Abstract
Background
The study examined the Lp-PLA2 activity at the patients presented to the emergency department with acute coronary syndrome (ACS) or acute ischemic stroke (AIS), as well as its diagnostic value.
Methods
The prospective study included consecutive male and female patients aged >18 years that presented to the our emergency department with ACS or AIS between November 2009 and January 2010. Blood samples were obtained immediately following diagnosis in the ACS and AIS groups. The diagnostic value of Lp-PLA2 was determined based on receiver operating characteristic curves, sensitivity, specificity, predictive values, likelihood ratios and accuracy rates.
Results
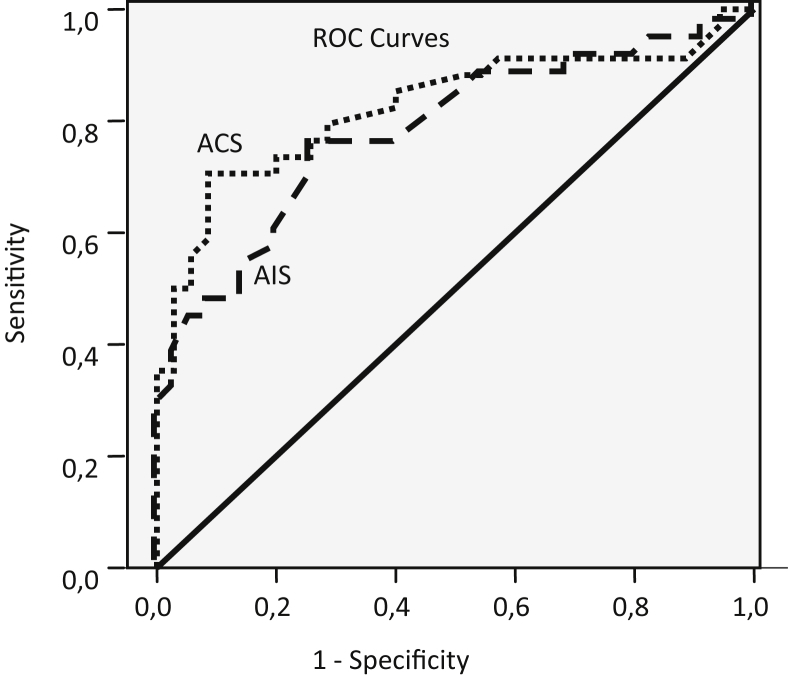
In all, 34 ACS and 32 AIS patients were included in the study, and the control group included 35 patients. Lp-PLA2 enzyme activity was significantly lower in the ACS and AIS groups than in the control group (26.7 ± 13.8, 31.4 ± 13.6, and 41.4 ± 8.1 nmol min−1·mL−1, respectively; p < 0.0001, p = 0.022). In the ACS group the area under the curve (AUC) was 0.825 (95%CI: 0.722–0.929), sensitivity was 71% for an optimal Lp-PLA2 cut-off value of 31.4 nmol min−1·mL−1, and specificity was 91%, whereas in the AIS group the AUC was 0.768 (95%CI: 0.652–0.884), sensitivity was 75% for an optimal Lp-PLA2 cut-off value of 38.1 nmol min−1·mL−1, and specificity was 74%.
Conclusions
Lp-PLA2 enzyme activity was significantly lower during the early stage of both ACS and AIS. The obtained statistic data suggest that low Lp-PLA2 enzyme activity can be used for diagnostic purposes.
Keywords: Lipoprotein-associated phospholipase-A2, Ischemia, Emergency medicine
1. Introduction
The lipoprotein-associated phospholipase-A2 (Lp-PLA2), which is also known as platelet-activating factor acetyl hydrolase (PAF-AH). Lp-PLA2 is a member of the phospholipase A2 super family. It is stated that Lp-PLA2 is produced by myeloid-originated inflammatory cells, is associated with atherogenic lipoproteins in the circulation, and is highly expressed in lesion regions.1
Lp-PLA2 hydrolyzes oxidized phospholipids, which form pro-inflammatory products that contribute to endothelial dysfunction, plaque inflammation and formation of the necrotic core in the plaque. Moreover, it is thought that Lp-PLA2 constitutes a link between oxidative modification of LDL (Low Density Lipoprotein) and increased inflammatory response in the arterial intima.2 Additionally; Lp-PLA2 exhibits pro-inflammatory characteristics. It hydrolyzes oxidized phospholipids into lysophosphatidylcholine and free fatty acids. The atherogenic potential of LDL is related to this high lysophosphatidylcholine content.3
In the last decade, many studies have examined the effects of Lp-PLA2 on atherosclerosis. The purpose of these studies was to determine the utility of Lp-PLA2 in risk prediction or risk classification of cardiovascular diseases (CVDs).4 To the best of our knowledge the literature contains few studies on the diagnostic use of the Lp-PLA2 level in patients with acute coronary syndrome (ACS) or acute ischemic stroke (AIS).5, 6 As such, the present study aimed to examine the Lp-PLA2 activity and its diagnostic potential in early period ACS and AIS patients.
2. Methods
2.1. Patients and process
The prospective study included consecutive male and female patients aged >18 years that presented to our emergency department with ACS or AIS between November 2009 and January 2010.The control group included consecutively patients without a history of or current thromboembolic events that presented to the emergency department with other complaints.The number of patients in each group was determined as about 30 considering the financial support of the study. The patients under 18 years were not included in the study in accordance with ethical rules. The patients who had the following were also excluded from the study due to their potential to affect lipoprotein metabolism, acute inflammatory response, cardiac troponin and CK-MB (Kreatin Kinaz–MB) levels and thereby the results of the study 7, 8, 9, 10: accompanying serious trauma, acute or chronic liver or renal failure, traumatic venous occlusion, hemorrhagic diathesis or coagulation disorders, hematologic malignancies, acute or chronic inflammatory disease, use of anti-aggregates, anticoagulants, anti-hyperlipidemia, or anti-inflammatory agents, and pregnancy. All ACS patients were examined by a cardiologist, whereas all AIS patients were examined by a neurologist and a neuroradiologist. In the diagnosis of ACS, the criteria which were published currently in guidelines were considered.11
For AIS group, all patients who had acute focal or systemic stroke signs (eg. alteration in consciousness, weakness of extremities, etc.) were determined with their first evaluation and blood sampling was taken. After the diagnosis of AIS was confirmed with computed tomography (CT) and/or diffusion weighted magnetic resonance imaging (DWI), those patients were included into AIS group.
Patients were enrolled in the study following verification of their diagnosis via appropriate diagnostic tests and provision of written informed consent. Blood samples were obtained from these patients for Lp-pLA2, Crp (C reaktif protein), Tn-I (Troponin I) and CK-MB analyses, and the Lp-pLA2 levels were compared to the other parameters. The sample drawn period was defined as the time from the onset of symptoms to blood drawn and it was categorized as ≤6 h, 6<−<12 h, and ≥12 h in the ACS and AIS groups. A possible difference between the sample drawn periods as well as Lp-PLA2 levels both within and between the ACS and AIS groups was investigated. The study protocol adhered to the Helsinki Declaration and was approved by the Ethics Committee of Selcuk University Meram Medicine School. The study was supported by the Scientific Research Projects Coordination Office of Selcuk University.
2.2. Sample drawn
Each patient provided 10 cc of venous blood, which was collected into test tubes containing EDTA (Etilendiamin tetraasetik asit). After plasma was separated from the obtained samples via cold centrifugation at 4 °C and 700–1000 g for 10 min, the plasma samples were placed in Eppendorf tubes and preserved at −80 °C until used.
2.3. Biochemical method
A commercially available colorimetric assay (PAF Acetyl hydrolase Assay Kit, Catalogue no. 760901, Cayman Chemical Company, USA) was used to measure the plasma PAF-AH. This method uses 2-thio PAF to serve as a substrate for all PAF-AHs. Free thiols emerging from hydrolysis of the acetyl thioester bond in the sn-2 position are identified using 5,5′ -dithio-bis-(2-nitrobenzoic acid) (DTNB; Ellman's reagent). All laboratory personnel were blinded to the study and control group patients' clinical data and outcomes. Electrochemiluminesans assay (Roche Hitachi, Cobas e 411, Roche Diagnostic Turkey Company) was used to measure CK-MB and Troponın I. Nephalometrically assay (BN2 SIEMENS) was used to measure CRP.
2.4. Statistical analysis
Study data were analyzed using Statistical Package for the Social Sciences (SPSS) for Windows v.19.0. Parametric testing was used for data with normal distribution (according to the Lilliefors test) and non-parametric testing was used for data not normally distributed. The chi-square test was used to evaluate the distribution of gender, hypertension (HT), diabetes mellitus (DM), coronary heart disease (CHD), hyperlipidemia (HL), and smoking, according to group. One-way analysis of variance (ANOVA) was used to compare patient age and Lp-PLA2 activity between groups. When ANOVA test results were significant Tukey's HSD post hoc test was used. Time is used as covariant in order to control the effect.
The relationship between the sample drawn period and LP-PLA2 activity within groups was assessed using the Kruskal-Wallis test and between groups using Pearson's correlation analysis. The correlation of level of Lp-PLA2 and other biochemical parameters between groups were performed by Spearman's Test and its' accuracy was tested by Scatter-Dot Graphs. The level of statistical significance was set at p < 0.05. ROC (Receiver Operating Characteristic) curves were used to determine the diagnostic properties of specific Lp-PLA2 activity values in the ACS and AIS groups, as compared to the control group. In addition to the area under the curve (AUC), the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive and negative likelihood ratios (LR), accuracy rate (AR), odd ratio and confidence interval (CI) were determined. In addition to cut-off values in which sensitivity and specificity were the highest in total, calculations also were made for probable cut-off values in which sensitivity or specificity would be higher. In ACS group, the second ROC curve was performed with reference to Tn-I and the diagnostic potential was calculated.
3. Results
In all, 34 ACS and 32 AIS patients, and 35 control patients were enrolled in the study. Mean age of the ACS patients was 62.2 ± 12.0 years (range 23–83), 28 (82.4%) of them were male; mean age of the AIS patients was 64.7 ± 13.9 years (range 40–91), 18 (56.3%) of them were male, and mean age of the control patients was 51.5 ± 17.2 years (range 20–90), 17 (48.6%) of them were male (Table 1). Mean age in the AIS group was significantly higher than that in the control group (p = 0.007). Similarly, male patients were significantly more prevalent than that in the other groups (p = 0.003). A correlation was not detected between Lp-PLA2 levels and age, gender of the groups (p = 0.72 ve p = 0.91 respectively). Mean sample drawn period in the ACS group was 11.2 ± 17.0 h, versus 9.2 ± 7.0 h in the AIS group (Table 2). We found no association between the sample drawn period and Lp-PLA2 activity in the ACS and AIS groups, both intra-group (p = 0.38 and p = 0.86, respectively) and between the two groups (p = 0.33). There was a significant statistical difference between ACS and control group in terms of TnI and CK-MB levels (p = 0.005, p < 0.0001 respectively). The level of CRP was higher in both ACS and AIS group when compared with control group, which had statistical significance (p = 0.02, p < 0.0001 respectively). But there was no significant difference found between ACS and AIS groups in terms of CRP levels (p = 0.26).
Table 1.
Demographic characteristics and medical history of the groups.
| ACS | AIS | Control | p | |
|---|---|---|---|---|
| n | 34 | 32 | 35 | |
| Sex (men) (%) | 82.4 | 56.3 | 48.6 | 0.003 |
| Age (mean ± SD) | 62.2 ± 12.0 | 64.7 ± 13.9 | 51.5 ± 17.2 | 0.007 |
| HT (%) | 16 (47.0) | 23 (71.9) | 15 (42.9) | NS |
| DM (%) | 11 (32.3) | 12 (37.5) | 7 (20.0) | NS |
| CHD (%) | 14 (41.2) | 12 (37.5) | – | 0.05 |
| HL (%) | 13 (38.2) | 11 (34.4) | 3 (8.6) | 0.04 |
| Smoking (current) | 9 (26.5) | 11 (34.4) | 11 (31.4) | NS |
ACS: Acute coronary syndrome, AIS: Acute ischemic stroke, HT: Hypertension, DM: Diabetes mellitus, CHD: Coronary heart disease, HL: Hyperlipidemia, NS: No significance.
Table 2.
Biochemical measurement values of the groups.
| ACS | AIS | Control | p | |
|---|---|---|---|---|
| Sample draw time (h) | ||||
| (Median) | 5.6 | 7.2 | NA | NS |
| (Range) | 1–86 | 1.5–28 | ||
| Tn I (ng/mL) (mean ± SD) | 14.8 ± 29.0 | NA | 0.02 ± 0.02 | 0.005 |
| CK-MB (ng/mL) (mean ± SD) | 84.9 ± 105.3 | NA | 2.4 ± 3.0 | <0.0001 |
| CRP (mg/L) (mean ± SD) | 22.0 ± 36.2 | 31.8 ± 25.0 | 5.3 ± 2.9 | 0.02 |
| LP-PLA2 enzym activity (nmol/min/mL) (mean ± SD) | 26.7 ± 13.8 | 31.4 ± 13.6 | 41.4 ± 8.1 | <0.0001 |
NA: Not available, NS: No significance.
Lp-PLA2 enzyme activity was significantly lower in the ACS and AIS groups than in the control group (p < 0.0001 and p = 0.022, respectively) (Table 2); however, it did not differ significantly between the ACS and AIS groups (p = 0.67). Although a significant correlation was found between levels of TnI and CK-MB in the ACS group (r = 89, p < 0.0001), there was not any correlation between TnI and Lp-PLA2, CK-MB and Lp-PLA2. Also, no significant correlation was found between levels of CRP and Lp-PLA2 in both the ACS and AIS groups.
Separate ROC curves were plotted and the AUC was estimated for each group in order to determine the capability of the serum Lp-PLA2 enzyme activity to detect the acute event (Fig. 1). In the ACS group the AUC was 0.825 (95% CI: 0.722–0.929) and the standard error was 0.053. Accordingly, the optimal Lp-PLA2 cut-off value of 31.4 nmol min−1 mL−1 had a sensitivity of 71%, specificity of 91% and odd ratio 25.60 (5.56–136.21). In the AIS group the AUC was 0.768 (95% CI: 0.652–0.884) and the standard error was 0.059. Accordingly, the optimal Lp-PLA2 cut-off value of 38.1 nmol min−1·mL−1 had a sensitivity of 75%, specificity of 74% and odd ratio 8.67 (2.55–30.92) (Table 3). When ROC analysis was performed with reference to TnI, AUC = 0,798 (CI%95, 0,688–0,907) and standard error of 0,056 were found. So that, when the cut-off point was accepted as 37.7 nmol/min/ml that was optimum Lp-PLA2 cut-off point, sensitivity was 76% and specificity was 72% (Table 4).
Fig. 1.
ROC curve for ACS and AIS patients in the Lp-PLA2 values.
Table 3.
Diagnostic performance of Lp-PLA2 enzyme activity according to different cut-points in the ACS and AIS patients.
| ACS | AIS | |||||
|---|---|---|---|---|---|---|
| AUC SE CI 95% |
0,825 0.053 0,722–0,929 |
0,768 0.059 0,652–0,884 |
||||
| Cut-offa (nmol/ml/min) | 26.1 | 31.4 | 41.1 | 35.5 | 38.1 | 41.9 |
| Sensitivity % CI 95% |
50 34–66 |
71 54–84 |
88 74–96 |
59 42–75 |
75 58–87 |
88 73–96 |
| Specificity % CI 95% |
97 87–100 |
91 79–98 |
49 33–65 |
80 65–91 |
74 55–84 |
46 30–62 |
| PPV % CI 95% |
94 77–99 |
89 73–98 |
63 48–75 |
73 54–87 |
71 54–84 |
60 45–73 |
| NPV % CI 95% |
67 53–78 |
76 62–87 |
81 61–93 |
68 53–81 |
76 59–88 |
80 59–93 |
| (+)LR CI 95% |
17.50 2.89–351.74 |
8.24 2.89–32.17 |
1.72 1.20–2.18 |
2.97 1.41–6.79 |
2.92 1.60–5.32 |
1.61 1.13–2.04 |
| (−)LR CI 95% |
0.52 0.47–0.71 |
0.32 0.24–0.52 |
0.24 0.07–0.66 |
0.51 0.34–0.80 |
0.34 0.17–0.63 |
0.27 0.08–0.75 |
| AR % CI 95% |
74 63–83 |
81 71–89 |
68 57–78 |
70 59–80 |
73 62–83 |
66 54–76 |
ACS: Acute coronary syndrome, AIS: Acute ischemic stroke, AUC: Area under curve, SE: Standard error, CI: Confidence interval, PPV: Positive predictive value, NPV: Negative predictive value, LR: Likelihood ratio, AR: Accuracy rate.
The analyzes based on the cut off with high sensitivity appear on the right side of the middle column and those based on cut off with high specificity appear on the left side of the middle column. Middle column represents the optimum cut off values.
Table 4.
The diagnostic value of Lp-PLA2 by reference to troponin I in ACS group.
| AUC SE CI 95% |
Lp-PLA2 Cut-off (nmol/ml/min) |
Sens. % CI 95% |
Spec. % CI 95% |
PPV % CI 95% |
NPV % CI 95% |
(+)LR CI 95% |
(−)LR CI 95% |
AR % CI 95% |
|---|---|---|---|---|---|---|---|---|
| 0,798 0.056 0,688–0,907 |
37.7 | 76 62–86 |
72 60–82 |
71 59–81 |
77 63–87 |
2.73 1.55–4.74 |
0.34 0.17–0.63 |
74 61–84 |
ACS: Acute coronary syndrome, AUC: Area under curve, SE: Standard error, CI: Confidence interval, PPV: Positive predictive value, NPV: Negative predictive value, LR: Likelihood ratio, AR: Accuracy rate.
4. Discussion
In our study, Lp-PLA2 enzyme activity was significantly lower in both patient groups (as more prominent in the ACS group) than in the control group.
It was reported that Lp-PLA2 levels in women during the premenopausal period are generally lower than in men and that the Lp-PLA2 level increases with age.12 In the present study mean age in the AIS group was higher than that in the control group, and the male ratio was higher in the ACS group; therefore, we think that a low Lp-PLA2 level become more significant in a population in which age and the male ratio are higher than in a control group. The literature contains few studies in which the Lp-PLA2 level was measured following acute events. In a study that included 12 male patients admitted to hospital with Acute Myocardial Infarction (AMI), the PAF-AH activity level decreased on days 2 and 7 of hospitalization.13 Another study reported that the PAF-AH level in blood samples taken from 18 patients with AMI 6 h after presentation was significantly lower than that in the control group.14
Elkind et al. measured both Lp-PLA2 mass and activity before and after acute events in 37 patients (20 with myocardial infarction (MI) and 17 with stroke).15 The researchers reported that Lp-PLA2 mass and activity were significantly lower after acute events both in MI and stroke patients than before. They stated that the median sample drawn period was 5 days (range 2–40 days). The median sample drawn period in the present study was approximately 6 h and the maximum was 3.5 days. The researchers argued that Lp-PLA2 level may have decreased in accordance with a decrease in LDL levels after MI due to co-localization of Lp-PLA2 and LDL or that acute phase proteins, such as albumin and fibrinogen, may have affected the binding and activity capacity of Lp-PLA2.
Jabor et al. found Lp-PLA2 mass levels higher in ACS patients who admit within 48 h following symptom onset compared to the recovery patients 12 weeks later.6 Lp-PLA2 levels were found higher in patients with AIS in the study of Kara et al. An association was found between Lp-PLA2 levels and stroke severity and infarction size. The authors state that they included the acute stroke patients who admit to emergency department within the first 24 h following symptom onset however they do not provide a detailed information about the time to obtain blood samples.5 Tai et al. reported that Lp-PLA2 mass and activity do not show a significant change in patients with acute cerebral ischemia. However this study is different from ours as almost half of the patients have TIA and median time to obtain samples was 23 h in that study.16
In the NOBIS-II study, multi-marker (troponin-I, NT-proBNP (N-terminal proBNP), CRP, D-dimer, and Lp-PLA2) testing was used in patients with suspected ACS that presented to the emergency department.17 The researchers investigated the role of the Lp-PLA2 level obtained during the acute stage in risk classification of ACS patients, and reported that Lp-PLA2 can be used for risk stratification not only in chronic CHD patients but also in ACS patients. It was also reported that samples were drawn from patients at the time of presentation and 6 h and 12–24 h after presentation; however, the time between the onset of symptoms and sample drawn was not clarified. Quantitative analysis in the study showed that the threshold value was 210 μg.L−1. It was considerable that the median Lp-PLA2 value of the 429 patients in the study was 205 μg L−1. In literature, an Lp-PLA2 mass ≥235 ng mL−1 is the suggested cut-off for increased risk.18 So, in the NOBIS-II study, the significant proportion of patients in the acute stage had Lp-PLA2 levels lower than this cut-off value. As there was no control group in the NOBIS-II study, comparison could not be possible.
In the PROVE IT-TIMI 22 study, no correlation was observed between the Lp-PLA2 level in blood samples obtained during the first 10 days (median 7 days) post-ACS and an increased risk of recurrent CV (cardiovascular) event.19 This situation raises the question if the cause of this result is decreased Lp-PLA2 levels in the early stage; as the researchers did not measure the Lp-PLA2 level at presentation and since there was no placebo or control group they did not know if the Lp-PLA2 level increased during the acute stage, and they could not conclude if there was a return to the basal level.
Research performed as a sub-study of the FRISC II, GUSTO IV, and SWISCH studies investigated the effect of the Lp-PLA2 levels on mortality and the development of new ischemic events.20 Blood samples were obtained at median 38 h (interquartile range 28–53 h) in the FRISC II study and at median 15 h (interquartile range 9.4–20 h) in the GUSTO IV study. The Lp-PLA2 level was higher in ACS patients (in both cohorts) than in the healthy control group; however, there was not a correlation between the Lp-PLA2 level, and the risk of new CV events or mortality. The authors also reported that the time from the onset of symptoms to sample drawn (up to 84 h) was not correlated with the Lp-PLA2 levels.
In consideration of the present study's results, it can be concluded that the Lp-PLA2 level significantly decreases during the early stage of ACS and AIS. Again, when the sample drawn periods and the obtained Lp-PLA2 levels are taken into consideration, it appears that the Lp-PLA2 levels decrease in hours after an acute event, remain low for days, and thereafter return to the basal level; however, this is merely supposition. The exact time of the decrease in Lp-PLA2 level after acute event, duration of decreased level's persistence and the exact time to return to the basal level are not known. As such, when performing prognostic assessment or risk stratification, it may be crucial to know if a patient has recently experienced any acute events. On the other hand, as stated above, there is a small number of papers reporting that Lp-PLA2 levels do not change or increase in the acute period in ACS and AIS patients.
Measurement of Lp-PLA2 mass versus activity, and differences in measurement methods and patient populations, patients' basal lipoprotein levels, medications used, and cut-off values may account for the inconsistency of published results. Moreover, although numerous theories have been suggested to explain the decrease in Lp-PLA2 level the actual cause is yet to be discerned. Enlightening these issues through further studies and reaching a certain standardization in the studies would eliminate this obscure.
4.1. Limitations
As this was a preliminary study with limited financial support, the number of patients was quite low. No power analysis was performed to determine the sample count of the groups. The impact of current diseases and patient use of pharmacological agents on the results has been ignored. The most important limitation of our study is the difference between the control group and study groups with regard to age and gender, despite the fact that the control group participants were selected among other patients who had presented to the emergency unit and consecutively included in the study. Furthermore, we have no data on a possible difference between Lp-pLA2 measurements according to age or gender in our country. The mass of the Lp-PLA2 enzyme could not be studied along with its activity; hence, we could not determine if there was a correlation in between mass and activity. Lp-PLA2 activity was measured only once and its change over time was not assessed; therefore, we could not determine how long this low level persisted. Finally, the relationship between the lipoprotein level and Lp-PLA2 level was not investigated. ″LDL-C″ levels that was unable to view relationship and correlation with it. Patients with ACS and AIS were not evaluated separately with regard to their etiology.
5. Conclusions
In the present study, AUC values, statistical data obtained for Lp-PLA2 measured in both the ACS and AIS groups show that the diagnostic performance of Lp-PLA2 was sufficient. Statistical results for optimum cut-off points showed that Lp-PLA2 can be more beneficial in the patients with ACS. The sensitivity and specificity of the optimal cut-off points were higher in the ACS group than in the AIS group. Although it was found that the levels of TnI and CK-MB were higher and the levels of Lp-LA2 were decreasing in the ACS group when compared with the control group, the fact that there was not any negative correlation made investigators think that independent factors had effect on those biomarkers. This was also true for CRP. In conclusion, a decrease in the acute stage Lp-PLA2 level in ACS and AIS patients may be used for diagnostic purposes in the emergency departments.
Funding
None declared.
Conflict of interest
None declared.
Footnotes
Peer review under responsibility of The Emergency Medicine Association of Turkey.
Contributor Information
Sedat Kocak, Email: drskocak06@hotmail.com.
Birsen Ertekin, Email: biceacil@hotmail.com.
Abdullah Sadik Girisgin, Email: sgirisgin@yahoo.com.
Zerrin Defne Dundar, Email: zerdef@hotmail.com.
Mehmet Ergin, Email: drmehmetergin@gmail.com.
Idris Mehmetoglu, Email: imehmetoglu@hotmail.com.
Said Bodur, Email: sbodur@hotmail.com.
Basar Cander, Email: basarcander@yahoo.com.
References
- 1.Zalewski A., Nelson J.J., Hegg L., Macphee C. Lp-PLA2: a new kid on the block. Clin Chem. 2006;52(9):1645–1650. doi: 10.1373/clinchem.2006.070672. [DOI] [PubMed] [Google Scholar]
- 2.The Lp-PLA2 Studies Collaboration Lipoprotein-associated phospholipase A2 and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375(9725):1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macphee C.H., Moores K.E., Boyd H.F., Dhanak D., Ife R.J., Leach C.A. Lipoprotein-associated phospholipase A2, platelet activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J. 1999;338:479–487. [PMC free article] [PubMed] [Google Scholar]
- 4.Garza C.A., Montori V.M., McConnell J.P., Somers V.K., Kullo I.J., Lopez-Jimenez F. Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin Proc. 2007;82(2):159–165. doi: 10.4065/82.2.159. [DOI] [PubMed] [Google Scholar]
- 5.Kara H., Akinci M., Degirmenci S., Bayir A., Ak A., Nayman A. High-sensitivity C-reactive protein, lipoprotein-related phospholipase A2, and acute ischemic stroke. Neuropsychiatr Dis Treat. 2014 Aug 6;10:1451–1457. doi: 10.2147/NDT.S67665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabor B., Choi H., Ruel I., Hafiane A., Mourad W., Genest J. Lipoprotein-associated phospholipase A(2) (Lp-PLA(2)) in acute coronary syndrome: relationship with low-density lipoprotein cholesterol. Can J Cardiol. 2013 Dec;29(12):1679–1686. doi: 10.1016/j.cjca.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 7.Pepys M.B., Gideon M.H. C-reactive protein: a critical update. J Clin Investig. 2003;111:1805–1811. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asanoa K., Okamotoa S., Fukunagaa K., Shiomia T., Moria T., Iwataa M. Cellular source(s) of platelet-activating-factor acetylhydrolase activity in plasma. Biochem Biophys Res Commun. 1999;261:511–514. doi: 10.1006/bbrc.1999.1066. [DOI] [PubMed] [Google Scholar]
- 9.Svetlov S.I., Howard K.M., Debuysere M.S., Olson M.S. Secretory PAF-acetylhydrolase of the rat hepatobiliary system: characterization and partial purification. Am J Physiol Gastrointest Liver Physiol. 1998;274:891–900. doi: 10.1152/ajpgi.1998.274.5.G891. [DOI] [PubMed] [Google Scholar]
- 10.De Gennaro L., Brunetti N.D., Cuculo A., Pellegrino P.L., Izzo P., Roma F. Increased troponin levels in nonischemic cardiac conditions and noncardiac diseases. J Interv Cardiol. 2008 Apr;21(2):129–139. doi: 10.1111/j.1540-8183.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K., Alpert J.S., Jaffe A.S., Simoons M.L., Chaitman B.R., White H.D. Third universal definition of myocardial infarction. Eur Heart J. Oct 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 12.Zalewski A., Macphee C.H. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005;25:923–931. doi: 10.1161/01.ATV.0000160551.21962.a7. [DOI] [PubMed] [Google Scholar]
- 13.Stephens C.J., Graham R.M., Sturm M.J., Richardson M., Taylor R.R. Variation in plasma platelet-activating factor degradation and serum lipids after acute myocardial infarction. Coron Artery Dis. 1993 Feb;4(2):187–193. doi: 10.1097/00019501-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Serebruany V.L., Gurbel P.A., Murugesan S.R., Lowry D.R., Sturm E., Svetlov S.I. Depressed plasma platelet-activating factor acetylhydrolase in patients presenting with acute myocardial infarction. Cardiology. 1998;90:127–130. doi: 10.1159/000006831. [DOI] [PubMed] [Google Scholar]
- 15.Elkind M.S.V., Leon V., Moon Y.P., Paik M.C., Sacco R.L. High-sensitivity C-Reactive protein and lipoprotein-associated phospholipase A2 stability before and after stroke and myocardial infarction. Stroke. 2009;40:3233–3237. doi: 10.1161/STROKEAHA.109.552802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tai W., Garcia M., Mlynash M., Kemp S., Albers G.W., Olivot J.M. Lipoprotein phospholipase A2 mass and activity are not associated with the diagnosis of acute brain ischemia. Cerebrovasc Dis. 2014 Nov 21;38(5):324–327. doi: 10.1159/000368218. [DOI] [PubMed] [Google Scholar]
- 17.Möckel M., Müller R., Vollert J.O., Müller C., Danne O., Gareis R. Lipoprotein-associated phospholipase A2 for early risk stratification in patients with suspected acute coronary syndrome: a multi-marker approach the north Wuerttemberg and Berlin infarction study-II (NOBIS-II) Clin Res Cardiol. 2007;96:604–612. doi: 10.1007/s00392-007-0540-x. [DOI] [PubMed] [Google Scholar]
- 18.Lanman R.B., Wolfert R.L., Fleming J.K., Jaffe A.S., Roberts W.L., Warnick G.R. Lipoprotein-associated phospholipase A2: review and recommendation of a clinical cut point for adults. Prev Cardiol. 2006;9:138–143. doi: 10.1111/j.1520-037x.2006.05547.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Donoghue M., Morrow D.A., Sabatine M.S., Murphy S.A., McCabe C.H., Cannon C.P. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (pravastatin or atorvastatin evaluation and infection therapy–thrombolysis in myocardial infarction. Trial Circ. 2006;113:1745–1752. doi: 10.1161/CIRCULATIONAHA.105.612630. [DOI] [PubMed] [Google Scholar]
- 20.Oldgren J., James S.K., Siegbahn A., Wallentin L. Lipoprotein-associated phospholipase A2 does not predict mortality or new ischaemic events in acute coronary syndrome patients. Eur Heart J. 2007;28:699–704. doi: 10.1093/eurheartj/ehl565. [DOI] [PubMed] [Google Scholar]