Abstract
BACKGROUND
High-dose, post-transplantation cyclophosphamide (PTCy) to prevent graft-versus-host disease (GVHD) has improved outcomes in haploidentical (HAPLO) stem cell transplantation (SCT). However, it remains unclear whether this strategy is effective in SCT from 1-antigen human leukocyte antigen (HLA)-mismatched unrelated donors (9/10 MUD) and how the outcomes of these patients compare with those of haploidentical transplantation recipients.
METHODS
A parallel, 2-arm, nonrandomized phase 2 clinical trial was conducted of melphalan-based reduced-intensity conditioning with PTCy, tacrolimus, and mycophenolate mofetil to prevent GVHD in patients with high-risk hematologic malignancies who underwent HAPLO (n = 60) or 9/10 MUD (n = 46) SCT.
RESULTS
The 1-year overall and progression-free survival rates were 70% and 60%, respectively, in the HAPLO arm and 60% and 47%, respectively, in the 9/10 MUD arm. The day +100 cumulative incidence of grade II to IV acute GVHD and grade III to IV acute GVHD was 28% and 3%, respectively, in the HAPLO arm and 33% and 13%, respectively, in the 9/10 MUD arm. The 2-year cumulative incidence of chronic GVHD was 24% in the HAPLO arm and 19% in the 9/10 MUD arm. The 1-year cumulative incidence of nonrelapse mortality was 21% in the HAPLO arm and 31% in the 9/10 MUD arm, and the 1-year relapse rate was 19% in the HAPLO arm and 25% in the 9/10 MUD arm.
CONCLUSIONS
Although this was a nonrandomized study and could not serve as a direct comparison between the 2 groups, the authors conclude that PTCy-based GVHD prophylaxis is effective for both HAPLO and 9/10 MUD SCTs. Prospective randomized trials will be required to compare the efficacies of alternative donor options for patients lacking HLA-matched donors.
Keywords: 9/10 matched unrelated donors, graft-versus-host disease, haploidentical transplantation, hematologic malignancies, post-transplantation cyclophosphamide
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (SCT) is a curative option for many patients with high-risk or advanced hematologic malignancies.1 However, donor availability remains an important limitation for a large proportion of patients.2 For patients without an HLA-matched sibling, an 8/8 human leukocyte antigen (HLA)-matched unrelated donor (MUD), matched at HLA-A, HLA-B, HLA-C, and HLA-D-related β1 (HLA-DRB1), is the current standard of care because of similar transplantation outcomes.3,4 The availability of an MUD is related to the race of the recipient; such a match can be identified for about 70% of Caucasians, 30% of Hispanics and Asians, and ≤20% of African Americans.5 When there is no MUD available, 1 of 3 alternative donor sources could be used: haploidentical donors, cord blood, or mismatched unrelated donors. It remains unclear whether 1 of these alternative sources is superior to the others.
The introduction of high-dose, post-transplantation cyclophosphamide (PTCy) in combination with tacrolimus and mycophenolate mofetil (MMF) to prevent graft-versus-host disease (GVHD) has significantly improved the outcomes of patients who undergo haploidentical SCTs.6 Moreover, 2 parallel Blood and Marrow Transplant Clinical Trials Network clinical studies using nonmyeloablative conditioning regimens reported similar outcomes between haploidentical SCTs and cord blood transplantations.7 However, outcomes of haploidentical transplantations have not been compared with outcomes of HLA-mismatched unrelated donor transplantations, and it is currently unknown whether PTCy can also reduce GVHD in patients who undergo a 1-antigen HLA-mismatched unrelated donor (9/10 MUD) SCT. To address this question, we initiated a nonrandomized phase 2 clinical trial with 2 parallel arms investigating the safety and efficacy of GVHD prophylaxis with PTCy, tacrolimus, and MMF after a melphalan-based, reduced-intensity conditioning regimen in patients with advanced hematologic malignancies who underwent SCT from a haploidentical donor or a MUD mismatched at a single HLA-A, HLA-B, HLA-C, HLA-DRB1, or HLA-DQ β1 (DQB1) antigen or allele. Patients were considered for study entry if they lacked a matched related donor or a 10/10 HLA-matched unrelated donor (including HLA-DQB1) typed by high-resolution methods. The melphalan-based regimen used in the current study was previously used by our group with T-cell–depleted haploidentical grafts and produced good disease control and an acceptable toxicity profile.8 Here, we report the results from this single-institution clinical trial.
MATERIALS AND METHODS
Study Design
This was a prospective nonrandomized phase 2 clinical trial for patients with hematologic malignancies and consisted of 2 parallel arms: haploidentical transplants (HAPLO arm) and 9/10 MUD transplants (9/10 MUD arm). The clinical trial was registered at ClinicalTrials.gov (National Clinical Trial NCT01010217). Patients were enrolled during the period from January 2010 through August 2014 and were assigned to the HAPLO arm or the 9/10 MUD arm based on donor availability and physician preference. The 2 arms received identical melphalan-based conditioning chemotherapy and GVHD prophylaxis, as described below. The primary objective of this study was to assess the safety and feasibility of this approach to prevent GVHD in mismatched related and unrelated donor transplants and determine the clinical outcomes of patients with advanced hematologic malignancies treated in this fashion with 2 donor sources. The study was reviewed and approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. All patients provided written informed consent according to the principles of the Declaration of Helsinki.
Treatment Protocol
The conditioning regimen consisted of 1 intravenous dose of 140 mg/m2 melphalan on day −7, 1 intravenous dose of 5 mg/kg thiotepa on day −6, and 4 intravenous doses of 40 mg/m2 fludarabine (1 daily on days −5 through −2) (FM140). Because thiotepa was intermittently available, it was replaced by total body irradiation at a dose of 2 grays on day −1. Older patients (aged >55 years) or those with significant comorbidities received a lower melphalan dose (100 mg/m2) (FM100). All patients who had B-lymphocyte antigen (CD20)-positive lymphoma received rituximab (375 mg/m2) on days −13, −6, +1, and +8.
The GVHD prophylaxis consisted of 50 mg/kg cyclophosphamide on days +3 and +4 and tacrolimus and MMF starting on day +5. Patients received MMF at a dose of 15 mg/kg 3 times daily (maximum, 1000 mg per dose), which was continued until day +100 and then tapered. The MMF was originally planned to stop on day +35; however, after the first 11 patients were enrolled, 5 patients developed grade II through IV acute GVHD (aGVHD) after the MMF was stopped. Subsequently, we amended the protocol to taper the MMF after day +100. Serum levels of tacrolimus were maintained between 5 and 15 ng/mL (goal, 8 ng/mL), and the tacrolimus was continued until day +180 followed by a weekly taper if GVHD was absent.
The stem cell source was unmodified bone marrow for both the HAPLO arm and the 9/10 MUD arm and was infused fresh on day 0. The goal of the bone marrow harvest was 3 × 108 total nucleated cells/kg recipient body weight. If obtaining a bone marrow graft was not feasible, then patients received a peripheral blood graft. Filgrastim (5 μg/kg/day) was started on day +7 and continued until the absolute neutrophil count (ANC) was >1000/μL for 3 consecutive days. Supportive care included standard antiviral, antifungal, and Pneumocystis carinii pneumonia prophylaxis.
Patients
Patients ages 18 to 65 years with advanced or high-risk hematologic malignancies, including patients with active disease, who lacked an HLA-matched related donor or a 10/10 MUD were eligible for the study. High-risk disease was defined according to established criteria and is described in the accompanying online materials (see online Supporting Information). Other criteria for inclusion in the study were a Karnofsky performance status ≥70 and adequate cardiac, pulmonary, hepatic, and renal function. The exclusion criteria were human immunodeficiency virus infection, active hepatitis B or C infection, active infections, cirrhosis, active disease involvement in the central nervous system, and pregnancy. HLA typing, HLA antibody testing, and donor selection were performed as previously described (see online Supporting Information).8–10
Immunologic Reconstitution Studies
We evaluated the reconstitution of lymphocyte subsets using flow cytometry for total lymphocytes (CD3+), total lymphocytes/T cells (CD3+/CD4+, CD3+/CD8+), T-regulatory cells (CD25+), B cells (CD19+), natural killer cells (CD56+), naive cells (CD45RA+), and memory cells (CD45RO+) on peripheral blood samples from recipients of HAPLO and 9/10 MUD transplantations on days +30, +90, +180, +270, and +365 after transplantation, as described in the online materials (see online Supporting Information).
Statistical Analysis
The primary objective of this study was to determine the safety and nonrelapse mortality (NRM) for each group. The study was monitored using the NRM rate at day +100 as the primary safety endpoint; a Bayesian monitoring scheme was used with early stopping rules in which the trial would be stopped if the predicted NRM rate at day +100 was >25%. The secondary endpoints were the cumulative incidence of neutrophil and platelet recovery; the 1-year overall survival (OS) rate; and the cumulative incidence of aGVHD, chronic GVHD (cGVHD), NRM, and relapse. Other endpoints were the progression-free survival (PFS) rate and the immunologic reconstitution of the T-cell subsets during the first year after transplantation. Further details of the statistical methods are outlined in the online materials (see online Supporting Information).
RESULTS
Patient Characteristics
The characteristics of patients in both the HAPLO arm (n = 60) and the 9/10 MUD arm (n = 46) of this study are summarized in Table 1. The median follow-up duration was 24 months in the HAPLO arm and 29 months in the 9/10 MUD arm. Because neither arm met the early stopping rule and because enrollment in the 9/10 MUD arm was slower than in the HAPLO arm, enrollment in the HAPLO arm was extended until accrual in the 9/10 MUD arm was completed. Table 2 summarizes the transplantation outcomes for each arm.
TABLE 1.
Patient Characteristics
| Characteristic | No. of Patients (%)
|
|
|---|---|---|
| HAPLO Arm, n = 60 | 9/10 MUD Arm, n = 46 | |
| Median age [range], y | 45 [20–63] | 51 [20–64] |
| Recipient sex | ||
| Men | 29 (48) | 23 (50) |
| Women | 31 (52) | 23 (50) |
| Karnofsky performance status | ||
| ≥90 | 53 (88) | 40 (87) |
| <90 | 7 (12) | 6 (13) |
| Donor sex | ||
| Men | 35 (58) | 22 (48) |
| Women | 25 (42) | 24 (52) |
| Donor relationship to recipient | ||
| Unrelated | NA | 46 (100) |
| Parent | 8 (13) | NA |
| Child | 24 (40) | NA |
| Sibling | 27 (45) | NA |
| Cousin | 1 (2) | NA |
| HLA locus mismatch location | ||
| A | 0 (0) | 20 (44) |
| B | 0 (0) | 6 (13) |
| C | 0 (0) | 16 (35) |
| DRB1 | 0 (0) | 2 (4) |
| DQB1 | 0 (0) | 2 (4) |
| A/B/C/DRB1/DQB1 | 42 (70) | 0 (0) |
| B/C/DRB1/DQB1 | 7 (12) | 0 (0) |
| A/B/DRB1/DQB1 | 4 (7) | 0 (0) |
| A/B/C/DQB1 | 2 (3) | 0 (0) |
| A/B/C/DRB1 | 1 (2) | 0 (0) |
| A/DRB1/DQB1 | 2 (3) | 0 (0) |
| A/B/DRB1 | 1 (2) | 0 (0) |
| DRB1/DQB1 | 1 (2) | 0 (0) |
| Hematopoietic cell transplant-comorbidity index | ||
| 0–1 | 31 (52) | 22 (48) |
| 2–3 | 19 (32) | 16 (35) |
| >3 | 10 (17) | 8 (17) |
| Disease risk indexa | ||
| Very high | 5 (8) | 3 (7) |
| High | 18 (30) | 15 (33) |
| Intermediate | 29 (48) | 12 (26) |
| Low | 8 (13) | 12 (26) |
| NA | 0 (0) | 4 (9)b |
| No. of prior autologous hematopoietic cell transplantations | ||
| 0 | 53 (88) | 38 (83) |
| 1 | 6 (10) | 6 (13) |
| 2 | 1 (2) | 2 (4) |
| No. of prior chemotherapy cycles | ||
| 0–3 | 48 (80) | 36 (78) |
| >3 | 12 (20) | 10 (22) |
| Conditioning intensity | ||
| FM100 | 20 (33) | 18 (39) |
| FM140 | 40 (67) | 28 (61) |
| Graft source | ||
| Bone marrow | 58 (97) | 38 (83) |
| Peripheral blood | 2 (3) | 8 (17) |
| CMV status | ||
| Patient and donor negative | 6 (10) | 5 (11) |
| Patient or donor positive | 54 (90) | 41 (89) |
| Diagnosis | ||
| AML/MDS | 33 (55) | 18 (39) |
| Secondary AML/MDSc | 7 (12) | 7 (15) |
| ALL | 7 (12) | 5 (11) |
| Non-Hodgkin lymphoma | 8 (13) | 11 (24) |
| Hodgkin lymphoma | 2 (3) | 2 (4) |
| Myeloproliferative disease | 9 (15) | 4 (9) |
| Multiple myeloma | 1 (2) | 2 (4) |
| Aplastic anemia | 0 (0) | 4 (9) |
| Disease stage For acute leukemia | ||
| CR1/CR2 | 24 (67) | 9 (56) |
| ≥CR3/CRp | 6 (17) | 5 (31) |
| Active disease | 6 (17) | 2 (13) |
| For lymphoma | ||
| CR | 3 (30) | 8 (62) |
| PR | 5 (50) | 3 (23) |
| Chemoresistant | 2 (20) | 2 (15) |
| For multiple myeloma | ||
| PR | 1 (100) | 1 (50) |
| Very good PR | 0 (0) | 1 (50) |
Abbreviations: 9/10 MUD, HLA-mismatched unrelated donor; ALL, acute lymphoblastic leukemia; AMS/MDS, acute myeloid leukemia/myelodysplastic syndrome; CMV, cytomegalovirus; CR1, first complete remission; CR2, second complete remission; CR3, third complete remission; CRp, complete remission with incomplete count recovery; DQB1, DQ β1; DRB1, D-related β1; FM100, the same regimen as FM140 with a lower melphalan dose of 100 mg/m2; FM140, 1 intravenous dose of 140 mg/m2 melphalan on day −7, 1 intravenous dose of 5 mg/kg thiotepa on day −6, and 4 intravenous doses of 40 mg/m2 fludarabine (1 daily on days −5 through −2); HAPLO, haploidentical donor; HLA, human leukocyte antigen; NA, not applicable; PR, partial remission.
Disease risk was assessed using the disease risk index described by Armand et al, 2012.30
These patients had aplastic anemia.
Secondary AML/MDS was defined as disease secondary to a previous hematologic disorder or therapy related.
TABLE 2.
Transplantation Outcomes
| Outcome | HAPLO Arm, n = 60 |
9/10 MUD Arm, n = 46 |
|---|---|---|
| Engraftment, % | 97 | 98 |
| Median time to platelet recovery, d | 25 | 28 |
| Median time to ANC >500/μL, d | 18 | 18 |
| Chimerism at day 30 excluding patients with graft failure or early death, no./no. evaluable | ||
| Full | 56/57 | 43/44 |
| Mixed | 1/57 | 1/44 |
| OS rate [95% CI], % | ||
| Day 100 | 90 [79–95] | 87 [73–94] |
| 1 y | 70 [55–79] | 60 [44–73] |
| 2 y | 55 [40–67] | 52 [35–65] |
| PFS rate [95% CI], % | ||
| Day 100 | 88 [77–94] | 80 [66–89] |
| 1 y | 60 [46–72] | 47 [32–61] |
| 2 y | 53 [38–65] | 42 [27–55] |
| Relapse cumulative incidence [95% CI], % | ||
| Day 100 | 2 [0.1–8] | 7 [2–16] |
| 1 y | 19 [10–31] | 25 [13–38] |
| 2 y | 24 [14–37] | 25 [13–38] |
| Grade II–IV aGVHD cumulative incidence [95% CI], % | ||
| Day 100 | 28 [18–40] | 33 [20–46] |
| 1 y | 33 [22–45] | 40 [25–54] |
| Grade III–IV aGVHD cumulative incidence [95% CI], % | ||
| Day 100 | 3 [0 6–10] | 13 [5–25] |
| 1 y | 5 [1–13] | 15 [7–27] |
| cGVHD cumulative incidence [95% CI], % | ||
| 1 y | 19 [10–30] | 19 [9–32] |
| 2 y | 24 [13–36] | 19 [9–32] |
| NRM cumulative incidence [95% CI], % | ||
| Day 100 | 10 [4–19] | 13 [5–25] |
| 1 y | 21 [11–32] | 31 [18–45] |
| 2 y | 23 [13–35] | 34 [20–48] |
| CMV reactivation cumulative incidence at day 100 [95% CI], % | 66 [52–76] | 67 [50–79] |
| Deaths, no. of patients (%) | 24 (100) | 23 (100) |
| Disease relapse | 11 (46) | 8 (35) |
| Infection | 4 (17) | 5 (22) |
| Organ damage | 4 (17) | 2 (9) |
| aGVHD | 1 (4) | 5 (22) |
| cGVHD | 2 (8) | 2 (9) |
| Graft failure | 2 (8) | 1 (4) |
Abbreviations: 9/10 MUD, HLA-mismatched unrelated donor; aGVHD, acute graft-versus-host disease; ANC, absolute neutrophil count; cGVHD, chronic graft-versus-host disease; CI, confidence interval; CMV, cytomegalovirus; NRM, nonrelapse mortality; OS, overall survival; PFS, progression-free survival.
Engraftment and Chimerism
In the HAPLO arm, the cumulative incidence of neutrophil recovery at day +45 was 95% (95% confidence interval [CI], 84%–99%), and the median time to neutrophil recovery was 18 days (range, 13–27 days). The cumulative incidence of platelet recovery at day +100 was 87% (95% CI, 74%–94%), and the median time to platelet recovery was 25 days (range, 18–114 days). There were 2 cases of primary graft failure (both patients received FM140 conditioning). One patient had a high level of donor-specific anti-HLA antibodies and could not undergo another transplantation, and the other patient underwent a second allogeneic transplantation from another haploidentical donor; however, both patients died of treatment-related causes. Another patient who received FM100 conditioning for chronic lymphocytic leukemia developed secondary graft failure with autologous hematopoietic reconstitution, underwent a second transplantation, and remained alive and in remission 3 years after the second transplantation. All other evaluable patients achieved full (>95%) donor T-cell and myeloid chimerism at day +30.
In the 9/10 MUD arm, the cumulative incidence of neutrophil recovery at day +45 was 98% (95% CI, 46%–99%), and the median time to neutrophil recovery was 18 days (range, 13–34 days). The cumulative incidence of platelet recovery at day +100 was 80% (95% CI, 63%–90%), and the median time to platelet recovery was 28 days (range, 18–141 days). One patient developed primary graft failure and subsequently died. Three patients had delayed engraftment, with ANC recovery at days +30, +31, and +34. One patient who received FM140 conditioning for multiple myeloma had mixed chimerism at day +30, achieved full chimerism at day +60, and remained in complete remission at the date of the last follow-up. All other evaluable patients achieved full (>95%) donor T-cell and myeloid chimerism at day +30.
GVHD
In the HAPLO arm, the cumulative incidence of grade II–IV aGVHD was 28% at day +100 and 33% at 1 year after transplantation, whereas the cumulative incidence of severe aGVHD (grade III–IV) was 3% at day +100 and 5% at 1 year after transplantation. The cumulative incidence of cGVHD at 2 years after transplantation was 24% (Table 2, Fig. 1).
Figure 1.
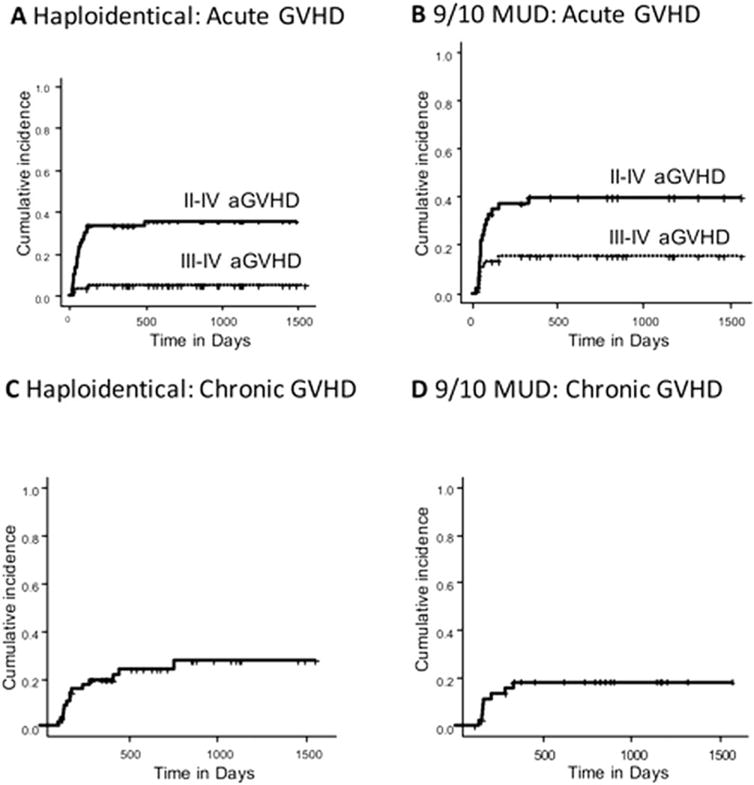
(A,B) The incidence of acute graft-versus-host disease (aGVHD) is illustrated in (A) the haploidentical donor arm and (B) the 1-antigen human leukocyte antigen-mismatched unrelated donor (9/10 MUD) arm. (C,D) The incidence of chronic GVHD is illustrated in (C) the haploidentical donor arm and (D) the 9/10 MUD arm.
In the 9/10 MUD arm, the cumulative incidence of grade II–IV aGVHD was 33% at day +100 and 40% at 1 year after transplantation, whereas the cumulative incidence of severe aGVHD (grade III–IV) was 13% at day +100 and 15% at 1 year after transplantation. The cumulative incidence of cGVHD at 2 years after transplantation was 19% (Table 2, Fig. 1).
NMR, Relapse, and Survival
In the HAPLO arm, the cumulative incidence of NRM at day +100 was 10%, and the 1-year cumulative incidence of NRM was 21%. The 1-year OS rate was 70%, and the 1-year PFS rate was 60%. The conditioning intensity (FM140 vs FM100) had no impact on the 1-year OS rate (FM140, 69%; FM100, 69%; P = .64) or the 1-year PFS rate (FM140, 64%; FM100, 53%; P = .8) (Table 2, Fig. 2). The 1-year cumulative incidence of disease relapse was 19%, with a 2-year cumulative incidence of relapse-related mortality of 23% (Table 2, Fig. 2).
Figure 2.
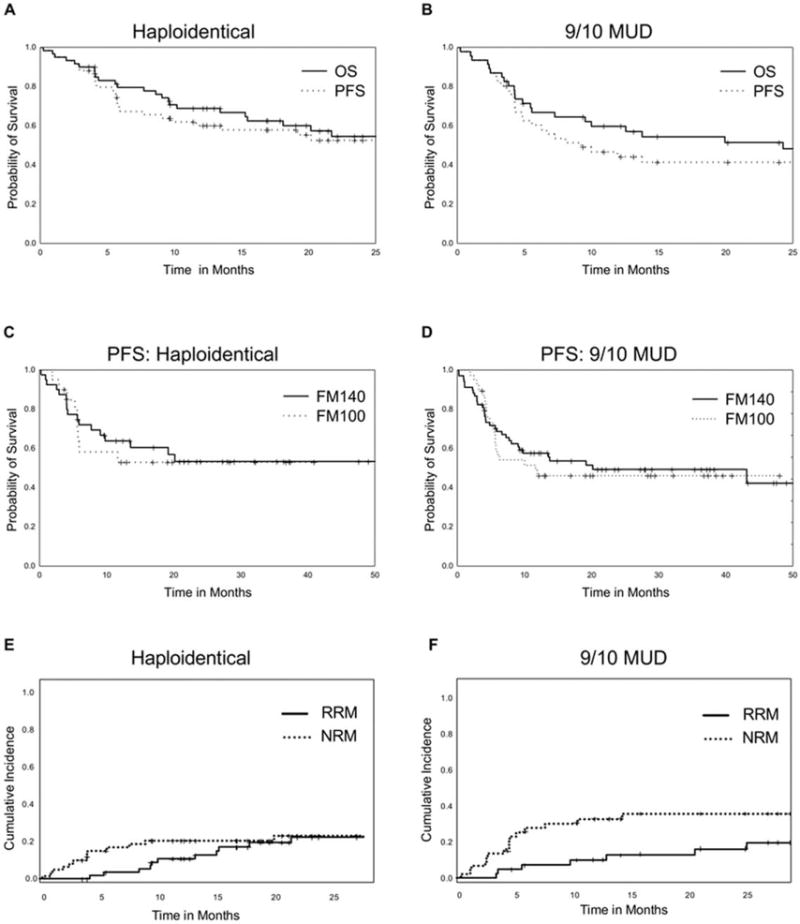
(A,B) Overall survival (OS) and progression-free survival (PFS) outcomes are illustrated in (A) the haploidentical donor arm and (B) the 1-antigen human leukocyte antigen-mismatched unrelated donor (9/10 MUD) arm. (C,D) PFS is illustrated according to conditioning intensity for those who received FM140 (1 intravenous dose of 140 mg/m2 melphalan on day −7, 1 intravenous dose of 5 mg/kg thiotepa on day −6, and 4 intravenous doses of 40 mg/m2 fludarabine [1 daily on days −5 through −2]) versus those who received FM100 (the same regimen with a lower melphalan dose of 100 mg/m2) in (C) the haploidentical donor arm and (D) the 9/10 MUD arm. (E,F) Relapse-related mortality (RRM) and nonrelapse-related mortality (NRM) are illustrated in (E) the haploidentical donor arm and (F) the 9/10 MUD arm.
In the 9/10 MUD arm, the cumulative incidence of NRM at day +100 was 13%, and the 1-year cumulative incidence of NRM was 31%. The 1-year OS rate was 60%, and the 1-year PFS rate was 47%. Like in the HAPLO arm, the conditioning intensity had no impact on the 1-year OS rate (FM140, 62%; FM100, 50%; P = .83) or the 1-year PFS rate (FM140, 49%; FM100, 39%; P = .9) (Table 2, Fig. 2). The 1-year cumulative incidence of disease relapse was 25%, with a 2-year cumulative incidence of relapse-related mortality of 14% (Table 2, Fig. 2).
Transplantation-Related Complications
Grade III–IV transplant-related toxicities are summarized in Table 3. The most common adverse events after transplantation in both arms were infectious complications. The cumulative incidence of cytomegalovirus (CMV) reactivation among patients at risk of CMV reactivation was similar in the 2 arms (HAPLO, 66%; 9/10 MUD, 67%). CMV organ disease occurred in 2 patients in the HAPLO arm (CMV colitis, CMV retinitis) and 5 patients in the 9/10 MUD arm (CMV colitis, n = 1; CMV pneumonitis, n = 4). Two patients in the HAPLO arm developed post-transplantation lymphoproliferative disease associated with Epstein-Barr virus reactivation. The causes of death are summarized in Table 2.
TABLE 3.
Severe Transplantation-Related Toxic Effects (From the Start of Conditioning Through Day 180)
| Toxic Effect | No. of Events (%)
|
|||
|---|---|---|---|---|
| HAPLO Arm, n = 60
|
9/10 MUD Arm, n = 46
|
|||
| Grade III | Grade IV | Grade III | Grade IV | |
| Bacterial infection | 37 (62) | 5 (8) | 26 (57) | 5 (11) |
| Viral infection | 18 (30) | 2 (3) | 9 (20) | 1 (2) |
| Fungal infection | 2 (3) | 2 (3) | 3 (7) | 0 (0) |
| Neutropenic fever | 29 (48) | 0 (0) | 13 (28) | 0 (0) |
| Hepatic effecta | 6 (10) | 0 (0) | 3 (7) | 3 (7) |
| Pulmonary effecta | 4 (7) | 0 (0) | 1 (2) | 1 (2) |
| Diffuse alveolar hemorrhage | 1 (2) | 0 (0) | 0 (0) | 0 (0) |
| Renal impairment | 2 (3) | 1 (2) | 5 (11) | 0 (0) |
| Mucositis | 3 (5) | 0 (0) | 2 (4) | 0 (0) |
| Nausea | 2 (3) | 0 (0) | 1 (2) | 0 (0) |
| Diarrhea | 3 (5) | 1 (2) | 9 (20) | 0 (0) |
| Skin rash | 1 (2) | 0 (0) | 1 (2) | 0 (0) |
| Neurologic effecta | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
| Headache | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Encephalopathy | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Thrombotic microangiopathy | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| Veno-occlusive disease | 0 (0) | 0 (0) | 1 (2) | 1 (2) |
| Cardiac dysfunction | 0 (0) | 0 (0) | 1 (2) | 1 (2) |
Abbreviations: 9/10 MUD, HLA-mismatched unrelated donor; HAPLO, haploidentical donor.
“Hepatic effect” indicates elevation of liver enzymes; “pulmonary effect” indicates hypoxia and/or dyspnea; and “neurologic effect” indicates cerebellar toxicity.
Immunologic Reconstitution
The median absolute numbers of lymphocyte subsets for total lymphocytes (CD3+), T cells (CD4+, CD8+), B cells (CD19+), natural killer cells (CD56+), T-regulatory cells (CD25+), naive T cells (CD45RA+), and memory T cells (CD45RO+) obtained during the first year post-transplantation are provided in Figure 3. In both arms, the median absolute lymphocyte count reached normal levels (≥1000 cells/μL) by day +180 after transplantation. A detailed analysis of the recovery of lymphocyte subsets in both arms is presented in the online Supporting Information.
Figure 3.
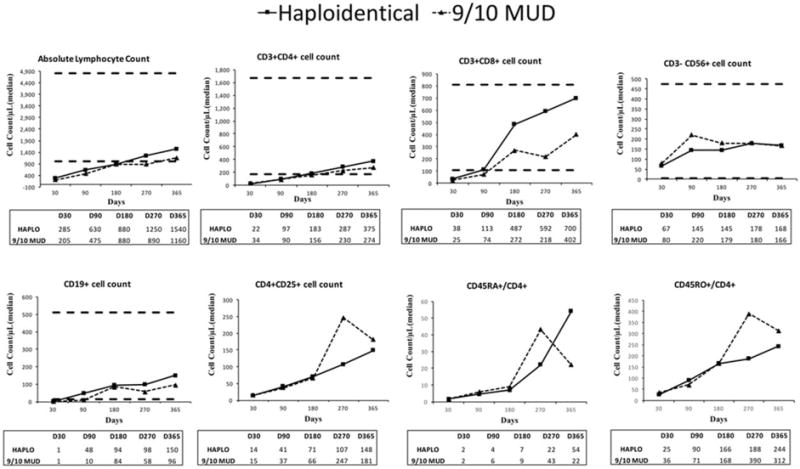
Charts illustrate immune reconstitution of lymphocyte subsets in the haploidentical donor (HAPLO) and 1-antigen human leukocyte antigen-mismatched unrelated donor (9/10 MUD) arms. Median absolute counts of T-lymphocyte subsets, B cells (cluster of differentiation 19-positive [CD19+]), natural killer cells (CD3−/CD56+), and T-regulatory cells (CD4+/CD25+) are shown for each donor type. Horizontal lines in the graphs indicate reference values, and tables below the graphs display median values at different time points. Reference values have not been established for CD4+/CD25+, CD45RA+/CD4+, or CD45RO+/CD4+ cells. D indicates day.
DISCUSSION
We investigated PTCy-based GVHD prophylaxis for haploidentical and 9/10 MUD transplantations using a melphalan-based, reduced-intensity conditioning regimen in a phase 2 clinical trial among patients who had advanced hematologic malignancies. With a 100-day NRM cumulative incidence of 10% in the HAPLO arm and 13% in the 9/10 MUD arm, the trial met its primary endpoint of an NRM rate <25%, establishing this regimen as a safe and feasible option for both donor sources.
Several alternative donor sources are currently available for patients who lack an HLA-matched donor, including haploidentical donors, cord blood, and HLA-mismatched unrelated donor grafts. The choice of stem cell source used in the absence of an HLA-matched donor is currently influenced largely by the individual institution’s experience and research priorities. Previously, 2 parallel, multicenter, nonrandomized phase 2 clinical trials conducted by the Blood and Marrow Transplant Clinical Trials Network reported outcomes of haploidentical and cord blood grafts using identical conditioning regimens.7 The same group is currently conducting a randomized study between these 2 donor sources. In that study, the NRM rate in the patients who underwent haploidentical transplantation was very low; however, the relapse rate appeared to be higher in the haploidentical group than in the cord blood graft group.7
It is important to note that, although our protocol was designed to evaluate this GVHD prophylaxis regimen with haploidentical and 9/10 MUD transplantations, the trial was nonrandomized and cannot serve as a head-to-head comparison of clinical outcomes between the 2 arms. Therefore, we did not assess statistical differences between the 2 groups. Overall, both arms had comparable clinical outcomes, with similar 2-year OS rates, although the NRM rate was somewhat higher in the 9/10 MUD arm (34% vs 23%). This higher NRM rate was driven by a higher proportion of patients experiencing grade II and IV aGVHD (40% vs 33%) and grade III and IV aGVHD (15% vs 5%). However, these results should be interpreted with caution, because the trial was not intended to compare both groups, and any differences in outcomes between the 2 groups may have occurred by chance.
The introduction of PTCy, which selectively eliminates alloreactive T cells, has significantly lowered the incidence GVHD in haploidentical transplantations.6,11–15 Similarly, in our study, the rate of grade II–IV aGVHD at 1 year was low in the HAPLO arm (33%) using a melphalan-based conditioning regimen. Our study builds on this concept and extends this GVHD prevention approach to mismatched MUD transplantations, which is a novel aspect of this trial. Some trials have investigated PTCy in HLA-matched related and unrelated transplantations with or without post-transplantation immune suppression.16–20 We observed a low grade of grade II–IV aGVHD at 100 days (33%) and cGVHD at 2 years (19%) in the 9/10 MUD arm, which establishes PTCy in combination with tacrolimus and MMF as an effective GVHD prevention strategy in patients who undergo mismatched MUD SCT. This compares favorably to other studies using PTCy without post-transplantation immune suppression in 10/10 HLA-matched unrelated transplantations, in which the incidence of grade II–IV GVHD at 100 days was 60% (95% CI, 45%–74%) and 22% (95% CI, 10%–34%), respectively, for cGVHD at 2 years.17 Overall, this suggests that post-transplantation immunosuppression is still important even after PTCy. However, we observed a seemingly higher rate of aGVHD in the 9/10 MUD arm compared with the HAPLO arm; however, this needs to be confirmed in a randomized trial, because it may have been the result of chance given the differences between both groups. For example, more patients in the 9/10 MUD arm received peripheral blood stem cells rather than bone marrow stem cells, which may have led to a seemingly higher incidence of aGVHD in the 9/10 MUD arm.21
Historically, unmanipulated T-cell–replete haploidentical transplantations with conventional GVHD prophylaxis were associated with high rates of aGVHD.22–24 This was overcome by ex vivo T-cell depletion, but immune recovery was significantly delayed, leading to high rates of infectious complications and NRM.25–27 In our study, we observed a more rapid recovery of all T-cell subsets in the first 6 months after transplantation in both arms using a T-cell–replete graft, which translated into an acceptable rate of infectious complications. This is attributable to PTCy, which selectively deletes only the alloreactive T cells, preserving nonalloreactive T cells, which likely helps augment immune recovery.
Disease relapse remains a significant cause of treatment failure. Prior studies using haploidentical transplantations with less intensive conditioning were associated with high rates of relapse, exceeding 60%.6 In the current trial and with the use of melphalan-based conditioning, we observed a relatively low incidence of relapse in both arms without excessive toxicity, considering that approximately one-third of patients with leukemia in both groups were beyond first or second complete remission and that 70% of patients in the HAPLO arm and 38% of those in the 9/10 MUD arm who had lymphoma were not in remission (20% and 15% of patients, respectively, were chemotherapy resistant at transplantation). At least in the HAPLO arm, the 1-year relapse rate was lower than in the Blood and Marrow Transplant Clinical Trials Network trial (19% vs 46%), in which a nonmyeloablative conditioning regimen was used.7
Another novel aspect of this trial was the prospective evaluation of a regimen in which a lower dose of melphalan (FM100) was used for older patients or for those who had comorbidities and no HLA-matched donors, a reduction in intensity from the original FM140 regimen.28 This lower dose was associated with low NRM and good disease control in the older population and did not produce significantly different outcomes compared with the FM140 conditioning dose intensity. Our group also reported favorable outcomes with lower melphalan doses in a previous study.29 With its lower incidence of aGVHD and NRM and its very good outcomes, this lower dose intensity could be a particularly good option for older patients or for those with comorbidities.29
In conclusion, this study establishes PTCy, tacrolimus, and MMF as an effective method for preventing GVHD in HLA-mismatched transplantation using both haploidentical and mismatched unrelated donor sources. The results also support melphalan-based conditioning as an effective regimen for a broad range of hematologic malignancies. Prospective randomized trials are required to compare the efficacy of alternative donor options for allogeneic hematopoietic transplantation in patients who lack an HLA-matched donor.
Acknowledgments
FUNDING SUPPORT
The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant P30CA016672 and used Cancer Center Support Grant shared resources.
Footnotes
Presented in abstract form at the 57th American Society of Hematology Annual Meeting; December 5–8, 2015; Orlando, Florida.
We are indebted to Sarah Bronson for excellent proofreading.
Additional supporting information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
Dean Lee reports licensing fees from Ziopharm Oncology and Intrexon Corporation; personal fees from Ziopharm Oncology, Courier Therapeutics, Intellia Therapeutics, Shire, and Sanofi Oncology; and holds ownership and is a board member of Therapeutics, all outside the submitted work. Qaiser Bashir reports research funding from Celgene and Takeda and serves on the Takeda and Spectrum Pharmaceuticals advisory boards, all outside the submitted work. Chitra Hosing reports a grant for clinical trials from Celgene, Inc; and personal fees from Sanofi, Aviara Pharmaceuticals, Seattle Genetics, and Cardinal Health, all outside the submitted work.
AUTHOR CONTRIBUTIONS
Sameh Gaballa: Data preparation and analysis, statistical analysis, and writing–first draft. Isabell Ge, Riad El Fakih, Jonathan E. Brammer, Piyanuch Kongtim, Ciprian Tomuleasa, Sa A. Wang, Dean Lee, Demetrios Petropoulos, Kai Cao, Aimee Hammerstrom, Lindsey Lombardi, Gheath Alatrash, Martin Korbling, Betul Oran, Partow Kebriaei, Sairah Ahmed, Nina Shah, Katayoun Rezvani, David Marin, Qaiser Bashir, Amin Alousi, Yago Nieto, Muzaffar Qazilbash, Chitra Hosing, Uday Popat, Elizabeth J. Shpall, Issa Khouri: Data review and interpretation and writing-editing final draft, Gabriela Rondon, and Julianne Chen: Data preparation. Richard E. Champlin and Stefan O. Ciurea: Study design, data review and interpretation, and writing–editing final draft. All authors approved the final manuscript.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Allan DS, Takach S, Smith S, Goldman M. Impact of declining fertility rates in Canada on donor options in blood and marrow transplantation. Biol Blood Marrow Transplant. 2009;15:1634–1637. doi: 10.1016/j.bbmt.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum FR. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia when a matched related donor is not available. Hematology Am Soc Hematol Educ Program. 2008:412–417. doi: 10.1182/asheducation-2008.1.412. [DOI] [PubMed] [Google Scholar]
- 4.Saber W, Opie S, Rizzo JD, Zhang MJ, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012;119:3908–3916. doi: 10.1182/blood-2011-09-381699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the US registry. N Engl J Med. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciurea SO, Saliba R, Rondon G, et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant. 2010;45:429–436. doi: 10.1038/bmt.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciurea SO, Thall PF, Milton DR, et al. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:1392–1398. doi: 10.1016/j.bbmt.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciurea SO, Champlin RE. Donor selection in T cell-replete haploidentical hematopoietic stem cell transplantation: knowns, unknowns, and controversies. Biol Blood Marrow Transplant. 2013;19:180–184. doi: 10.1016/j.bbmt.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj K, Pagliuca A, Bradstock K, et al. Peripheral blood hematopoietic stem cells for transplantation of hematological diseases from related, haploidentical donors after reduced-intensity conditioning. Biol Blood Marrow Transplant. 2014;20:890–895. doi: 10.1016/j.bbmt.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosso D, Gaballa S, Alpdogan O, et al. A 2-step approach to myeloablative haploidentical transplantation: low nonrelapse mortality and high survival confirmed in patients with earlier stage disease. Biol Blood Marrow Transplant. 2015;21:646–652. doi: 10.1016/j.bbmt.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Bashey A. Peripheral blood stem cells for T cell-replete nonmyeloablative hematopoietic transplants using post-transplant cyclophosphamide. Biol Blood Marrow Transplant. 2014;20:598–599. doi: 10.1016/j.bbmt.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Alousi AM, Brammer JE, Saliba RM, et al. Phase II trial of graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide after reduced-intensity busulfan/fludarabine conditioning for hematological malignancies. Biol Blood Marrow Transplant. 2015;21:906–912. doi: 10.1016/j.bbmt.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Stasi A, Milton DR, Poon LM, et al. Similar transplantation outcomes for acute myeloid leukemia and myelodysplastic syndrome patients with haploidentical versus 10/10 human leukocyte antigen-matched unrelated and related donors. Biol Blood Marrow Transplant. 2014;20:1975–1981. doi: 10.1016/j.bbmt.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanakry CG, O’Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32:3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alousi AM, Brammer JE, Saliba RM, et al. Phase II trial of graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide after reduced-intensity busulfan/fludarabine conditioning for hematological malignancies. Biol Blood Marrow Transplant. 2015;21:906–912. doi: 10.1016/j.bbmt.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanakry CG, Tsai HL, Bolanos-Meade J, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124:3817–3827. doi: 10.1182/blood-2014-07-587477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mielcarek M, Furlong T, O’Donnell PV, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–1508. doi: 10.1182/blood-2015-10-672071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313:765–771. doi: 10.1056/NEJM198509263131301. [DOI] [PubMed] [Google Scholar]
- 23.Clift RA, Hansen JA, Thomas ED, et al. Marrow transplantation from donors other than HLA-identical siblings. Transplantation. 1979;28:235–242. doi: 10.1097/00007890-197909000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Powles RL, Morgenstern GR, Kay HE, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983;1:612–615. doi: 10.1016/s0140-6736(83)91793-2. [DOI] [PubMed] [Google Scholar]
- 25.Ball LM, Lankester AC, Bredius RG, Fibbe WE, van Tol MJ, Egeler RM. Graft dysfunction and delayed immune reconstitution following haploidentical peripheral blood hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35(suppl 1):S35–S38. doi: 10.1038/sj.bmt.1704842. [DOI] [PubMed] [Google Scholar]
- 26.Rizzieri DA, Koh LP, Long GD, et al. Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol. 2007;25:690–697. doi: 10.1200/JCO.2006.07.0953. [DOI] [PubMed] [Google Scholar]
- 27.O’Reilly RJ, Keever C, Kernan NA, et al. HLA nonidentical T cell depleted marrow transplants: a comparison of results in patients treated for leukemia and severe combined immunodeficiency disease. Transplant Proc. 1987;19:55–60. [PubMed] [Google Scholar]
- 28.Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 29.Gaballa S, Kongtim P, Rondon G, et al. Feasibility and outcomes of haploidentical transplantation for elderly patients with advanced hematological malignancies: the MD Anderson Cancer Center experience [abstract] Blood. 2014;124:1245. [Google Scholar]
- 30.Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120:905–913. doi: 10.1182/blood-2012-03-418202. [DOI] [PMC free article] [PubMed] [Google Scholar]


