 | 19.3 | 20,011 | 22.3 | 19,585 | 25.0 | 19,195 | 28.7 | 20,350 | 32.6 | 20,816 | 35.1 |
|
| ||||||||||||||
| Stress or imaging test performed | 273,237 | 68.7 | 26,720 | 65.1 | 57,942 | 64.6 | 53,045 | 67.7 | 47,420 | 70.9 | 45,041 | 72.1 | 43,069 | 72.5 |
| Stress test results (among those with a test) | ||||||||||||||
| Unavailable | 40,046 | 15.1 | 5,053 | 19.6 | 10,328 | 18.4 | 8,373 | 16.3 | 6,442 | 14.0 | 5,142 | 11.7 | 4,708 | 11.2 |
| Low risk* | 37,316 | 14.0 | 4,272 | 16.5 | 9,548 | 17.0 | 7,855 | 15.2 | 5,953 | 12.9 | 5,171 | 11.8 | 4,517 | 10.7 |
| Intermediate risk** | 116,078 | 43.7 | 10,756 | 41.6 | 23,920 | 42.5 | 22,416 | 43.5 | 20,319 | 44.1 | 19,709 | 44.8 | 18,958 | 44.9 |
| High risk# | 72,463 | 27.3 | 5,759 | 22.3 | 12,460 | 22.2 | 12,893 | 25.0 | 13,373 | 29.0 | 13,960 | 31.7 | 14,018 | 33.2 |
|
| ||||||||||||||
| Fractional flow reserve among patients with 40–70% lesion | 14,636 | 18.0 | 706 | 8.1 | 1,987 | 10.2 | 2,285 | 13.8 | 2,824 | 21.6 | 3,369 | 28.2 | 3,465 | 30.8 |
|
| ||||||||||||||
| No. of diseased vessels | ||||||||||||||
| 0 | 2,758 | 0.7 | 350 | 0.9 | 741 | 0.8 | 587 | 0.8 | 407 | 0.6 | 358 | 0.6 | 315 | 0.5 |
| 1 | 214,960 | 54.1 | 23,162 | 56.5 | 49,732 | 55.4 | 42,445 | 54.2 | 35,963 | 53.8 | 32,790 | 52.5 | 30,868 | 52.0 |
| 2 | 116,447 | 29.3 | 11,656 | 28.4 | 25,908 | 28.9 | 23,008 | 29.4 | 19,578 | 29.3 | 18,539 | 29.7 | 17,758 | 29.9 |
| 3 | 63,572 | 16.0 | 5,856 | 14.3 | 13,323 | 14.9 | 12,288 | 15.7 | 10,901 | 16.3 | 10,770 | 17.2 | 10,434 | 17.6 |
Includes July 1, 2009 to December 31, 2009.
Abbreviations: CABG, coronary artery bypass graft; CCS, Canadian Cardiovascular Society; PCI, percutaneous coronary intervention.
Low-risk (<1% annual mortality rate) includes: low-risk treadmill score (score ≥5); normal or small myocardial perfusion defect at rest or with stress; normal stress echocardiographic wall motion or no change of limited resting wall motion abnormalities during stress.
Intermediate-risk (1% to 3% annual mortality rate) includes: mild/moderate resting left ventricular dysfunction (LVEF 35% to 49%); intermediate-risk treadmill score (score between −11 and <5); stress-induced moderate perfusion defect without LV dilation or increased lung intake (thallium-201); limited stress echocardiographic ischemia with a wall motion abnormality only at higher doses of dobutamine involving less than or equal to 2 segments.
High-risk (>3% annual mortality rate) includes: severe resting left ventricular dysfunction (LVEF <35%); high-risk treadmill score (score ≤−11); severe exercise left ventricular dysfunction (exercise LVEF <35%); stress-induced large perfusion defect (particularly if anterior); stress-induced multiple perfusion defects of moderate size; large, fixed perfusion defect with LV dilation or increased lung uptake (thallium-201); stress-induced moderate perfusion defect with LV dilation or increased lung uptake (thallium-201); echocardiographic wall motion abnormality (involving >2 segments) developing at low dose of dobutamine (≤10 mg/kg/min) or at a low heart rate (<120 beats/min); stress echocardiographic evidence of extensive ischemia.
Among patients in the overall study cohort, the absolute number and relative proportion of patients undergoing PCI with CCS 1 or 2 angina decreased over time while the absolute number and relative proportion of patients with CCS 4 angina increased over the study period. The numbers of patients undergoing PCI in the setting of an acute coronary syndrome (ACS) (unstable angina, NSTEMI, and STEMI) were stable (367,253 in 2010 to 368,574 in 2014) with increases in the number of NSTEMI patients (94,097 in 2010 to 107,225 in 2014) and decreases in the number of unstable angina patients (194,008 in 2010 to 183,735 in 2014). Use of anti-anginal therapy increased over the study period while use of non-invasive testing remained stable. The number and relative proportion of patients with unavailable or low-risk results on stress testing declined while there was an increase in the number and relative proportion of patients with intermediate and high-risk findings. The burden of coronary artery disease on angiography was similar over the study period.
Among patients undergoing non-acute PCI, the absolute number and relative proportion of patients without symptoms or with CCS 1 or 2 angina decreased over time. There was an increase in both the absolute number and relative proportion of patients undergoing non-acute PCI with CCS 3 angina (13,442 and 15.0% in 2010 to 20,727 and 34.9% in 2014). There was an increase in the use of anti-anginal therapy with 80.6% of patients undergoing non-acute PCI in 2014 reported to be on at least 1 anti-anginal medication and 35.1% receiving 2 or more anti-anginal medications as compared with 69.8% and 22.3% respectively in 2010. Performance of non-invasive testing and fractional flow reserve testing increased over the study interval from 64.6% and 8.1% in 2010 to 72.5% and 30.8% in 2014. Moreover, the extent of ischemia with non-invasive testing changed over time with 64.7% of patients having intermediate or high-risk findings in 2010 as compared with 78.1% in 2014. The proportion of patients with multi-vessel coronary artery disease was 43.7% in 2010 and 47.5% in 2014.
Trends in Inappropriate PCI
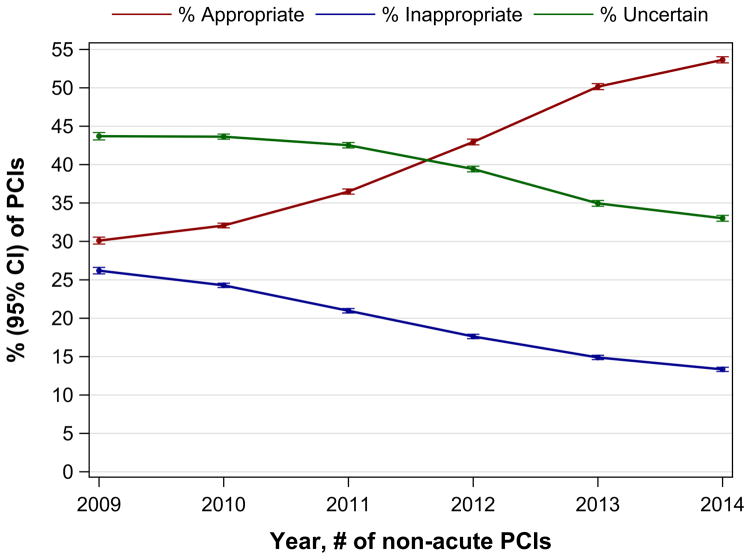
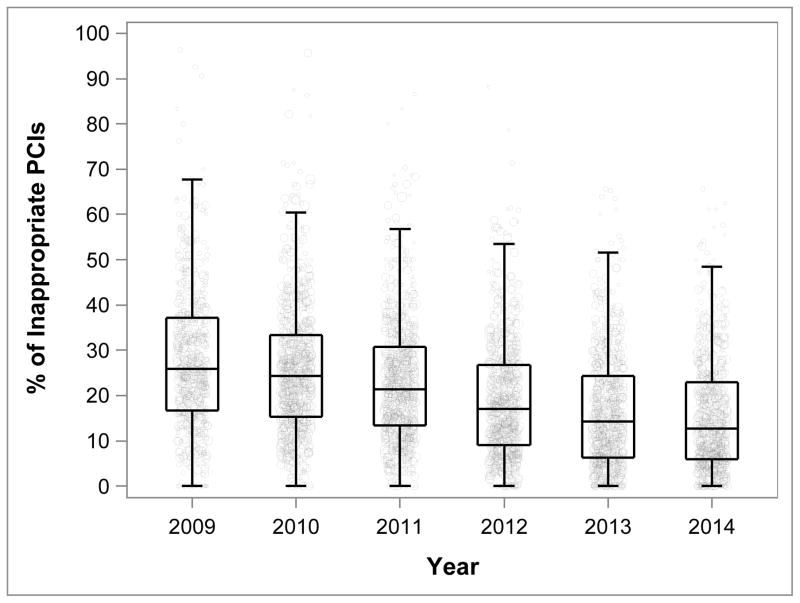
Between July 2009 and December 2014, the proportion (95% CI) of non-acute PCIs classified as inappropriate decreased from 26.2% (95% CI, 25.8%–26.6%) to 13.3% (95% CI, 13.1%–13.6%), p<0.001, Figure 2A). The absolute number of inappropriate PCIs decreased from 21,781 in 2010 to 7,921 in 2014. The percentage (95% CI) of non-acute PCIs classified as appropriate increased from 30.1% (95% CI, 29.7%–30.6%) to 53.6% (95% CI, 53.2%-54.0%) and those considered uncertain decreased from 43.7% (95% CI, 43.2%–44.2%) to 33.0% (95% CI, 32.6%–33.4%) (Figure 2A). Hospital-level trends in the proportion of inappropriate non-acute PCIs are shown in Figure 2B. The median hospital proportion of non-acute PCIs considered inappropriate decreased from 25.8% in 2009 to 12.6% in 2014. There was persistent variation in hospital level proportion of non-acute PCIs classified as inappropriate over the study interval (IQR 16.7% to 37.1% in 2009; IQR 5.9% to 22.9% in 2014).
Figure 2. Proportions of Appropriate, Inappropriate, and Uncertain PCI at the Patient-level (A) and Proportions of Inappropriate PCI at the Hospital-level (B) among Non-acute PCIs from July 1, 2009 to December 31, 2014.
Figure 2A/B. Rates of Appropriate, Inappropriate, and Uncertain PCI at the Patient-level (A) and Rate of Inappropriate PCI at the Hospital-level (B) among non-acute PCIs from July 1, 2009 to December 31, 2014 at 766 hospitals participating continuously in the NCDR-CathPCI Registry over the study period. For each classification of procedural appropriateness, the point estimate and 95% CI are plotted in Figure 2A. For each box-plot in Figure 2B, the horizontal line in the center of the rectangle represents the median, the bottom and top horizontal lines of each rectangle represent the 25th and 75th percentiles respectively, and the horizontal lines capping the vertical lines extending from the rectangle represent 1.5-times the interquartile range. Each hospital is represented as a point in the box-plot, the size of the point reflects the hospital volume. Note: Results from 2009 include 6-months of data.
Temporal Patterns Across Hospitals
Among hospitals in the highest quartile for proportion of non-acute PCI deemed inappropriate from July 2009 to December 2010 (n=191), we observed 4 distinct trajectories in changes in rates of inappropriate PCI from January 2011 to December 2014 (Figure 3). Hospitals in groups 1, 2 and 4 had similar baseline rates of inappropriate PCI, however, hospitals in group 4 (n=108) demonstrated immediate and steady declines in inappropriate PCI rates from 43.9% (95% CI, 42.4%–45.3%) in 2009–10 to 15.5% (95% CI, 14.0%–17.0%) in 2014. In contrast, hospitals in group 1 (n=18) had minimal change in the first two years, but demonstrated lower rates of inappropriate PCI in the last two years of the study period. Hospitals in group 2 (n=50) demonstrated steady but smaller absolute declines in inappropriate PCI over the study period than groups 1 and 4, with the proportion of inappropriate non-acute PCIs decreasing from 40.9% (95% CI, 39.7%–42.1%) in 2009–10 to 32.2% (95% CI, 30.4%–34.1%) in 2014. Finally, hospitals in group 3 (n=15) had the highest initial rates of inappropriate PCI, but also the largest absolute decline over the study period from 70.6% (95% CI, 68.5%–72.7%) in 2009–10 to 9.4% (95% CI, 7.6%–11.1%) in 2014. There were no systematic differences in hospital characteristics, geographic location, financial status, or teaching status across hospital groups (eTable 3).
Figure 3. Trends in Inappropriate Non-Acute PCI at Hospitals with the Highest Initial Proportion of Inappropriate PCI (>34% from July 2009 to December 2010).
Observed Rates (95% CI) of Inappropriate non-acute PCI for 4 groups of hospitals identified by latent growth curve analysis. The analysis was restricted to hospitals with the highest initial rates of inappropriate non-acute PCI from July 2009 to December 2010 (>34%, n=191). Note: Results shown for 2010 include data for 2009 and 2010.
Discussion
Among patients undergoing PCI between July 2009 and December 2014, we found that volumes of non-acute PCIs declined significantly from 89,704 in 2010 to 59,375 in 2014 while the volume of acute PCIs remained stable, 377,540 in 2010 to 374,543 in 2014. In addition, we observed significant reductions in the proportion of non-acute PCIs classified as inappropriate from 26.2% in 2009 to 13.3% in 2014. However, there was persistent hospital-level variation in the rate of inappropriate PCIs with an IQR of 5.9% to 22.9% in 2014. Collectively these findings suggest that the practice of interventional cardiology has evolved since the introduction of Appropriate Use Criteria in 2009.
Our analysis provides details about changes in the clinical profiles of patients undergoing PCI and suggests that the observed reductions in inappropriate PCI in part reflect improvements in patient selection and clinical decision-making as well as better documentation of the key elements used to determine procedural appropriateness. Trends consistent with improvements in patient selection include the reduction in non-acute PCI volume and changes in the clinical profile of patients undergoing non-acute PCI. We observed significant declines in the proportions of non-acute PCI patients who were asymptomatic or had minimal symptoms; who were not receiving or only receiving minimal anti-anginal therapy; and who had low or intermediate risk findings on non-invasive testing. We identified increased use of FFR among patients with intermediate stenosis. These findings may indicate that clinicians are doing a better job of identifying and limiting non-acute PCI procedures to those patients most likely to benefit from revascularization.
We cannot exclude the possibility that reductions in inappropriate PCI may reflect changes in documentation or even intentional up-coding, particularly of subjective data elements such as symptom severity. Temporal trends in anginal symptom burden raise the possibility that this data element, which is largely subjective, may be overstated. Specifically, despite significant reductions in the volume of non-acute PCI, we observed increases in the numbers and proportions of patients reported to have CCS 3 and 4 angina but minimal change in extent of CAD. Nevertheless, we did not see evidence that patients were being systematically shifted from non-acute to acute indications for PCI. The number of acute PCIs were stable over time, and the proportion of acute PCI patients reported to have unstable angina decreased.
The appropriateness of PCI has garnered attention from clinicians, insurers, and policymakers. It has been the subject of national quality improvement initiatives and incorporated into pay-for-performance programs. In our analysis, the observed reductions in inappropriate PCI appeared to accelerate in 2011, which coincided with the publication of a high profile paper on PCI appropriateness, the NCDR’s inclusion of procedural appropriateness in its benchmarking reports, and the launch of national performance improvement campaigns.3,7 Our findings are consistent with an analysis of PCI appropriateness in Washington State.21 However, because the registry was not configured to characterize PCI appropriateness until July 2009, our analyses are limited to cases performed after the release of the Appropriate Use Criteria. As such we could not evaluate the impact of the Appropriate Use Criteria, and our findings are best considered a description of changes in patterns of care and procedural appropriateness over this period. It is likely that many factors such as the publication of the COURAGE and BARI2D trials influenced clinical practice during this timeframe.22,23
We observed persistent variation in hospital-level performance of inappropriate PCI. Among better performing hospitals (lowest quartile), fewer than 5% of non-acute PCIs in 2014 were classified as inappropriate. In contrast, among worse performing hospitals (highest quartile), more than 25% of non-acute PCIs were classified as inappropriate. These findings suggest the need for ongoing performance improvement initiatives and hospital benchmarking. Among hospitals with the highest rates of inappropriate non-acute PCI from July 2009 and December 2010, we observed distinct trajectories from January 2011 to December 2014. Although the majority of hospitals with the highest baseline rates of inappropriate PCI demonstrated large reductions in the proportion of PCIs classified as inappropriate, we identified a group of hospitals with less than 10% absolute reduction in the performance of inappropriate PCI over the study period. The observed differences in timing and pace of change suggest both that Appropriate Use Criteria-related quality metrics are actionable and that the specific approach adopted by a hospital impacts its performance. Identifying the organizational strategies and enabling structures most strongly associated lower rates of inappropriate PCI remain a potentially important area for future research.
There are several limitations to our analysis. First, not all hospitals that perform PCI in the United States participate in the registry. Furthermore, we excluded hospitals that did not participate in the registry throughout the entire study period and these hospitals may have different rates of inappropriate PCI. Regardless, our analysis included nearly 2.7 million procedures performed across 766 facilities and represents the most comprehensive examination of PCI appropriateness to date. In addition, only including hospitals participating in the registry over the entire study period enabled us to more rigorously investigate temporal changes in PCI utilization, clinical characteristics, and appropriateness. Second, our analysis focused mostly on trends in potential overuse (i.e. inappropriate) PCI. Understanding whether Appropriate Use Criteria have introduced new barriers to the performance of medically necessary procedures remains an important topic that could not be addressed in our study. Relatedly, we only have information on patients undergoing PCI, rather than the larger population of patients with CAD who might be considered for revascularization. As such, we cannot determine whether the observed changes truly reflect improved patient selection or overestimation of patient symptoms. The integration of more objective assessments of patient-reported health status into routine clinical care may provide a way to reduce the chances of misclassifying symptom burden.24
Conclusions
Since the publication of the Appropriate Use Criteria in 2009, there have been significant reductions in non-acute PCI volume. The proportion of non-acute PCIs classified as inappropriate has declined though hospital-level variation in inappropriate PCI persists, suggesting the need for ongoing quality improvement initiatives.
Supplementary Material
Acknowledgments
Dr. Nihar Desai and Dr. Jeptha Curtis had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Craig Parzynski (Center for Outcomes Research and Evaluation, Yale-New Haven Hospital, New Haven, CT) conducted the analysis and is responsible for the data analysis.
Funding
Dr. Desai is supported by grant K12 HS023000-01 from the Agency for Healthcare Research and Quality. Drs. Krumholz and Curtis are supported by grant U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. Dr. Bradley is supported by a Career Development Award (HSR&D-CDA2 10-199) from Veterans Affairs Health Services Research and Development. This research was supported by the NCDR. The analytic work for this investigator-initiated study was performed by the Yale Center for Outcomes Research and Evaluation Data Analytic Center with financial support from the American College of Cardiology.
Footnotes
Role of the Sponsors
The NCDR CathPCI Registry is an initiative of the ACC Foundation and the Society for Cardiovascular Angiography and Interventions. The manuscript was reviewed by the NCDR for compliance with registry description and representation but the sponsor did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation of the manuscript; or decision to submit the manuscript for publication.
Disclaimer
The views expressed in this article represent those of the authors and do not necessarily represent the official views of the NCDR or its associated professional societies, identified at http://www.ncdr.com
Conflicts of Interest
Drs. Desai and Krumholz are recipients of a research agreement from Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing. Drs. Desai, Krumholz and Curtis receive funding from the Centers for Medicare & Medicaid Services to develop and maintain performance measures that are used for public reporting. Dr. Krumholz receives research support from Medtronic, through Yale University, to develop methods of clinical trial data sharing and of a grant from the Food and Drug Administration to develop methods for post-market surveillance of medical devices. Dr. Krumholz chairs a cardiac scientific advisory board for UnitedHealth. Dr. Spertus discloses funding from the American College of Cardiology to analyze the NCDR registries, membership on the United Healthcare cardiac scientific advisory board and an equity interest in Health Outcomes Sciences. Dr. Patel has research grants through Duke University with Johnson and Johnson, AstraZeneca, Maquet, National Heart Lung and Blood Institute, AHRQ, and is on the Advisory Board for Bayer Healthcare, Jansen, and Genzyme. Dr. Curtis discloses equity interest in Medtronic. No other disclosures were reported.
References
- 1.Patel MR, Dehmer GJ, Hirshfeld JW, et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. Journal of the American College of Cardiology. 2009 Feb 10;53(6):530–553. doi: 10.1016/j.jacc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed June 15, 2015];Proceedings from the National Summit on Overuse. http://www.jointcommission.org/assets/1/6/National_Summit_Overuse.pdf.
- 3.Chan PS, Patel MR, Klein LW, et al. Appropriateness of percutaneous coronary intervention. Jama. 2011 Jul 6;306(1):53–61. doi: 10.1001/jama.2011.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley SM, Maynard C, Bryson CL. Appropriateness of percutaneous coronary interventions in Washington State. Circulation. Cardiovascular quality and outcomes. 2012 Jul 1;5(4):445–453. doi: 10.1161/CIRCOUTCOMES.111.964320. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed July 2, 2015];Heart Treatment Overused. http://www.wsj.com/articles/SB10001424052702304760604576428323005864648.
- 6.Inappropriate heart procedures are expensive and risky. [Accessed July 2, 2015];And studies show thousands happen every year. http://www.washingtonpost.com/blogs/wonkblog/wp/2012/08/08/inappropriate-heart-procedures-are-expensive-and-risky-and-studies-show-thousands-happen-every-year/
- 7.http://www.choosingwisely.org/societies/society-for-cardiovascular-angiography-and-interventions/
- 8.Blue Cross Blue Shield of Michigan. [Accessed June 20, 2015];Hospital Pay-for-Performance Program. 2014 https://www.bcbsm.com/content/dam/public/Providers/Documents/value/p4p-hospital-cqi-performance-index-guide.pdf.
- 9.New York State Medicaid Update. [Accessed June 20, 2015];Percutaneous Coronary Intervention Coverage Guidelines. https://www.health.ny.gov/health_care/medicaid/program/update/2013/2013-06.htm#ous.
- 10.Weintraub WS, McKay CR, Riner RN, et al. The American College of Cardiology National Database: progress and challenges. American College of Cardiology Database Committee. Journal of the American College of Cardiology. 1997 Feb;29(2):459–465. doi: 10.1016/s0735-1097(96)00545-1. [DOI] [PubMed] [Google Scholar]
- 11.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. Journal of the American College of Cardiology. 2001 Jun 15;37(8):2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 12.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 Appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. Journal of the American College of Cardiology. 2012 Feb 28;59(9):857–881. doi: 10.1016/j.jacc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Hendel RC, Patel MR, Allen JM, et al. Appropriate use of cardiovascular technology: 2013 ACCF appropriate use criteria methodology update: a report of the American College of Cardiology Foundation appropriate use criteria task force. Journal of the American College of Cardiology. 2013 Mar 26;61(12):1305–1317. doi: 10.1016/j.jacc.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Patel MR, Spertus JA, Brindis RG, et al. ACCF proposed method for evaluating the appropriateness of cardiovascular imaging. Journal of the American College of Cardiology. 2005 Oct 18;46(8):1606–1613. doi: 10.1016/j.jacc.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol Methods. 1999 Jun;4(2):139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Bradley SM, Rao SV, Curtis JP, et al. Change in Hospital-Level Use of Transradial Percutaneous Coronary Intervention and Periprocedural Outcomes Insights from the National Cardiovascular Data Registry. Circ-Cardiovasc Qual. 2014 Jul;7(4):550–559. doi: 10.1161/CIRCOUTCOMES.114.001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cochran WG. Some methods for strengthening the common chi-squared tests. Biometrics (International Biometric Society) 1954;10(4):417–451. doi: 10.2307/3001616.JSTOR3001616. [DOI] [Google Scholar]
- 18.Armitage P. Tests for Linear Trends in Proportions and Frequencies. Biometrics (International Biometric Society) 1955;11(3):375–386. doi: 10.2307/3001775.JSTOR3001775. [DOI] [Google Scholar]
- 19.Jonckheere A. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- 20.Sidak Z. Rectangular Confidence Regions for Means of Multivariate Normal Distributions. J Am Stat Assoc. 1967;62(318):626. –&. [Google Scholar]
- 21.Bradley SM, Bohn CM, Malenka DJ, et al. Temporal Trends in Percutaneous Coronary Intervention Appropriateness: Insights From the Clinical Outcomes Assessment Program. Circulation. 2015 Jul 7;132(1):20–26. doi: 10.1161/CIRCULATIONAHA.114.015156. [DOI] [PubMed] [Google Scholar]
- 22.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. The New England journal of medicine. 2007 Apr 12;356(15):1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 23.Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. The New England journal of medicine. 2009 Jun 11;360(24):2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circulation. Cardiovascular quality and outcomes. 2014 Sep;7(5):640–647. doi: 10.1161/CIRCOUTCOMES.114.000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.