Summary
Post-transplantation cyclophosphamide (PTCy) is an effective strategy to prevent graft-versus-host disease (GVHD) after haploidentical haematopoietic cell transplantation (HCT). We determined the efficacy of PTCy-based GVHD prophylaxis in human leucocyte antigen (HLA)-mismatched unrelated donor (MMUD) HCT. We analysed 113 adult patients with high-risk haematological malignancies who underwent one-antigen MMUD transplantation between 2009 and 2013. Of these, 41 patients received PTCy, tacrolimus and mycophenolate mofetil (MMF) for GVHD prophylaxis; 72 patients received conventional prophylaxis with anti-thymocyte globulin, tacrolimus and methotrexate. Graft source was primarily bone marrow (83% PTCy vs. 63% conventional group). Incidence of grade II–IV (37% vs. 36%, P = 0.8) and grade III–IV (17% vs. 12%, P = 0.5) acute GVHD was similar at day 100. However, the incidence of grade II–IV acute GVHD by day 30 was significantly lower in the PTCy group (0% vs. 15%, P = 0.01). Median time to neutrophil (18 days vs. 12 days, P < 0.001) and platelet (25.5 days vs. 18 days, P = 0.05) engraftment was prolonged in PTCy group. Rates of graft failure, chronic GVHD, 2-year non-relapse mortality, relapse, progression-free survival or overall survival were similar. Our results demonstrate that PTCy, tacrolimus and MMF for GVHD prophylaxis is safe and produced similar results as conventional prophylaxis in patients with one antigen HLA-MMUD HCT.
Keywords: HLA-mismatched transplantation, post transplantation cyclophosphamide, MMUD, unrelated donor, GVHD
Despite the availability of more than 10 million potential haematopoietic cell transplantation (HCT) donors in the National Marrow Donor Program registry (NMDP; https://bethematch.org/), the probability of finding a suitable human leucocyte antigen (HLA)-matched donor for HCT varies considerably, from 75% in Caucasians to 16% among other races (Gragert et al, 2014). One of the alternative options in such cases is the use of HLA-mismatched unrelated donor (MMUD) HCT, but at the expense of increased risk of graft-versus-host disease (GVHD) and non-relapse mortality (NRM) with reduced progression-free survival (PFS) and overall survival (OS) compared to HLA-matched HCT (Sasazuki et al, 1998; Flomenberg et al, 2004; Lee et al, 2007; Woolfrey et al, 2011; Saber et al, 2012).
The standard pharmacological GVHD prophylaxis regimen for HLA-matched unrelated (MUD) or related donor (MRD) HCT includes a calcineurin inhibitor (commonly tacrolimus or ciclosporin) and methotrexate (Nash et al, 2000; Hiraoka et al, 2001; Perkins et al, 2010; Saber et al, 2012). This is often intensified with in vivo T-cell depletion (TCD), generally with antithymocyte globulin (ATG) or alemtuzumab in MMUD HCT (Finke et al, 2003; Ayuk et al, 2008; Devillier et al, 2014; Fuji et al, 2015). With this intensive regimen, the incidence of grade II–IV acute GVHD (20–35%), grade III–IV acute GVHD (4–20%) and chronic GVHD (22–67%) in MMUD HCT approaches comparable levels to those seen after MUD HCT (Finke et al, 2003; Ayuk et al, 2008; Kim et al, 2009; Devillier et al, 2014; Fuji et al, 2015). However, in vivo TCD delays T-cell immune reconstitution (Small et al, 1997; Duval et al, 2002; Bosch et al, 2012) and poses heightened risk of bacterial and viral infections, including herpes simplex virus, cytomegalovirus (CMV), Epstein–Barr virus, and infection-related deaths (Bacigalupo et al, 2001), as well as fatal post-transplant lymphoproliferative disorder (PTLD) (Small et al, 1997; van Esser et al, 2001; Finke et al, 2009). Alternative improved GVHD prophylaxis regimens are needed. One potential method is the use of high dose post-transplantation cyclophosphamide (PTCy) given on days +3 and +4, which induces transplantation tolerance by inhibiting rapidly proliferating ‘alloreactive’ T-cells (Luznik et al, 2012), thereby reducing the risk of GVHD. Several studies reported encouraging outcomes with PTCy in haploidentical HCT in combination with tacrolimus and MMF (O’Donnell et al, 2002; Luznik et al, 2008), and a number of studies demonstrated its efficacy as the sole GVHD prevention method after myeloablative conditioning in 10/10-MUD and MRD HCT (Luznik et al, 2010; Kanakry et al, 2014). Its safety and efficacy in MMUD setting is undefined.
The aim of the present retrospective study was to compare the incidence of acute or chronic GVHD in patients who received PTCy in combination with tacrolimus and mycofenolate mofetil (MMF) as a GVHD prophylaxis regimen versus those who received standard GVHD prophylaxis using in vivo TCD, tacrolimus and methotrexate after one-antigen HLA-MMUD (9/10 or 7/8 HLA-matched) HCT.
Methods
Study protocol and objectives
A phase-II three arm clinical trial was initiated at the M.D. Anderson Cancer Center in 2009 to assess the safety and efficacy of PTCy after T-cell replete haploidentical, MMUD/MMRD or MUD HCT (protocol 2009-0266, ClinicalTrials.gov Identifier: NCT01010217). The present study is focused on the outcomes of a subset of those patients who underwent one-antigen MMUD HCT. The primary objective of the present study was to compare the incidence of acute or chronic GVHD in these patients to the rates in a separate contemporaneous cohort of patients who received conventional GVHD prophylaxis at our institution.
Patient population
We included all consecutive adult patients with haematological malignancies who received 9/10 HLA-MUD HCT at our institution between 2009 and 2013 after myeloablative or reduced-intensity conditioning regimen (n = 113). Of these, 41 patients received PTCy as a part of GVHD prophylaxis (study group) and 72 patients received conventional GVHD prophylaxis (control group). Participation in the clinical trial was based on preferences of patients and their treating physician and was also contingent on insurance approval. Among the study group, the majority of patients (n = 36/41, 88%) were enrolled in the above mentioned clinical trial (ClinicalTrials.gov Identifier: NCT01010217), whereas five patients (12%) did not qualify for the phase II clinical trial due to insurance reasons, but received the PTCy-based GVHD prophylaxis regimen off-study and were included in the current retrospective analysis. Out of 113 patients, 29 had HLA-DQB1 mismatches. As isolated donor-recipient mismatch at HLA-DQ does not affect survival (Flomenberg et al, 2004), we performed a separate analysis of 84 patients who underwent 7/8 HLA-MUD HCT. Out of these, 46 patients received conventional GVHD prophylaxis and 38 patients received PTCy-based prophylaxis. All patients gave signed informed consent according to the Declaration of Helsinki and an Institutional Review Board – approved protocol was obtained for this retrospective study.
Transplantation procedure
Patients in the PTCy group received a conditioning regimen of fludarabine and melphalan with either thiotepa or 200 cGy of total body irradiation (TBI). Melphalan [140 mg/m2 intravenous (IV) with myeloablative regimen or 100 mg/m2 with reduced intensity regimen] was given on day −8, followed by fludarabine 40 mg/m2 IV for 4 days (day −6 to −3). In addition, patients either received thiotepa 5 mg/kg IV on day −7 or TBI 200 cGy on day −1. Reduced doses of melphalan were used for older patients (aged above 55 years) or those with significant comorbidities. Cyclophosphamide 50 mg/kg/day IV was administered on days +3 and +4. Additional GVHD prophylaxis in this arm was provided with tacrolimus and MMF, as previously reported by us (Ciurea et al, 2012).
Patients in the conventional GVHD prophylaxis group received various conditioning regimens, such as those based on busulfan/fludarabine (Bu/Flu; 37.5%), fludarabine/melphalan (22.2%), fludarabine/cyclophosphamide (18.1%), and others. Prophylaxis against GVHD was provided with tacrolimus (dose and schedule as above) and methotrexate 5 mg/m2 IV on days +1, +3, +6 and +11. Almost all patients received in vivo TCD (97.2%) using rabbit ATG (n = 68/72) or alemtuzumab (n = 2/72).
Granulocyte colony-stimulating factor (Filgrastim) 5 μg/kg was administered subcutaneously daily starting day +7 until absolute neutrophil count (ANC) was >1.0 × 109/l.
Statistical analysis
Definitions and assessments
High resolution HLA typing was performed for all donor-recipient pairs matching for HLA-A, -B, -C, -DRB1 and –DQB1. Any single antigen or allele mismatch at these loci was defined as “9/10 match,” while single antigen or allele mismatch at HLA-A, -B, -C, or -DRB1 was defined as ‘7/8 match’. The time to neutrophil engraftment was defined as the first of three consecutive days after HCT with an ANC ≥0.5 × 109/l, and the time to platelet engraftment as the first of seven consecutive days with a platelet count ≥20 × 109/l without platelet transfusion. Primary graft failure was defined as the failure to attain an ANC >0.5 × 109/l by day +28 that was maintained for three consecutive measurements, with no evidence of donor-derived cells by bone marrow chimerism studies and no evidence of persistent or relapsing disease. Secondary graft failure was defined as a decline in ANC to <0.5 × 109/l for three consecutive days after initial engraftment. Diagnosis and grading of acute and chronic GVHD was defined based on standard criteria (Glucksberg et al, 1974; Shulman et al, 1980; Przepiorka et al, 1995). Chimerism analysis was performed on days 30 and 100 after transplantation and every 3 months thereafter, using a polymerase chain reaction with primer sets flanking microsatellite repeats. Complete donor chimerism was defined as the detection of >95% donor DNA in a sample.
Endpoints
The primary outcome of interest was incidence of acute or chronic GVHD. We also assessed GVHD occurring within 20 and 30 days of HCT (‘early acute’ GVHD). Secondary outcomes included rates of graft failure, time to neutrophil and platelet engraftments, attainment of donor chimerism, NRM, PFS and OS, and causes of deaths. NRM was defined as death without evidence of disease persistence or recurrence. PFS was defined as the time from HCT to either death or relapse. OS was defined as the time from HCT to death from any cause.
Statistical procedure
Baseline patient characteristics were compared between the groups using the Wilcoxon rank-sum test for continuous variables and Chi-square test or Fisher’s exact test for dichotomous variables. The cumulative incidence of acute GVHD, chronic GVHD, NRM and disease progression were estimated accounting for competing risks. Disease progression or death before GVHD were considered competing risks in the estimation of GVHD incidence. Death with persistent disease or disease progression were competing risks in the estimation of the rate of NRM, and NRM was a competing risk in the estimation of disease progression. Actuarial probabilities of PFS and OS were estimated using the Kaplan–Meier estimator. Cox proportional hazards regression analysis and log rank test were used to compare outcomes between the PTCy and conventional GVHD prophylaxis groups. The proportionality of the hazards assumption was tested statistically in assessment of the rate of GVHD between the two groups and was found to be met. Predictors of grade II–IV acute GVHD were assessed using Cox proportional hazards analysis. Predictors considered included gender, age, graft source, disease type, disease risk index, conditioning regimens, time between diagnosis and transplant, number of prior chemotherapy regimens, number of prior autologous transplants and year of transplantation. Due to significant differences in the graft source (peripheral blood (PB) or bone marrow (BM)) between the conventional group and the PTCy group, a planned subgroup analysis was performed that was restricted to patients that received only BM grafts. All analyses were performed using STATA 12 [StataCorp LP, College Station, TX, USA and statistical significance was defined at the 0.05 level.
Results
Outcomes of patients with 9/10 HLA-MUD HCT
Patients
A total of 113 consecutive adult patients met the retrospective study inclusion criteria. The PTCy group (n = 41) received GVHD prophylaxis with PTCy, tacrolimus and MMF. This group was compared to the conventional GVHD prophylaxis group (n = 72) that received in vivo TCD (98%) with tacrolimus and methotrexate (94.4%) (Table I). Patient and transplant characteristics were comparable between these groups with the exception of age at transplantation, stem cell source and donor-recipient HLA class mismatch. Patients in the conventional group were marginally older (median age 54 years; range 19–74) than those in the PTCy group (median age 50 years; range 20–64, P = 0.05). Also, PB was used more frequently as a graft source in the conventional group (38% vs. 17%, P = 0.02). Approximately 88% of patients in the PTCy group had HLA class-I donor-recipient mismatch, compared with about 57% in the control group (P = 0.001). Half of the patients in the conventional group and 56% in the PTCy group received myeloablative conditioning regimens. There were no other differences between the groups, including CD34+ and CD3+ cell dose, donor-recipient gender match, donor-recipient CMV serostatus, disease type, disease risk index and prior treatments. The median follow-up in surviving patients was 24 (range 3–49) months in the conventional group and 20 (range 4–43) months in the PTCy group.
Table I.
Baseline patient characteristics.
| Variable | Conventional GVHD prophylaxis (n = 72) | Post-transplantation cyclophosphamide (n = 41) | P-value |
|---|---|---|---|
| Age, years; median (range) | 54 (19–74) | 50 (20–64) | 0.05 |
| Diagnosis, n (%) | |||
| AML/MDS | 30 (42%) | 15 (37%) | |
| ALL | 4 (6%) | 7 (17%) | |
| CLL | 10 (14%) | 3 (7%) | |
| CML/MPD | 8 (11%) | 2 (5%) | |
| Non-Hodgkin lymphoma | 14 (19%) | 8 (20%) | |
| Hodgkin lymphoma | 4 (6%) | 1 (2%) | |
| Aplastic Anaemia | 2 (3%) | 4 (10%) | |
| MM | 0 (0%) | 1 (2%) | |
| Lymphoid malignancies | 32 (44%) | 20 (49%) | 0.7 |
| Myeloid malignancies | 40 (56%) | 21 (51%) | |
| Disease risk index, n (%) | |||
| Very High | 9 (13%) | 6 (15%) | 0.4 |
| High | 19 (26%) | 13 (32%) | |
| Intermediate | 26 (36%) | 7 (17%) | |
| Low | 16 (22%) | 11 (27%) | |
| Missing | 2 (3%) | 4 (10%) | |
| Median (range) time to HCT from diagnosis, months | 27 (5–319) | 15 (3–162) | 0.3 |
| Donor/Recipient gender, n (%) | |||
| Female/Female | 9 (14%) | 13 (32%) | 0.3 |
| Female/Male | 18 (25%) | 7 (17%) | |
| Male/Female | 20 (28%) | 8 (20%) | |
| Male/Male | 25 (35%) | 13 (32%) | |
| Donor/Recipient CMV, n (%) | |||
| Non-reactive/Non-reactive | 5 (7%) | 2 (5%) | 0.5 |
| Reactive/Reactive | 26 (36%) | 14 (34%) | |
| Non-reactive/Reactive | 36 (50%) | 22 (54%) | |
| Reactive/Non-reactive | 5 (7%) | 3 (7%) | |
| Conditioning regimens, n (%) | |||
| Myeloablative | 36 (50%) | 23 (56%) | 0.5 |
| Reduced-intensity | 36 (50%) | 18 (44%) | |
| HLA mismatches | |||
| HLA class-I mismatch | 41 (57%) | 36 (88%) | <0.001 |
| HLA class-II mismatch | 31 (43%) | 5 (12%) | |
| Graft source, n (%) | |||
| Bone marrow | 45 (63%) | 34 (83%) | 0.02 |
| Peripheral blood | 27 (38%) | 7 (17%) | |
| Cell dose (bone marrow), median (range) | |||
| CD34+ (× 106/kg) | 2.4 (0.9–8.5) | 2.2 (0.7–7.63) | 0.3 |
| CD3+ (× 105/kg) | 17 (2.6–39) | 16 (0.9–47) | 0.2 |
| Cell dose (peripheral blood), median (range) | |||
| CD34+ (× 106/kg) | 6.44 (2.45–16) | 13.12 (3.5–41) | 0.06 |
| CD3+ (× 105/kg) | 154 (27–505) | 284 (86–381) | 0.2 |
| Median (range) number of prior chemotherapies | 2 (0–10) | 2 (0–7) | 0.4 |
| Prior autologous HCT, n (%) | |||
| None | 65 (90%) | 36 (88%) | |
| One | 7 (10%) | 4 (10%) | |
| Two | 0 (0%) | 1 (2%) | |
| Year of HCT, n (%) | |||
| 2009 | 18 (25%) | 1 (2%) | |
| 2010 | 21 (29%) | 9 (22%) | |
| 2011 | 18 (25%) | 13 (32%) | |
| 2012 | 10 (14%) | 14 (34%) | |
| 2013 | 5 (7%) | 4 (10%) | |
| Median (range) follow-up, in months | 24 (3–49) | 20 (4–43) |
AML, acute myeloid leukaemia; ALL, acute lymphoblastic leukaemia; CML, chronic myeloid leukaemia; CLL chronic lymphocytic leukaemia; CMV, Cytomegalovirus; GVHD, graft-versus-host disease; HLA, Human Leucocyte Antigen; HCT, haematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; MM, multiple myeloma; MPD, myeloproliferative disorder.
Acute and chronic GVHD
The overall cumulative incidences of grade II–IV (37% vs. 36%) or grade III–IV (17% vs. 12%) acute GVHD at day 100 did not differ between the PTCy and the conventional groups, respectively (Fig 1). However, the cumulative incidence of grade II–IV acute GVHD by day 20 was 8% in the conventional arm compared with 0% in the PTCy arm (P = 0.075). The corresponding numbers by day 30 were 15% and 0%, respectively, P = 0.01. Consistent with these data, the incidence of grade III–IV acute GVHD by day 30 was 8% in the conventional group and 0% in the PTCy group (P = 0.08). On the other hand, the cumulative incidence of chronic GVHD was similar between the two groups at 6 months (20% vs. 15%), at 1 year (30% vs. 31%) or 2 years (30% vs. 42%) post-transplant (Table II). Risk factors analysis showed that the use of PTCy was the sole independent predictor of lower risk of grade II–IV acute GVHD by day 30 (P = 0.01). None of the risk factors evaluated, including PTCy use, were shown to predict the rate of grade II–IV acute GVHD within day 100 post-transplant (Table III).
Fig 1.
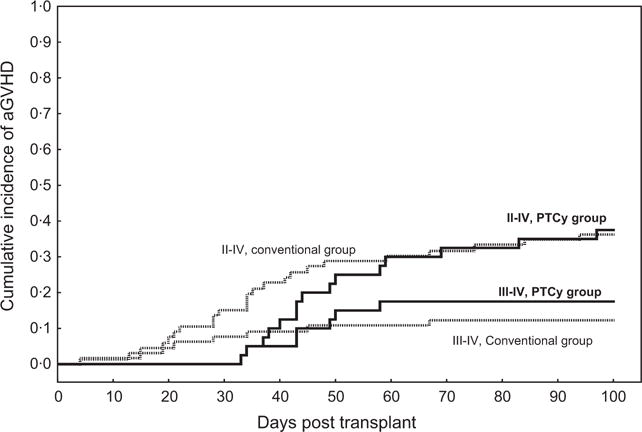
Cumulative incidence of grade II–IV and III–IV acute graft-versus-host disease (aGVHD) by day 100 in the post-transplant cyclophosphamide (PTCy) group (solid line) compared with the conventional group (dotted line), all patients.
Table II.
Acute and chronic GVHD.
| Variable | All patients
|
|||
|---|---|---|---|---|
| Cumulative incidence (95% CI)
|
||||
| Conventional GVHD prophylaxis (n = 65)` | PTCy (n = 40) | HR; 95% CI | P-value | |
| Acute GVHD, day +30 | ||||
| Grade II–IV | 15% (9–27%) | 0% | NE | 0.01 |
| Grade III–IV | 8% (3–18%) | 0% | NE | 0.08 |
| Acute GVHD, day +100 | ||||
| Grade II–IV | 36% (26–50) | 37% (25–56) | 0.9 (0.5–1.8) | 0.8 |
| Grade III–IV | 12% (6–23) | 17% (9–34) | 1.4 (0.5–3.9) | 0.5 |
| Chronic GVHD | ||||
| 6 months | 15% (8–31) | 20% (9–44) | 1 (0.6–1.7) | 0.9 |
| 1 year | 31% (19–50) | 30% (16–57) | 0.95 (0.6–1.5) | 0.8 |
| 2 years | 42% (27–68) | 30% (16–57) | 0.9 (0.5–1.4) | 0.6 |
| Variable | Bone marrow grafts only
|
7/8-HLA matched patients (n = 84)
|
||
|---|---|---|---|---|
| PTCy (n = 34) vs. Conventional GVHD prophylaxis (n = 45)
|
PTCy (n = 38) vs. Conventional GVHD prophylaxis (n = 46)*
|
|||
| HR; 95% CI | P-value | HR; 95% CI | P-value | |
| Acute GVHD, day +30 | ||||
| Grade II–IV | NE | 0.03 | NE | 0.005 |
| Grade III–IV | NE | 0.2 | – | – |
| Acute GVHD, day +100 | ||||
| Grade II–IV | 0.9 (0.4–1.8) | 0.8 | 1 (0.5–2.1) | 0.9 |
| Grade III–IV | 1.9 (0.5–6.7) | 0.3 | 1.1 (0.3–3.3) | 0.9 |
| Chronic GVHD | ||||
| 6 months | 1.2 (0.3–4.6) | 0.8 | 0.8 (0.2–2.9) | 0.7 |
| 1 year | 0.8 (0.3–2.6) | 0.8 | 0.8 (0.3–2.2) | 0.6 |
| 2 years | 0.6 (0.2–1.9) | 0.4 | 0.7 (0.2–1.9) | 0.5 |
CI, Confidence Interval; HR, Hazard Ratio; GVHD, graft-versus-host disease; PTCy, Post-transplant cyclophosphamide; HLA, Human Leucocyte Antigen; NE, not evaluable.
Acute GVHD data missing for one engrafted patient in the conventional GVHD prophylaxis group.
Table III.
Univariate analysis of acute and chronic GVHD at day 30 and day 100 (all patients).
| N | Acute GVHD II–IV, day30
|
Acute GVHD II–IV, day 100
|
|||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Group | |||||||
| PTCy group | 41 | NE | 0.01 | 0.97 | 0.5–1.8 | 0.9 | |
| Conventional group | 72 | Ref. | |||||
| Gender mismatch | |||||||
| Female-Male | 22 | 1.7 | 0.4–6.3 | 0.45 | 1.3 | 0.6–2.6 | 0.5 |
| Others | 84 | Ref. | Ref. | ||||
| Age, years | |||||||
| ≤40 | 26 | Ref. | Ref. | ||||
| 41–50 | 19 | 1.4 | 0.2–10.2 | 0.7 | 1.3 | 0.5–3.2 | 0.5 |
| 51–55 | 17 | 0.7 | 0.1–8.1 | 0.8 | 0.7 | 0.2–2.2 | 0.6 |
| 56–60 | 17 | 1.5 | 0.2–10 | 0.7 | 1.5 | 0.6–3.7 | 0.3 |
| >60 | 27 | 1.4 | 0.2–8.4 | 0.7 | 0.7 | 0.3–1.8 | 0.5 |
| Graft source | |||||||
| Bone marrow | 74 | Ref. | Ref. | ||||
| Peripheral blood | 32 | 1.5 | 0.4–5.4 | 0.5 | 0.7 | 0.3–1.5 | 0.4 |
| Diagnosis | |||||||
| Lymphoid malignancies | 49 | 1.8 | 0.5–6.3 | 0.4 | 1.4 | 0.8–2.6 | 0.3 |
| Myeloid malignancies | 57 | Ref. | Ref. | ||||
| Disease Risk Index | |||||||
| Very High | 14 | Ref. | Ref. | ||||
| High | 31 | 0.5 | 0.1–3.2 | 0.4 | 1.3 | 0.5–3.6 | 0.6 |
| Intermediate | 30 | 0.7 | 0.1–4.1 | 0.7 | 1.2 | 0.4–3.3 | 0.8 |
| Low | 27 | 0.8 | 0.1–4.4 | 0.8 | 0.9 | 0.3–2.7 | 0.8 |
| Missing | 4 | NE | 0.7 | 0.1–6.4 | 0.7 | ||
| Conditioning regimen | |||||||
| Myeloablative | 55 | Ref. | Ref. | ||||
| Reduced-intensity | 51 | 1.6 | 0.5–5.8 | 0.4 | 1.2 | 0.6–2.1 | 0.6 |
| Year of transplantation | |||||||
| 2009 | 16 | Ref. | Ref. | ||||
| 2010 | 29 | 0.3 | 0.06–2.0 | 0.2 | 0.7 | 0.2–2.0 | 0.5 |
| 2011 | 30 | 0.7 | 0.1–2.9 | 0.6 | 1.05 | 0.4–2.9 | 0.9 |
| 2012 | 23 | NE | 0.9 | 0.3–2.7 | 0.9 | ||
| 2013 | 8 | 0.6 | 0.1–5.9 | 0.7 | 2 | 0.6–7.1 | 0.3 |
| 2009 vs. >2009 | 2.6 | 0.7–10 | 0.2 | ||||
CI, Confidence Interval; HR, Hazard Ratio; NE, Not evaluable; PTCy, Post-transplant cyclophosphamide, GVHD, graft-versus-host disease; Ref., reference.
Because of differential use of BM or PB as a graft source between the groups, a subgroup analysis was performed including only those patients who received BM grafts. Again, the rate of grade II–IV acute GVHD by day 30 was significantly lower in the PTCy group; six patients in the conventional group (n = 45) experienced GVHD within 30 days of transplantation compared with none in the PTCy group (n = 34), P = 0.03. Yet again, there were no significant differences in the rate of grade III–IV acute GVHD by day 30, grades II–IV or grade III–IV acute GVHD by day 100, and chronic GVHD at 0.5, 1 and 2 years (Table II).
As described in Table I, the PTCy group included significantly more patients with HLA class-I mismatches (87.8%) compared with the conventional group (56.9%). Therefore, a separate analysis was performed to determine the effect of GVHD prophylaxis regimens based on HLA class mismatch. In patients with HLA class-I mismatch, PTCy was associated with significantly reduced risk of grade II–IV, but not grade III–IV, acute GVHD by day 30 (P = 0.01). However, there were no significant differences in acute grade II–IV GVHD [Hazards Ratio (HR) 1.1, 95% confidence interval (CI) 0.5–2.5, P = 0.7] or acute grade III–IV GVHD (HR 1.5, 95% CI 0.4–5.4, P = 0.5) by day 100 between PTCy and the conventional groups. It is noteworthy that only five patients in the PTCy group had HLA class-II mismatch. With the confinements of small subgroups of patients, we did not find any difference in the incidence of grade II–IV (P = 0.4) or grade III–IV (P = 0.5) acute GVHD by day 30, grade II–IV (HR 0.4, 95% CI 0.05–3.1, P = 0.4) or grade III–IV (HR 1.4, 95% CI 0.2–13, P = 0.8) acute GVHD by day 100, chronic GVHD at 1-year between the PTCy and the conventional prophylaxis group in patients with HLA class-I mismatch (HR 0.8, P = 0.7) or HLA class-II mismatch (HR 0.9, P = 0.9).
Engraftment
The risk of graft failures did not differ between the PTCy group (primary 2%, secondary 2.4%) and the conventional group (primary 8%, secondary 4%). Two patients in the PTCy group and three patients in the conventional group experienced early death before engraftment could be assessed. The time to neutrophil engraftment was significantly faster in the conventional group (median 12 days; range 8–29 days) compared with the PTCy group (median 18 days; range 13–34 days), P < 0.001. Delayed neutrophil engraftment after PTCy was more pronounced in patients who received BM grafts [median 19 days (range 14–34) vs. 12 days (range 9–25), P < 0.001]. In patients with PB grafts, the median time to neutrophil engraftment was 14 days (range 13–17) in the PTCy group compared with 12 days (range 8–25) in the conventional group, P = 0.01.
Similarly, platelet engraftment was more rapid in the conventional group compared with the PTCy group (median 18 days (range 9–125) vs. 25.5 days (range 11–141), P = 0.05). Delayed platelet engraftment with PTCy was observed only in patients who received BM grafts [median 28 days (range 13–141) vs. 19 days (range 12–125), P = 0.045], but not in those who received PB grafts (median 13 days in both groups).
Analysis of BM graft recipients showed that 88% (30/34) of the patients in PTCy group and 51% (23/45) in the conventional GVHD prophylaxis group had attained full donor chimerism by day 30. Most patients in both groups achieved complete donor chimerism by 1 year post-transplant – 91% (31/34) and 71% (32/45) in the PTCy and conventional groups, respectively. Higher frequency of mixed chimerism seen in the conventional arm was attributed primarily to the use of busulfan-based conditioning regimens, as we had previously observed (de Lima et al, 2004; Alatrash et al, 2011).
Other outcomes
Two-year cumulative incidences of NRM (35% vs. 25%), disease progression (20% vs. 31%), PFS (42% vs. 38%) and OS (52% vs. 40%) were similar in the PTCy and the conventional groups, respectively (Fig 2). Likewise, subgroup analysis of BM graft recipients showed comparable outcomes between the groups (Table IV).
Fig 2.
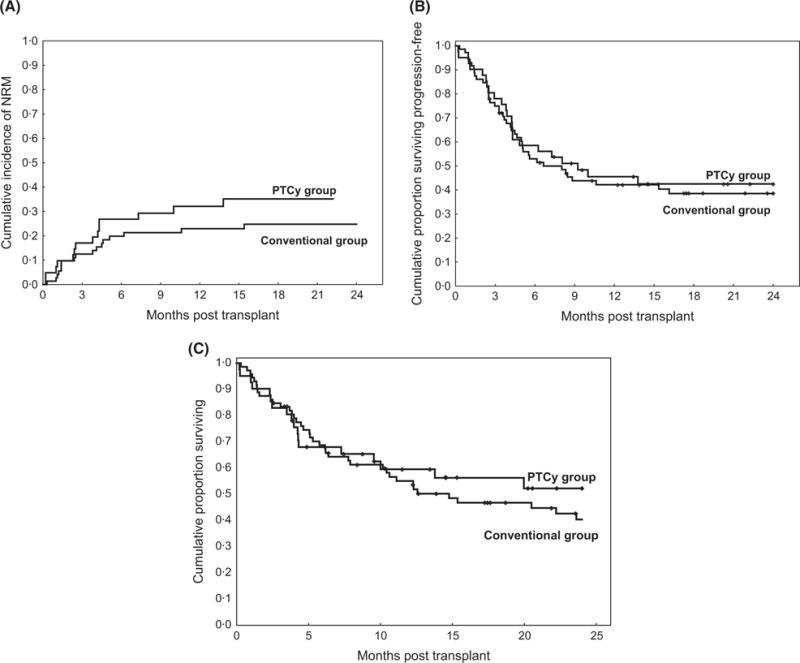
(A) Cumulative incidence of non-relapse mortality (NRM), (B) progression-free survival and (C) overall survival in the post-transplant cyclophosphamide (PTCy) group compared with the conventional group, all patients.
Table IV.
Secondary outcomes.
| All patients (n = 113)
|
Subgroup analysis with BM graft (n = 79)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Cumulative incidence (95% CI)
|
HR; 95% CI | P-value | Cumulative incidence (95% CI)
|
HR; 95% CI | P-value | ||
| Conventional GVHD prophylaxis (n = 72) | PTCy (n = 41) | Conventional GVHD prophylaxis (n = 45) | PTCy (n =34) | |||||
| NRM, 2 years, | 25% (16–38) | 35% (23–54) | 1.4; 0.7–2.8 | 0.3 | 1.6 (0.6–3.8) | 0.3 | ||
| Progression, 2 years | 31% (22–44) | 20% (22–37) | 0.6; 0.3–1.4 | 0.2 | 0.6 (0.3–1.5) | 0.3 | ||
| PFS, 2 years | 38% (27–50) | 42% (27–57) | 0.9; 0.5–1.5 | 0.7 | 43% (28–58) | 56% (37–72) | 0.9 (0.5–1.6) | 0.8 |
| OS, 2 years | 40% (28–52) | 52% (35–67) | 0.8; 0.5–1.4 | 0.5 | 38% (24–52) | 44% (27–60) | 0.8 (0.4–1.6) | 0.5 |
BM, bone marrow; CI, Confidence Interval; HR, Hazard Ratio; GVHD, graft-versus-host disease; PTCy, Post-transplant cyclophosphamide; NRM, non-relapse mortality; PFS, progression-free survival; OS, overall survival.
Causes of deaths
Disease recurrence or persistence was the leading cause of death in the entire cohort, accounting for about 46% of all deaths. In patients with BM grafts, four deaths occurred due to graft failure or rejection – three in the conventional arm and one in the PTCy arm. Overall, approximately 17% of deaths in the conventional group and 21% in the PTCy group were attributed to infections. Further causes of deaths are summarized in Table V.
Table V.
Causes of death, by graft source and GVHD prophylaxis
| Bone marrow graft
|
Peripheral blood graft
|
|||
|---|---|---|---|---|
| Conventional group (n = 25) | PTCy group (n = 15) | Conventional group (n = 17) | PTCy group (n = 4) | |
| Disease recurrence/persistence | 16 (64%) | 5 (33.3%) | 7 (41.2%) | – |
| Acute GVHD | – | 3 (20%) | 1 (5.9%) | 2 (50%) |
| Chronic GVHD | 1 (4%) | – | 2 (11.7%) | 2 (50%) |
| Graft failure/rejection | 3 (12%) | 1 (6.7%) | – | – |
| Infections | 3 (12%) | 4 (26.7%) | 4 (23.5%) | – |
| Pneumonia | 2 (8%) | – | – | – |
| Organ failure | – | 1 (6.7%) | 1 (5.9%) | – |
| Prior malignancy | – | – | 1 (5.9%) | – |
| Other | – | 1 (6.7%) | 1 (5.9%) | – |
GVHD, graft-versus-host disease; PTCy, Post-transplant cyclophosphamide.
Outcomes of patients with 7/8 HLA-MUD HCT
After exclusion of 29 patients with isolated HLA-DQ mismatches, 84 patients were identified as ‘7/8 HLA-MUD’ HCT recipients. Out of these, 38 patients received PTCy-based GVHD prophylaxis while 46 patients received the conventional prophylaxis. No patient in the PTCy group developed acute GVHD by day 30 compared with eight patients in the conventional group (P = 0.005) (Table II). Yet again, there were no differences in the incidence of grade II–IV (HR 1, 95% CI 0.5–2.1, P = 0.9) or grade III–IV (HR 1.1, 95% CI 0.3–3.3, P = 0.9) acute GVHD at day 100, or chronic GVHD at 6 months (HR 0.8, 95% CI 0.2–2.9, P = 0.7), 1 year (HR 0.8, 95% CI 0.3–2.2, P = 0.6) or 2 years (HR 0.7, 95% CI 0.2–1.9, P = 0.5) between the groups. The median time to neutrophil engraftment was 18 days (range 6–34) in the PTCy group and 12 days (range 8–25) in the conventional group, P = 0.001. The cumulative incidence of PFS (40% vs. 35%, HR 0.9, 95% CI 0.5–1.5, P = 0.6) and OS (51% vs. 41%, HR 0.8, 95% CI 0.5–1.6, P = 0.6) was similar between the PTCy group and the conventional GVHD prophylaxis group, respectively. With a median follow-up of 18 months (range 4–43) in the PTCy group and 27 months (range 3–49) in the conventional GVHD prophylaxis group, there were no differences in NRM (HR 1.3, 95% CI 0.6–2.7, P = 0.5) or disease progression (HR 0.6, 95% CI 0.3–1.5, P = 0.3) between the groups.
Discussion
In this single-institution retrospective analysis, we analysed the outcomes of one antigen HLA-MMUD (9/10-HLA matched) HCT with the use of PTCy as GVHD prophylaxis strategy along with tacrolimus and MMF, as compared to the conventional GVHD prophylaxis with in vivo TCD, tacrolimus and methotrexate. Overall, the rates of acute or chronic GVHD were similar between the groups.
Next, we evaluated differences in the incidences of early acute GVHD between the groups. It is known that the occurrence of ‘hyperacute’ GVHD is associated with lower response rate to GVHD treatment and higher NRM (Saliba et al, 2007). ‘Hyperacute’ GVHD was defined previously as GVHD occurring within 14 days of transplantation, which was evaluated soon after neutrophil engraftment. However, this conventional definition is inapplicable to our cohort of mismatched HCT, the majority of who received BM grafts. In fact, the median time to neutrophil engraftment was 18 days in the PTCy group, and no patient in that group developed grade II–IV acute GVHD by day 20 compared with the conventional group (8%), P = 0.075. Additionally, there was statistically significant reduction in the incidence of grade II–IV acute GVHD by day 30 in the PTCy group (0%) compared to 15% in the conventional group, P = 0.01.
However, reduced incidence of early acute GVHD did not translate into improvements in incidences of acute GVHD by day 100, chronic GVHD or NRM. As such, the clinical significance of early reduction of GVHD risk is presently uncertain, but may be evident as more patients are enrolled into this trial. Nevertheless, the PTCy-based regimen was at least as effective as the conventional GVHD prophylaxis regimen. Similar results were noted after excluding patients with isolated HLA-DQ mismatched (7/8-HLA matched) unrelated donor HCT; or analysing patients by graft source (BM versus PB) or HLA-class mismatch (class I versus class II).
In contrast to the rates of GVHD observed in haploidentical HCT recipients receiving PTCy (Luznik et al, 2008; Bacigalupo et al, 2015; Ciurea et al, 2015; Sugita et al, 2015), we found higher than expected rates of GVHD in our study. The burden of alloreactive T cells is much higher in the major histocompatibility complex (MHC)-mismatched setting and it is pertinent to assume that a greater degree of mismatch at major HLA antigens will result in a more profound T cell proliferation and more efficient clonal deletion by post-transplant cyclophosphamide. Moreover, in the setting of a one antigen MMUD transplant, the higher rates of acute GVHD after PTCy may be related to a higher degree of minor antigen mismatching in addition to less efficient in vivo deletion of effector T cells. Indeed, studies of tolerance-induction using MHC-matched skin grafts that differed only in expression of non-MHC, ‘minor’ histocompatibility antigens followed by cyclophosphamide administration failed to induce complete tolerance (Nirmul et al, 1971, 1973).
As noted in prior studies in MUD and MRD HCT (Luznik et al, 2010; Kanakry et al, 2014), we also observed prolonged time to neutrophil engraftment with the use of PTCy, especially in patients who received BM grafts, where the median time to engraftment was delayed by a week. Nevertheless, almost 90% of BM graft recipients in the PTCy group had achieved complete donor chimerism by day 30, compared with about half of the patients in the conventional GVHD prophylaxis. Although debatable, early achievement of complete donor chimerism, especially in the T-cell compartment, is shown to be associated with better long term disease control in many studies (Molloy et al, 1996; Gardiner et al, 1997; Bader et al, 1998; Shaffer et al, 2013). Besides, the overall rates of graft failures were similar between the PTCy and the conventional GVHD prophylaxis groups. This suggests that the use of PTCy is safe in this setting and is not associated with inferior graft function compared to the conventional GVHD prophylaxis.
With a study cohort including more than 50% of high- or very high-risk haematological malignancies, we observed similar 2-year progression (20% vs. 31%), PFS (42% vs. 38%) and OS (52% vs. 40%) in the PTCy group and the conventional group, respectively. A previous study in MUD and MRD HCT patients receiving Bu/Flu myeloablative conditioning and PTCy experienced higher rates of NRM than what is generally expected with Bu/Flu conditioning (Kanakry et al, 2014). The authors speculated that high dose PTCy may add to the toxicity of the conditioning regimen. We used fludarabine and melphalan based conditioning regimen with either thiotepa or TBI 200 cGy in the PTCy group and did not observe any difference in NRM compared to that of the conventional group.
We acknowledge certain limitations in our study. In addition to the inherent flaws of a retrospective analysis, small numbers of patients in different subgroups limited the power of the analysis and our ability to determine the benefits of PTCy in patients with HLA class-I versus class-II mismatches. The impact of different types of mismatches on outcomes after MMUD HCT is well described (Flomenberg et al, 2004). Additionally, comparison of data on tempo of immune reconstitution in both the groups is of interest and will be considered in future studies.
In conclusion, our study demonstrates that the use of PTCy, tacrolimus and MMF as GVHD prophylaxis in patients who receive single-antigen MMUD HCT after myeloablative or reduced-intensity conditioning, is safe and at least as effective as the conventional GVHD prophylaxis with in vivo TCD, tacrolimus and methotrexate. The PTCy-based regimen results in significantly lower risk of earlier occurrence of acute GVHD, the long-term significance of which is unclear at this time. However, as more patients are being treated it is possible that this early benefit could translate to an improvement in NRM. The use of PTCy does not contribute to additional toxicities; it may be associated with faster and better likelihoods of achieving complete donor chimerism and circumvent the need for in vivo TCD. In contrast, engraftment is delayed with PTCy in the recipients of BM grafts. Larger studies are needed to confirm our results.
Acknowledgments
The authors are grateful to the patients and their families. The authors would like to acknowledge our PharmDs, the nursing staff, research coordinators, and cell therapy laboratory staff.
Footnotes
Authorship contributions
R.S.M. contributed to data collection and interpretation of the results, and wrote the manuscript; R.M.S. performed the statistical analysis, interpreted the results and contributed to manuscript writing; J.C. contributed to data collection, reviewed and approved the manuscript; G.R. contributed to data collection, reviewed and approved the manuscript; A.E.H. contributed to patient care, reviewed and approved the manuscript; A.A. contributed to patient care, reviewed and approved the manuscript; M.Q. contributed to patient care, reviewed and approved the manuscript; Q.B. contributed to patient care, reviewed and approved the manuscript; S.A. contributed to patient care, reviewed and approved the manuscript; U.P. contributed to patient care, reviewed and approved the manuscript; C.H. contributed to patient care, reviewed and approved the manuscript; I.K. contributed to patient care, reviewed and approved the manuscript; E.J.S. contributed to patient care, reviewed and approved the manuscript; R.E.C. contributed to study design, reviewed, edited and approved the manuscript; S.O.C. contributed to study design, data collection and interpretation and manuscript writing.
Disclosure of conflicts of interest
The authors do not have any conflicts of interest.
References
- Alatrash G, de Lima M, Hamerschlak N, Pelosini M, Wang X, Xiao L, Kerbauy F, Chiattone A, Rondon G, Qazilbash MH, Giralt SA, de Padua Silva L, Hosing C, Kebriaei P, Zhang W, Nieto Y, Saliba RM, Champlin RE, Andersson BS. Myeloablative reduced-toxicity i.v. busulfan-fludarabine and allogeneic hematopoietic stem cell transplant for patients with acute myeloid leukemia or myelodysplastic syndrome in the sixth through eighth decades of life. Biology of Blood and Marrow Transplantation. 2011;17:1490–1496. doi: 10.1016/j.bbmt.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuk F, Diyachenko G, Zabelina T, Panse J, Wolschke C, Eiermann T, Binder T, Fehse B, Erttmann R, Kabisch H, Bacher U, Kroger N, Zander AR. Anti-thymocyte globulin overcomes the negative impact of HLA mismatching in transplantation from unrelated donors. Experimental Hematology. 2008;36:1047–1054. doi: 10.1016/j.exphem.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, Oneto R, Bruno B, Barbanti M, Sacchi N, Van Lint MT, Bosi A. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98:2942–2947. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- Bacigalupo A, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, Bregante S, Van Lint MT, Geroldi S, Luchetti S, Grasso R, Pozzi S, Colombo N, Tedone E, Varaldo R, Raiola AM. Unmanipulated haploidentical bone marrow transplantation and post-transplant cyclophosphamide for hematologic malignanices following a myeloablative conditioning: an update. Bone Marrow Transplantation. 2015;50:S37–S39. doi: 10.1038/bmt.2015.93. [DOI] [PubMed] [Google Scholar]
- Bader P, Beck J, Frey A, Schlegel PG, Hebarth H, Handgretinger R, Einsele H, Niemeyer C, Benda N, Faul C, Kanz L, Niethammer D, Klingebiel T. Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transplantation. 1998;21:487–495. doi: 10.1038/sj.bmt.1701119. [DOI] [PubMed] [Google Scholar]
- Bosch M, Dhadda M, Hoegh-Petersen M, Liu Y, Hagel LM, Podgorny P, Ugarte-Torres A, Khan FM, Luider J, Auer-Grzesiak I, Mansoor A, Russell JA, Daly A, Stewart DA, Maloney D, Boeckh M, Storek J. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy. 2012;14:1258–1275. doi: 10.3109/14653249.2012.715243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, Wang SA, Konopleva M, Fernandez-Vina M, Montes N, Bosque D, Chen J, Rondon G, Alatrash G, Alousi A, Bashir Q, Korbling M, Qazilbash M, Parmar S, Shpall E, Nieto Y, Hosing C, Kebriaei P, Khouri I, Popat U, de Lima M, Champlin RE. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation. 2012;18:1835–1844. doi: 10.1016/j.bbmt.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, Armand P, Antin JH, Chen J, Devine SM, Fowler DH, Luznik L, Nakamura R, O’Donnell PV, Perales MA, Pingali SR, Porter DL, Riches MR, Ringden OT, Rocha V, Vij R, Weisdorf DJ, Champlin RE, Horowitz MM, Fuchs EJ, Eapen M. Haploidentical transplant with post-transplant cyclophosphamide versus matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–1040. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillier R, Furst S, Crocchiolo R, El-Cheikh J, Castagna L, Harbi S, Granata A, D’Incan E, Coso D, Chabannon C, Picard C, Etienne A, Calmels B, Schiano JM, Lemarie C, Stoppa AM, Bouabdallah R, Vey N, Blaise D. A conditioning platform based on fludarabine, busulfan, and 2 days of rabbit antithymocyte globulin results in promising results in patients undergoing allogeneic transplantation from both matched and mismatched unrelated donor. American Journal of Hematology. 2014;89:83–87. doi: 10.1002/ajh.23592. [DOI] [PubMed] [Google Scholar]
- Duval M, Pedron B, Rohrlich P, Legrand F, Faye A, Lescoeur B, Bensaid P, Larchee R, Sterkers G, Vilmer E. Immune reconstitution after haematopoietic transplantation with two different doses of pre-graft antithymocyte globulin. Bone Marrow Transplantation. 2002;30:421–426. doi: 10.1038/sj.bmt.1703680. [DOI] [PubMed] [Google Scholar]
- van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF, van Loon AM, Frassoni F, Bacigalupo A, Schaefer UW, Osterhaus AD, Gratama JW, Lowenberg B, Verdonck LF, Cornelissen JJ. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell–depleted SCT. Blood. 2001;98:972–978. doi: 10.1182/blood.v98.4.972. [DOI] [PubMed] [Google Scholar]
- Finke J, Schmoor C, Lang H, Potthoff K, Bertz H. Matched and mismatched allogeneic stem-cell transplantation from unrelated donors using combined graft-versus-host disease prophylaxis including rabbit anti-T lymphocyte globulin. Journal of Clinical Oncology. 2003;21:506–513. doi: 10.1200/JCO.2003.03.129. [DOI] [PubMed] [Google Scholar]
- Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, Kolbe K, Mayer J, Maertens JA, Linkesch W, Holler E, Koza V, Bornhauser M, Einsele H, Kolb HJ, Bertz H, Egger M, Grishina O, Socie G, A.T.-F.T. Group Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncology. 2009;10:855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, Hurley C, Kollman C, Anasetti C, Noreen H, Begovich A, Hildebrand W, Petersdorf E, Schmeckpeper B, Setterholm M, Trachtenberg E, Williams T, Yunis E, Weisdorf D. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- Fuji S, Kanda J, Kato S, Ikegame K, Morishima S, Miyamoto T, Hidaka M, Kubo K, Miyamura K, Tsudo M, Kobayashi H, Maesako Y, Eto T, Adachi S, Ichinohe T, Atsuta Y, Kanda Y, HLA Working Group of the Japan Society for Hematopoietic Cell Transplantation A Single high-resolution HLA mismatch has a similar adverse impact on the outcome of related hematopoietic stem cell transplantation as a single low-resolution HLA mismatch. American Journal of Hematology. 2015;90:618–623. doi: 10.1002/ajh.24028. [DOI] [PubMed] [Google Scholar]
- Gardiner N, Lawler M, O’Riordan J, De’Arce M, McCann SR. Donor chimaerism is a strong indicator of disease free survival following bone marrow transplantation for chronic myeloid leukaemia. Leukemia. 1997;11(Suppl 3):512–515. [PubMed] [Google Scholar]
- Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, Hartzman R, Rizzo JD, Horowitz M, Confer D, Maiers M. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. New England Journal of Medicine. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka A, Ohashi Y, Okamoto S, Moriyama Y, Nagao T, Kodera Y, Kanamaru A, Dohy H, Masaoka T, Japanese FK506 BMT(Bone Marrow Transplantation) Study Group Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplantation. 2001;28:181–185. doi: 10.1038/sj.bmt.1703097. [DOI] [PubMed] [Google Scholar]
- Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, Mielcarek M, Champlin RE, Jones RJ, Thall PF, Andersson BS, Luznik L. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. Journal of Clinical Oncology. 2014;32:3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Min WS, Cho BS, Eom KS, Kim YJ, Min CK, Lee S, Cho SG, Jin JY, Lee JW, Kim CC. Successful prevention of acute graft-versus-host disease using low-dose antithymocyte globulin after mismatched, unrelated, hematopoietic stem cell transplantation for acute myelogenous leukemia. Biology of Blood and Marrow Transplantation. 2009;15:704–717. doi: 10.1016/j.bbmt.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, Fernandez-Vina M, Flomenberg N, Horowitz M, Hurley CK, Noreen H, Oudshoorn M, Petersdorf E, Setterholm M, Spellman S, Weisdorf D, Williams TM, Anasetti C. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, Shpall EJ, Shahjahan M, Pierre B, Giralt S, Korbling M, Russell JA, Champlin RE, Andersson BS. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, Matsui W, Bolanos-Meade J, Borrello I, Powell JD, Harrington E, Warnock S, Flowers M, Brodsky RA, Sandmaier BM, Storb RF, Jones RJ, Fuchs EJ. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biology of Blood and Marrow Transplantation. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, Huff CA, Borrello I, Matsui W, Powell JD, Kasamon Y, Goodman SN, Hess A, Levitsky HI, Ambinder RF, Jones RJ, Fuchs EJ. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Seminars in Oncology. 2012;39:683–693. doi: 10.1053/j.seminoncol.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy K, Goulden N, Lawler M, Cornish J, Oakhill A, Pamphilon D, Potter M, Steward C, Langlands K, Humphries P, McCann SR. Patterns of hematopoietic chimerism following bone marrow transplantation for childhood acute lymphoblastic leukemia from volunteer unrelated donors. Blood. 1996;87:3027–3031. [PubMed] [Google Scholar]
- Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, Przepiorka D, Davies S, Petersen FB, Bartels P, Buell D, Fitzsimmons W, Anasetti C, Storb R, Ratanatharathorn V. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- Nirmul G, Severin C, Taub RN. Cyclophosphamide-induced immunologic tolerance to skin homografts. Surgical Forum. 1971;22:287–288. [PubMed] [Google Scholar]
- Nirmul G, Severin C, Taub RN. Mechanisms and kinetics of cyclophosphamide-induced specific tolerance to skin allografts in mice. Transplantation Proceedings. 1973;5:675–678. [PubMed] [Google Scholar]
- NMDP. National Marrow Donor Program. 2015 Available at http://bethematch.org/
- O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, Rhubart P, Cowan K, Piantados S, Fuchs EJ. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biology of Blood and Marrow Transplantation. 2002;8:377–386. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- Perkins J, Field T, Kim J, Kharfan-Dabaja MA, Fernandez H, Ayala E, Perez L, Xu M, Alsina M, Ochoa L, Sullivan D, Janssen W, Anasetti C. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biology of Blood and Marrow Transplantation. 2010;16:937–947. doi: 10.1016/j.bbmt.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 consensus conference on acute GVHD Grading. Bone Marrow Transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- Saber W, Opie S, Rizzo JD, Zhang MJ, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. 2012;119:3908–3916. doi: 10.1182/blood-2011-09-381699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba RM, de Lima M, Giralt S, Andersson B, Khouri IF, Hosing C, Ghosh S, Neumann J, Hsu Y, De Jesus J, Qazilbash MH, Champlin RE, Couriel DR. Hyperacute GVHD: risk factors, outcomes, and clinical implications. Blood. 2007;109:2751–2758. doi: 10.1182/blood-2006-07-034348. [DOI] [PubMed] [Google Scholar]
- Sasazuki T, Juji T, Morishima Y, Kinukawa N, Kashiwabara H, Inoko H, Yoshida T, Kimura A, Akaza T, Kamikawaji N, Kodera Y, Takaku F. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. New England Journal of Medicine. 1998;339:1177–1185. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- Shaffer BC, Modric M, Stetler-Stevenson M, Arthur DC, Steinberg SM, Liewehr DJ, Fowler DH, Gale RP, Bishop MR, Pavletic SZ. Rapid complete donor lymphoid chimerism and graft-versus-leukemia effect are important in early control of chronic lymphocytic leukemia. Experimental Hematology. 2013;41:772–778. doi: 10.1016/j.exphem.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, Hackman R, Tsoi MS, Storb R, Thomas ED. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. American Journal of Medicine. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- Small TN, Avigan D, Dupont B, Smith K, Black P, Heller G, Polyak T, O’Reilly RJ. Immune reconstitution following T-cell depleted bone marrow transplantation: effect of age and posttransplant graft rejection prophylaxis. Biology of Blood and Marrow Transplantation. 1997;3:65–75. [PubMed] [Google Scholar]
- Sugita J, Kawashima N, Fujisaki T, Kakihana K, Ota S, Matsuo K, Miyamoto T, Akashi K, Taniguchi S, Harada M, Teshima T, Japan Study Group for Cell Therapy and Transplantation HLA-haploidentical peripheral blood stem cell transplantation with post-transplant cyclophosphamide after Busulfan-Containing reduced-intensity conditioning. Biology of Blood and Marrow Transplantation. 2015;21:1646–1652. doi: 10.1016/j.bbmt.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Woolfrey A, Klein JP, Haagenson M, Spellman S, Petersdorf E, Oudshoorn M, Gajewski J, Hale GA, Horan J, Battiwalla M, Marino SR, Setterholm M, Ringden O, Hurley C, Flomenberg N, Anasetti C, Fernandez-Vina M, Lee SJ. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biology of Blood and Marrow Transplantation. 2011;17:885–892. doi: 10.1016/j.bbmt.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


