Abstract
Flash pulmonary edema frequently develop in case of bilateral renal artery stenosis and unilateral renal artery stenosis with functional solitary kidney. In some rare cases, unilateral renal artery stenosis with bilaterally functional kidneys may also lead to flash pulmonary edema. Here, we present a case of flash pulmonary edema caused by accessory renal artery stenosis. To our knowledge, it is the first case reported in the literature.
Keywords: Pulmonary edema, Renal artery stenosis, Renal circulation
1. Introduction
Hypertensive flash pulmonary edema (FPE) results from rapid elevation of the left ventricular end-diastolic pressure. Absence of underlying valve disease or cardiomyopathy, it is usually caused by renal vascular disease. It may frequently develop in case of bilateral renal artery stenosis (RAS), unilateral RAS and accompanying functional solitary kidney.1 However, unilateral RAS with bilaterally functional kidneys may also lead to a clinical picture of pulmonary edema.2, 3 This paper presents a case of FPE caused by accessory RAS in a hypertensive patient with bilaterally functional kidneys.
2. Case presentation
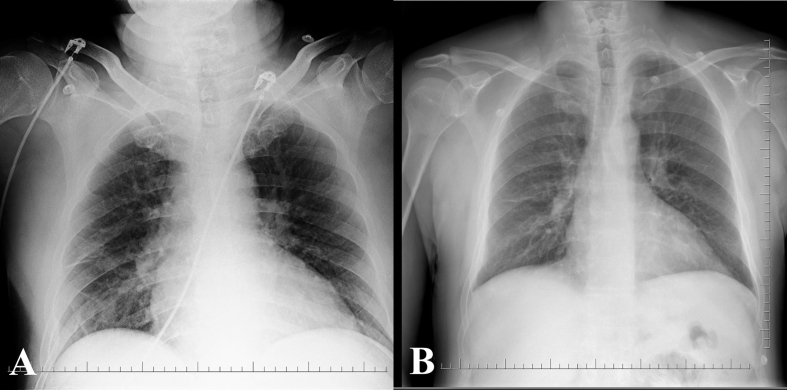
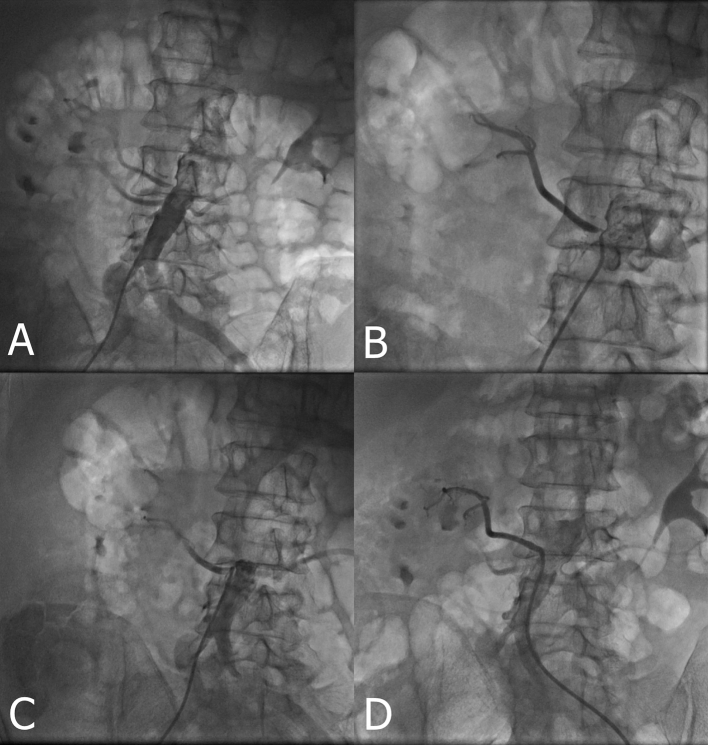
A 59-year-old male patient presented to the emergency department with rapidly developing shortness of breath and impaired consciousness after a 12-hour long road trip. The patient was hypertensive for 4 years but not taking any medication. He was a current smoker (46 pack-years). On initial presentation, blood pressure was 255/133 mm Hg, heart rate was 110 beats/min, and peripheral oxygen saturation measured with pulse oximeter was 50%. Infusions of intravenous nitroglycerine at the rate of 30 mcg/min and intravenous furosemide were administered. Initial blood tests showed slight elevation in blood glucose (243 mg/dL), blood urea (51 mg/dL), and creatinine (1.28 U/L) levels. Blood gas analysis revealed severe hypoxemia (pO2: 42.5 mm Hg, sO2: 50%), hypercarbia (pCO2: 73.6 mm Hg), and mixed acidosis (pH: 7.007). Complete blood count was normal except increased level of leukocytes (17 × 103/μL). Troponin level was within the reference range. NT-proBNP was elevated (3340 ng/L). D-dimer (2160 ng/mL, reference: 0-243) and fibrinogen (678 mg/dL, reference: 200-393) levels were increased. The telecardiogram showed patchy alveolar infiltrates indicative of pulmonary edema (Fig. 1A, B). In transthoracic echocardiography, left ventricular end-diastolic diameter was 5.0 cm and ejection fraction was 55%. Right chambers of the heart were not dilated. Minimal mitral regurgitation was observed. Left ventricular hypertrophy (septum: 1.4 cm, posterior wall: 1.1 cm) and ascending aortic dilatation (4.5 cm) were other co-existing abnormalities. The symptoms of the patients gradually decreased with non-invasive mechanical ventilation and medical treatment. The following day, heart failure symptoms were completely resolved and patient was hospitalized. The follow-up echocardiography revealed grade 2 diastolic dysfunction. In renal ultrasonography, right kidney was measured as 110 × 62 × 36 mm, left kidney as 118 × 56 × 44 mm; parenchymal disease and collecting system dilatation were absent. In Doppler sonography examination, the left renal artery flow rates were normal while the right renal artery could not be assessed due to rotational anomaly. The patient underwent coronary and renal angiography. No occlusive lesion was present in the coronary arteries. Double renal arteries were supplying the right kidney and the upper accessory renal artery had an ostial stenosis of 95% (Fig. 2A, B and C). Percutaneous renal artery revascularization was planned. Intrarenal nitroglycerin was administered and following a 3.0 × 17-mm balloon predilatation, a 4.0 × 12-mm coronary stent was successfully implanted (Fig. 2D). Post-procedural one-month follow-up demonstrated that the arterial pressure was under control with antihypertensive monotherapy.
Fig. 1.
Chest radiograph demonstrates bilateral interstitial shadowing and patchy alveolar infiltrates compatible with pulmonary edema (A) that subsided soon after admission (B).
Fig. 2.
Non-selective (A), accessory (B) and lower right renal artery (C) angiography of patient. The renal arteriography shows critical stenosis of the origin of the right accessory renal artery; percutaneous transluminal intervention was performed (D).
3. Discussion
Pulmonary edema is one of the major clinical pictures resulting in admission to emergency room.4 It usually accompanies an underlying problem impairing cardiac systolic or diastolic functions. It was described for the first time by Pickering et al in 1988 as a clinical picture of recurrent edema of the lungs due to bilateral renovascular disease.5 Even though its pathogenesis is not thoroughly understood, it is thought to arise from the volume overload due to the suppression of natriuresis.6 A similar mechanism was proposed in RAS in functional single kidney.7 However, it was suggested that pulmonary edema in the case of bilaterally functional kidneys and unilateral RAS might not be exclusively due to volume overload.2 In this case, the unaffected kidney could function as compensatory even though increased renin excretion from the stenotic kidney could reduce natriuresis through angiotensin II and aldosterone. Yet, these compensatory natriuresis and diuresis might be insufficient. As in our patient, when promoting factors such as diastolic dysfunction and lack of antihypertensive treatment exist, sudden excessive increases in the renin-angiotensin-aldosterone (RAA) axis can evoke FPE. To our knowledge, ours is the first case reported in association with accessory RAS. Vascular supply of the large part of the glomeruli is normal. Nevertheless, the triggering cause of rapid and severe increase in blood pressure is not well-recognized. We suggest that inadequate fluid intake during the long road trip and intravascular volume depletion could lead to present clinical picture by further inducing renin activity. Previous studies reported that intravascular volume decreased in acute and chronic heart failure even before treatment.8, 9 Immobility, especially, is acknowledged to promote the reduction in intravascular volume.8 Increased activation of the RAA system due to volume loss might be the reason for sudden elevation of blood pressure and FPE. Further investigations are needed to demonstrate this hypothesis.
In conclusion, FPE can occur in accessory RAS with bilaterally functional kidneys. In such cases, percutaneous renal intervention can have a part in the control of blood pressure and pulmonary edema.
Funding
We declared that this study has received no financial support.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
References
- 1.Messerli F.H., Bangalore S., Makani H. Flash pulmonary oedema and bilateral renal artery stenosis: the Pickering syndrome. Eur Heart J. 2011;32:2231–2235. doi: 10.1093/eurheartj/ehr056. [DOI] [PubMed] [Google Scholar]
- 2.Noh H.J., Jo H.C., Yang J.H. Flash pulmonary edema in a patient with unilateral renal artery stenosis and bilateral functioning kidneys. Korean Circ J. 2010;40:42–45. doi: 10.4070/kcj.2010.40.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan A.A., McFadden E.P. Emergent unilateral renal artery stenting for treatment of flash pulmonary edema: fact or fiction? Case Rep Cardiol. 2015;2015:659306. doi: 10.1155/2015/659306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rimoldi S.F., Yuzefpolskaya M., Allemann Y. Flash pulmonary edema. Prog Cardiovasc Dis. 2009;52:249–259. doi: 10.1016/j.pcad.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Pickering T.G., Herman L., Devereux R.B. Recurrent pulmonary oedema in hypertension due to bilateral renal artery stenosis: treatment by angioplasty or surgical revascularisation. Lancet. 1988;2:551–552. doi: 10.1016/s0140-6736(88)92668-2. [DOI] [PubMed] [Google Scholar]
- 6.Garovic V.D., Textor S.C. Renovascular hypertension and ischemic nephropathy. Circulation. 2005;112:1362–1374. doi: 10.1161/CIRCULATIONAHA.104.492348. [DOI] [PubMed] [Google Scholar]
- 7.Pun E., Dowling R.J., Mitchell P.J. Acute presentations of renal artery stenosis in three patients with a solitary functioning kidney. Australas Radiol. 2004;48:523–527. doi: 10.1111/j.1440-1673.2004.01372.x. [DOI] [PubMed] [Google Scholar]
- 8.Figueras J., Weil M.H. Blood volume prior to and following treatment of acute cardiogenic pulmonary edema. Circulation. 1978;57:349–355. doi: 10.1161/01.cir.57.2.349. [DOI] [PubMed] [Google Scholar]
- 9.Feigenbaum M.S., Welsch M.A., Mitchell M. Contracted plasma and blood volume in chronic heart failure. J Am Coll Cardiol. 2000;35:51–55. doi: 10.1016/s0735-1097(99)00530-6. [DOI] [PubMed] [Google Scholar]