Abstract
Objectives
One of the irritating features of migraine is emesis that can compromise taking oral medications. We designed this study to compare the effectiveness of granisetron and metoclopramide in reducing pain and treating emesis in migraine patients.
Methods
We included a total of 148 patients with migraine headache presenting to two referral hospitals in a prospective, double-blinded randomized controlled trial. We compared the effect of granisetron (2 mg intravenous) with metoclopramide (10 mg intravenous). Pain intensity and emesis episodes were recorded before drug administration, one, two and four 4 h after drug administration.
Results
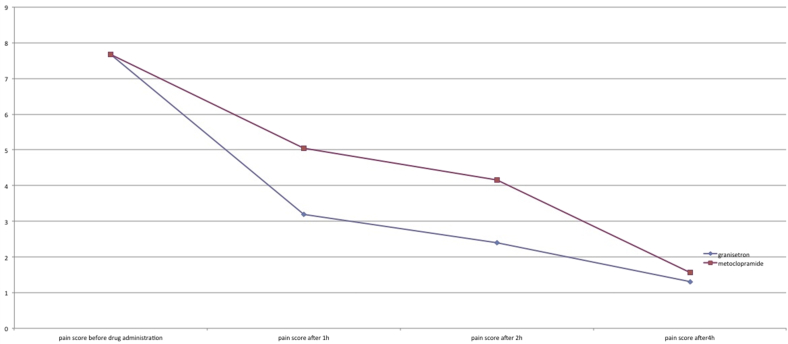
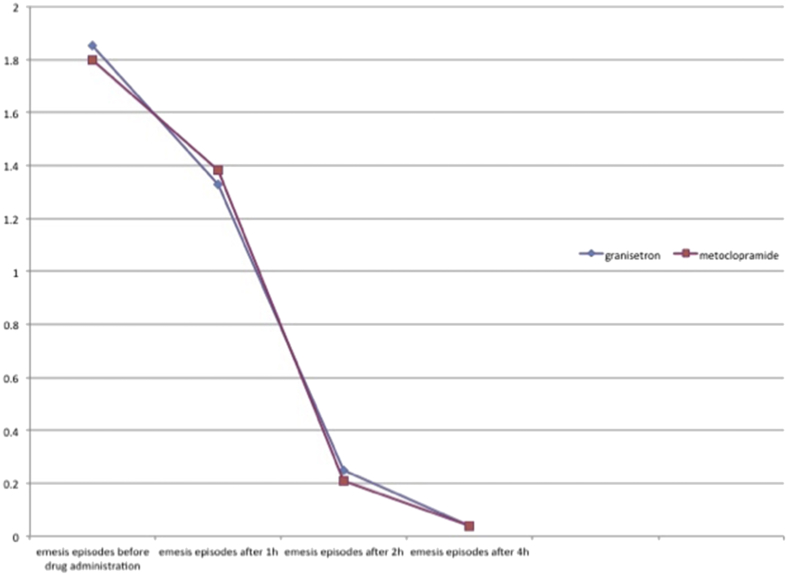
Of the 148 patients, 47 were male and 101 were female. 75 patients received granisetron and 73 metoclopramide. Mean pain intensity before the administration of the medications was 7.67 ± 1.30 in granisetron group and 7.68 ± 1.13 in metoclopramide group with an insignificant difference. Mean pain intensity at one, two, and 4 h after drug administration was 3.20 ± 1.37, 2.39 ± 1.28, and 1.31 ± 0.52 in granisetron group and 5.04 ± 1.77, 4.1 ± 1.8, and 1.56 ± 0.68 in metoclopramide group (P = 0.03). Mean emesis episodes before drug administration were 1.85 ± 0.81 and 1.80 ± 0.77 in granisetron and metoclopramide groups, respectively. These episodes were 1.33 ± 0.66, 0.25 ± 0.49, and 0.04 ± 0.19 in granisetron group and 1.38 ± 0.73, 0.21 ± 0.47, and 0.41 ± 0.19 in metoclopramide group at one, two, and 4 h after the drug administration (P = 0.7).
Conclusion
To came in conclusion, compared to metoclopramide, granisetron is a better choice in acute migraine ATTACK because it decreases the patients' pain as well as their emesis.
Keywords: Migraine, Headache, Granisetron, Metoclopramide, Pain
1. Introduction
Migraine is a common, chronic, and occasionally incapacitating neurovascular headache. This headache is often unilateral, pulsating in quality, and moderate to severe in intensity. Possible associated signs and symptoms include nausea, vomiting, anorexia, photophobia, phonophobia, osmophobia (aversion to odors), blurred vision, lightheadedness, and nasal congestion. Migraine affects 10% of the world population and accounts for approximately 1 million visits to the emergency departments (EDs) every year [1], [2]. The frequency of the migraine headache is quite variable. Some patients experience several episodes per month. Early theories postulated abnormal vasculature as the root cause of migraine headaches with vasoconstriction to be responsible for the aura and rebound vasodilatation for the pounding headache, nausea and vomiting, anorexia, photophobia and phonophobia [2]. Migraine is incapacitating because of both severe headache and other related symptoms. One of the irritating features of migraine is emesis which can compromise taking oral medications and can be resistant to therapy.
Metoclopramide is used as an antiemetic drug in migraine. It also has a prokinetic effect due to the agonism of the 5HT4 receptors (3). Metoclopramide inhibits the dopamine effects in the central nervous system and other organs. Its effects on the chemoreceptor trigger zone (CTZ) of the medulla makes it beneficial as a usual anti-emetic drug while it may also be a 5HT3 receptor antagonist [4]. Metoclopramide, with this mechanism, may be effective in the treatment of migraine. Adverse effects of metoclopramide include restlessness, Akathisia, sedation, extrapyramidal symptoms, anxiety, dystonia, headache, seizure, and hallucination [3].
Granisetron is a potent selective antagonist of 5-HT3 receptor used mainly for the treatment of chemotherapy-induced emesis. Its adverse effects include headache, diarrhea, constipation, anxiety, and insomnia [5].
Regarding the fact that many patients with migraine headache do not respond to metoclopramide administration, this study was designed to compare the effectiveness of granisetron and metoclopramide in reducing pain and treating emesis in migraine patients.
2. Material and methods
After approval of Ethics Committee of Iran University of Medical Sciences, registration of the study and taking written consent form we enrolled the patients. In this prospective, double-blinded randomized controlled trial, we evaluated patients with migraine headache presenting to the EDs of two referral centers in Tehran. We included all patients older than 18 years old presenting with headache with a previous history of migraine headache diagnosed by a neurologist. We excluded the patients if they were pregnant or breast-feeding, had suddenly initiated headache (different from the previous attacks), had abnormal neurologic findings or head trauma within the last month, were uncooperative, needed for additional doses of morphine and had an uncertain diagnosis.
The patients were randomly assigned into metoclopramide (10 mg/intravenously bolus [IV]) or granisetron (2mg/IV bolus) based on random block design. The drugs were stored in syringes with A and B tags and both the patients and the administrating physician were blind to the type of the medication in the syringe. Pain intensity and emesis episodes were evaluated before and 1, 2, and 4 h after drug administration. The chief investigator carefully examined all patients and recorded any drug adverse reactions. If there was a need for additional doses of morphine or if the clinician had used another analgesic, the patient's data was not used for pain intensity analysis. Demographic data including age and sex were recorded. A VAS (visual analogue scale) was used to analyze the pain intensity by the investigator. The Number of emesis episodes was asked from the patient and recorded, as well.
The data was analyzed using statistical package for social sciences (SPSS) software version 15. Descriptive statics and sample T-test were used to analyze data. A P value less than 0.05 were considered to be statistically significant.
3. Results
During 16 months, 148 patients fulfilled the inclusion criteria and were enrolled in the study (47 males and 101 females). Mean age was 33.5 years. A total of 75 patients received 2 mg IV granisetron and 73 patients received 10 mg IV metoclopramide.
Mean pain intensity before the administration of the medications was 7.67 ± 1.30 in granisetron group and 7.68 ± 1.13 in metoclopramide group with an insignificant difference. Mean pain intensity at one, two, and 4 h after drug administration was 3.20 ± 1.37, 2.39 ± 1.28, and 1.31 ± 0.52 in granisetron group and 5.04 ± 1.77, 4.1 ± 1.8, and 1.56 ± 0.68 in metoclopramide group (P = 0.03; Fig. 1).
Fig. 1.
Pain intensity in the study groups at 1, 2, and 4 h after the drug administration.
Mean emesis episodes before drug administration were 1.85 ± 0.81 and 1.80 ± 0.77 in granisetron and metoclopramide groups, respectively. These episodes were 1.33 ± 0.66, 0.25 ± 0.49, and 0.04 ± 0.19 in granisetron group and 1.38 ± 0.73, 0.21 ± 0.47, and 0.41 ± 0.19 in metoclopramide group at one, two, and 4 h after the drug administration (P = 0.7, Fig. 2). Mean pain intensity and emesis episodes showed no significant difference between the different age and gender groups, either (all P values greater than 0.05).
Fig. 2.
Emesis episodes in the study groups at 1, 2, and 4 h after the drug administration.
4. Discussion
Migraine headache is a complex, recurrent headache disorder that is one of the most common complaints in medicine. WHO estimates the worldwide prevalence of current migraine to be 10% and approximately 3000 migraine attacks per million persons occur every day [6]. Various phenomenon exist for pathophysiology of migraine but the popular vascular theory of migraine, which suggested that migraine headache was caused by the dilatation of blood vessels, while the aura of migraine resulted from vasoconstriction, is no longer considered viable. Recent researches suggest a primary neuronal dysfunction leads to a sequence of changes intracranially and extracranially that account for migraine, including the four phases of premonitory symptoms, aura, headache, and postdrome. Cortical spreading depression phenomenon can explain aura and headache phase; cellular depolarization cause primary cortical phenomenon or aura phase that activates trigeminal fibers causing the headache phase [7], [8], [9], [10].
We should mention that there is a wide variety of signs and symptoms for migraine but it typically occurs with a unilateral pulsing moderate to severe headache that lasts for 4–72 h and intensifies with movement, also Nausea and vomiting usually occur later in about 80% of patients [11], [12].
Pharmacologic agents used for the treatment of migraine can be classified as abortive or prophylactic. There are many options for acute migraine attack treatment. We concentrated on two drugs; metoclopramide and granisetrone, for treating headache, nausea and vomiting in acute migraine attack. Metoclopramide is an antiemetic and prokinetic agent that blocks dopamine receptors and serotonin receptors in chemoreceptor trigger zone of CNS. Granisetron is a selective 5-HT3 antagonist that binds to receptors both in the peripheral and central nervous system with primary effects in GI tract. There exist researches about these medications [13], [14]. Aleyasin A et al. concluded that three anti-nausea and vomiting agents, granisetron, its brand (Kytril), and generic metoclopramide, have a similar effect to manage PONV in obstetrics and gynecological surgeries and there is no Superiority of Granisetron Over Metoclopramide in Prevention of Post-operative Nausea and Vomiting [15]. Opposite of that, we found granisetron better that metoclopramide for treating emesis of migraine attack.
According to our results, mean episodes of emesis were less in the granisetron group; however, this differences was not statistically significant. This is while severity of headache had clinically and statistically decreased significantly in the granisetron group in comparison with the metoclopramide group. Although we could not find any study in the literature that has examined the same effect in migraine patients, in a study performed by Rowat and colleagues [16].
The positive effect of granisetron on alleviating the severity of headache compared to the placebo had also been reported. The same results were withdrawn in a pilot study performed by Couturier and associates on seven cases, as well [17]. We, with a larger study group, have obtained the same results and indicated that administration of granisetron could improve nausea and vomiting as well as the headache.
Given the accessibility of metoclopramide and its relative low price, it was the first antiemetic drug of choice for the treatment of emesis in migraine. But regarding the results of this study, granisetron may be a better choice because it decreases the patients' pain as well as their emesis. Our results regarding the effect of granisetron on headache intensity may be promising and additional research in this regard is warranted.
4.1. Limitation
Studies with larger samples are needed to define more accurate results.
5. Conclusion
To came in conclusion, compared to metoclopramide, granisetron is a better choice in acute migraine attack because it decreases the patients' pain as well as their nausea.
Funding
None.
Conflict of interest
All authors declare that they had no conflict of interest.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
References
- 1.Kwiatkowski T., Alagappan K. Headache. In: Marx J., Hockberger R., Missouri Walls R., editors. Rosen's emergency medicine. Mosby; 2010. pp. 1356–1359. [Google Scholar]
- 2.Schull M.J., Benny C.J. Headache and facial pain. In: Tintinalli J.E., Stapczynski J., John Ma O., Cline D., Cydulka R., Meckler G., editors. Tintinalli's emergency medicine. McGrawHill; New York: 2010. pp. 1113–1118. [Google Scholar]
- 3.The internet drug index, reglan (metoclopramide) drug. 2010. http://www.rxlist.com/reglan-drug/clinical-pharmacology.htm.] [Google Scholar]
- 4.Albibi R., McCallum R.W. Metoclopramide: pharmacology and clinical application. Ann Intern Med. 1983 Jan;98(1):86–95. doi: 10.7326/0003-4819-98-1-86. [DOI] [PubMed] [Google Scholar]
- 5.The internet drug index, kytril (granisetron) drug. 2010. http://www.rxlist.com/reglan-drug/clinical-pharmacology.htm.] [Google Scholar]
- 6.Sun-Edelstein C., Mauskop A. Role of magnesium in the pathogenesis and treatment of migraine. Expert Rev Neurother. 2009 Mar;9(3):369–379. doi: 10.1586/14737175.9.3.369. [DOI] [PubMed] [Google Scholar]
- 7.Cutrer F.M. Pathophysiology of migraine. Semin Neurol. 2006;26:171. doi: 10.1055/s-2006-939917. [DOI] [PubMed] [Google Scholar]
- 8.Charles A. Vasodilation out of the picture as a cause of migraine headache. Lancet Neurol. 2013;12:419. doi: 10.1016/S1474-4422(13)70051-6. [DOI] [PubMed] [Google Scholar]
- 9.Cutrer F.M., Charles A. The neurogenic basis of migraine. Headache. 2008 Oct;48(9):1411–1414. doi: 10.1111/j.1526-4610.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 10.Charles A. Advances in the basic and clinical science of migraine. Ann Neurol. 2009;65:491. doi: 10.1002/ana.21691. [DOI] [PubMed] [Google Scholar]
- 11.Lance J.W., Anthony M. Some clinical aspects of migraine. A prospective survey of 500 patients. Arch Neurol. 1966;15:356. doi: 10.1001/archneur.1966.00470160022003. [DOI] [PubMed] [Google Scholar]
- 12.Goadsby P.J., Lipton R.B., Ferrari M.D. Migraine–current understanding and treatment. N Engl J Med. 2002;346:257. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 13.Mouch I., Brouwers J.R., van 't Riet E. 5-HT3-antagonists as a substitute for metoclopramide and domperidone: a literature review. Ned Tijdschr Geneeskd. 2016;160(0):A9887. [PubMed] [Google Scholar]
- 14.Becker W.J. Acute migraine treatment in adults. Headache. 2015 Jun;55(6):778–793. doi: 10.1111/head.12550. [DOI] [PubMed] [Google Scholar]
- 15.Aleyasin A., Hayatshahi A., Saffarieh E. No superiority of granisetron over metoclopramide in prevention of post-operative nausea and vomiting: a randomized clinical trial. J Obstet Gynaecol India. 2014 Feb;64(1):59–62. doi: 10.1007/s13224-013-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowat B.M., Merrill C.F., Davis A., South V. A double-blind comparison of granisetron and placebo for the treatment of acute migraine in the emergency department. Cephalalgia. 1991;11:207–213. doi: 10.1046/j.1468-2982.1991.1105207.x. [DOI] [PubMed] [Google Scholar]
- 17.Couturier E.G., Hering R., Foster C.A., Steiner T.J., Clifford Rose F. First clinical study of the selective 5-HT3 antagonist, granisetron (BRL 43694), in the acute treatment of migraine headache. Headache. 1991;31:296–297. doi: 10.1111/j.1526-4610.1991.hed3105296.x. [DOI] [PubMed] [Google Scholar]