Abstract
Glucocorticoid (GC)-responsive epithelial–mesenchymal interactions regulate lung development. The GC receptor (GR) mediates GC signaling. Mice lacking GR in all tissues die at birth of respiratory failure. To determine the specific need for epithelial GR in lung development, we bred triple transgenic mice that carry SPC/rtTA, tet-O-Cre, and floxed, but not wild-type, GR genes. When exposed to doxycycline in utero, triple transgenic (GRepi−) mice exhibit a Cre-mediated recombination event that inactivates the floxed GR gene in airway epithelial cells. Immunofluorescence confirmed the elimination of GR in Cre-positive airway epithelial cells of late gestation GRepi− mice. Embryonic Day 18.5 pups had a relatively immature appearance with increased lung cellularity and increased pools of glycogen in the epithelium. Postnatal Day 0.5 pups had decreased viability. We used quantitative RT-PCR to demonstrate that specific elimination of epithelial immunoreactive GR in GRepi− mice is associated with reduced mRNA expression for surfactant proteins (SPs) A, B, C, and D; β- and γ-ENaC; T1α; the 10-kD Clara cell protein (CCSP); and aquaporin 5 (AQP5). Western blots confirmed reduced levels of AQP5 protein. No reduction in the levels of the GR transport protein importin (IPO)-13 was observed. Our findings demonstrate a requirement for lung epithelial cell GR in normal lung development. We speculate that impaired epithelial differentiation, leading to decreased SPs, transepithelial Na, and liquid absorption at birth, may contribute to the reduced survival of newborn mice with suppressed lung epithelial GR.
Keywords: perinatal lung development, conditional knockout, epithelial–mesenchymal interactions
CLINICAL RELEVANCE
This work demonstrates that glucocorticoid signaling in the respiratory epithelium plays a vital role in lung maturation. This sets the stage for the identification of the process and the targets by which it does so, which are likely to be of relevance to chronic lung disease of prematurity and its long-term sequelae.
Glucocorticoid (GC) hormones regulate a wide range of physiological processes in the developing lung, including cytodifferentiation (1, 2), branching morphogenesis (3), vasculogenesis (4), and alveolarization (5). The effects of GCs are mediated by the GC receptor (GR, Nr3c1), a prototype member of the nuclear receptor superfamily of transcriptional regulators. The absence of GC (2, 6) or of GR (7, 8) delays mammalian lung development, leading to respiratory insufficiency at birth. Prominent among GC-GR responsive developmental processes in the lung are those involving interactions between cell types, particularly interactions between the epithelium and mesenchyme (9), but our understanding of the temporal–spatial requirements for GC-GR remains incomplete.
Based on studies of mice deficient in GR in all tissues, Cole and colleagues have suggested that GR activation is not essential for differentiation of airway epithelial cells (AECs) into the type-II cell phenotype that synthesizes, stores, and secretes pulmonary surfactant. Their findings pointed to a role for GR in regulating lung cell proliferation and lung structural development, and in enhancing the differentiation of primordial AECs (10). More recent evidence from their group suggests that glucocorticoid signaling regulates hypercellularity of the fetal lung by affecting genes involved specifically in proliferation but not in apoptosis (11).
GCs act on the pulmonary mesenchyme to influence maturation in the respiratory epithelium (reviewed in Ref. 9). Little information is available regarding the potential role of epithelial-specific GC-GR signaling in lung maturation. Interestingly, we showed that expression in GR-deficient mice of a functional rat GR under the control of a human epithelial surfactant protein (SP)-C promoter is not sufficient to restore neonatal viability (12). These findings suggested that GC-GR signaling specifically in AECs may not be essential to the development of viable lung function at birth.
To test the hypothesis that epithelial-specific disruption of GC-GR signaling in vivo would inhibit fetal lung development, we bred pre-existing transgenic mice to create a new line of triple transgenic mice in which exposure to doxycycline (dox) suppresses GR expression in AECs (GRepi− mice). As indicators of the adequacy of lung development, we compared newborn GRepi− mice at birth with littermate controls with respect to the presence of respiratory distress, viability, and lung wet:dry weight ratio. In addition, in late gestation (Embryonic Day [E]18.5) GRepi− mice we evaluated measures of the degree of lung cellularity, epithelial cell differentiation (surfactant protein mRNA levels, glycogen content, fluid and electrolyte channels), and markers of airway cell sub-types.
MATERIALS AND METHODS
Generation of Triple Transgenic Mice
Three existing lines of mice were crossed, as illustrated in Figure E1 in the online supplement. Mice A and B were bred to give double transgenic Mouse C (a kind gift from J. Whitsett, Cincinnati Children's Hospital Medical Center). Briefly, in Mouse A (13) the SP-C promoter directs expression of the dox-inducible reverse tetracycline transactivator (rtTA) exclusively in AECs. Mouse B (14) has a (tetO)7-CMV promoter that regulates the expression of Cre recombinase (Cre). In Mouse C, dox induces rtTA, which binds to the tet-O sequence, activating Cre expression (13). Mouse D has a “floxed” GR allele in the place of the wild-type (WT) GR sequence. This was created by knocking the floxed allele into the normal GR locus (15). The floxed GR contains two loxP sites that flank exons 1c and 2 of the GR sequence; in the presence of Cre recombinase the loxP sites are recognized and excised in a recombination event. Mice B and D were bred to give our novel triple transgenic Mouse E, designed to suppress AEC GR expression in the presence of dox (GRepi− mice).
GRepi− Mice
Animals were housed (on a 12 h:12 h light:dark cycle with ad libitum access to rodent chow and water) and bred in the Lab Animal Services of the Hospital for Sick Children (HSC; Toronto, ON, Canada). All procedures were in accordance with the Canadian Council on Animal Care and were approved by the HSC Animal Care Committee. The presence of a vaginal plug was recorded as E0.5. Dox was administered to dams in the food at a concentration of 600 to 625 mg/kg (Haralan Teklad, Madison, WI) from E6.5 to E18.5 and was changed every 2 days due to light sensitivity. After pregnant dams were killed by diethyl ether excess, their fetuses were delivered by hysterectomy on E18.5. Pups were genotyped by polymerase chain reaction (PCR) looking for four gene products: SPC-rtTA, (tetO)7-Cre, WT GR, and floxed GR, using the primer sequences given in Table E1. PCR products were electrophoretically separated on a 1.5% agarose gel with ethidium bromide.
To establish epithelial-specific suppression of GR and to determine its effect on survival of newborn pups, GRepi− mice were compared exclusively with dox-treated littermates that were heterozygous for WT and floxed GR, while expressing both SPC-rtTA and (tetO)7-Cre.
Tissue Collection and Lung Wet:Dry Weight Ratio
Newborn lungs were excised, inflated, fixed, and processed as previously described (16) after note was taken of the respiratory status at birth. Lungs of E18.5 pups were not inflated. Coronal sections 5 μm thick were cut, mounted on coated glass slides, and then stored at room temperature until needed for analysis. For lung wet:dry weight ratios, the Postnatal Day (PN)0.5 lungs were removed from the chest cavity en bloc and immediately weighed. They were then placed in a 60°C oven, and weighed every 24 hours until no change in weight was observed.
Immunofluorescence
Staining was performed according to a standard protocol obtained from Jackson ImmunoResearch Laboratories (Mississauga, ON, Canada) for detection of two unlabeled primary antibodies from the same host species (http://www.jacksonimmuno.com/technical/examplec.asp). Briefly, tissue sections were dewaxed in xylene, rehydrated through a decreasing ethanol series (100% to 70%), and then washed in 1× PBS/0.03% (vol/vol) Triton-100-X. Tissue permeabilization was achieved by boiling in 10 mM sodium citrate (pH 6) for 15 minutess at 95°C, and then cooling at room temperature for 30 minutes. Nonspecific antibody binding was blocked by incubation with solution containing 10% (vol/vol) normal donkey serum (Jackson ImmunoResearch Laboratories) and 1% (vol/vol) bovine serum albumin in PBS at room temperature for 1 hour. Primary antibodies used were anti-GR rabbit polyclonal antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and anti-Cre rabbit polyclonal antibody (1:200; Novagen, Madison, WI) for 1 hour at room temperature. Secondary antibodies used were donkey anti-rabbit IgG conjugated to either Cy3 (1:400) or Alexa-488 (1:450; Jackson ImmunoResearch Laboratories) for 30 minutes at room temperature.
Tissue to Airspace Ratio and Periodic Acid Schiff Staining for Glycogen
Left lobe sections were dewaxed in xylene and rehydrated through a decreasing ethanol series, and then stained using Periodic Acid Schiff (PAS; Surgipath, Richmond, IL) for presence of glycogen according to the manufacturer's instructions, or with hematoxylin and eosin to determine tissue to airspace ratios as previously described by our lab (12).
Image Acquisition
Images from fluorescently labeled tissue were digitally captured using a Leica (Heerbrugg, Switzerland) DMIRE2 inverted fluorescence microscope equipped with a Hamamatsu (Hamamatsu City, Japan) Back-Thinned EM-CCD Camera. Images were viewed at ×400 magnification and analyzed using Volocity Acquisition software (PerkinElmer, Waltham, MA). Light microscope images were digitally captured at ×250 magnification using a light microscope camera (Leica DC 200) with a video interactive display system (Image Manager Version 1.20; Leica).
RNA Isolation and Real-Time PCR Assays
Total RNA was isolated from frozen lung samples using the Qiagen RNeasy Mini Column extraction kit (Qiagen, Inc., Mississauga, ON, Canada), treated with DNase DNA-free kit (Ambion, Austin, TX) according to manufacturer's instructions, and then reverse transcribed with SuperScript II reverse transcriptase (Invitrogen, Burlington, ON, Canada). A quantity of 5 ng of template cDNA (0.01 ng for 18S) was used for quantitative real-time PCR (ABI Prism 7900; Appled Biosystems by Life Technologies, Carlsbad, CA) using SYBR Green in conjunction with murine gene specific primer sets, or Taqman Assays.
Primer sequences are listed in Table E2. Differences in mRNA levels were compared between groups after normalization to 18S rRNA. Relative expression was calculated using the using the 2−(ΔCt(exp)−ΔCt(control)) method. Fold change was calculated according to Livak and Schmittgen (17). Data from real-time RT-PCR are presented as mean 2−ΔΔCt ± SEM. With respect to mRNA levels for a subset of the genes studied (GR, AQP5, and SPB), GRepi− mice (n = 4) were compared with dox-treated littermate controls that expressed rtTA (n = 6) or not (n = 8). In each case, tested mRNA levels were significantly reduced in GRepi− mice compared with both control groups (P < 0.05), but no significant differences were detected between the two control groups (Figure E2). Therefore, for all other tested genes, mRNA level levels were compared between GRepi− mice and dox-treated littermate controls lacking rtTA.
Western Blot
Measurement of protein expression in right lung lobes was performed as previously described (12). Equal amounts of protein were loaded on 7% resolving (for Cre) or 12.5% resolving (for AQP5 or IPO13) SDS-PAGE. Primary antibodies used were rabbit polyclonal anti-Cre antibody (1/10,000), AQP5 antibody (1/500; Alomone, Jerusalem, Israel), IPO13 antibody (1/5,000; Covance, Princeton, NJ) or GAPDH (1/4,000; Santa Cruz Biotechnology, Santa Cruz, CA). For all Western blot analyses, protein levels were quantitated by densitometry analysis on a Fluorchem 8000 Gel Documentation System (Alpha Innotech Corporation, San Leandro, CA) using Alpha Innotech software, and normalized to the expression of GAPDH or βactin.
Statistical Analysis
Statistical analyses were made using Sigma Stat software ver 2.0 (Jandel Scientific, Chicago, IL). Comparisons between two groups involved either Student's t test or a Mann-Whitney U test (for data that failed a normality test). Neonatal viability was compared using Fisher's Exact test. If data passed a normality test, comparisons between GRepi− mice and multiple control groups (with or without expression of rtTA) were made using ANOVA. In cases of overall statistical significance, post hoc pairwise comparisons between groups were then done using the Holm-Sidak test. When data failed a normality test, comparisons were made using Kruskal-Wallis One-Way Analysis of Variance on Ranks, followed in cases of overall significance by post hoc pairwise comparisons between groups using Dunn's method. P < 0.05 was considered to be statistically significant.
RESULTS
Dox Suppresses GR in AECs of GRepi− Mice
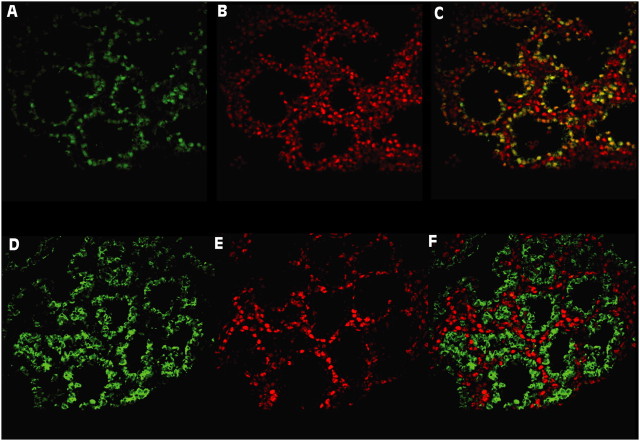
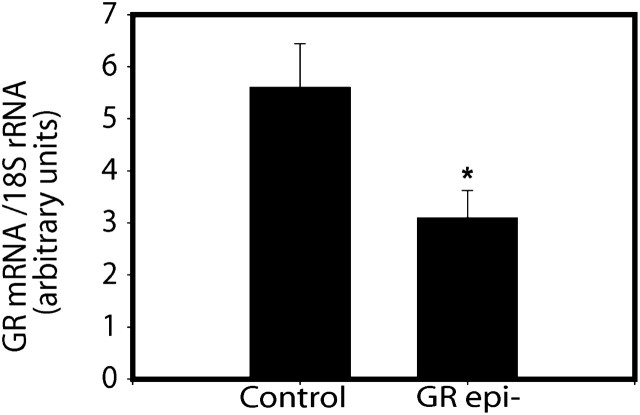
We determined the effect of dox on GR protein in the AECs of fetal triple transgenic mice. Pregnant mice received dox in their regular food from E6.5 (before the onset of lung formation) until E18.5. Immunoreactive Cre recombinase protein expressed under the control of the SP-C promoter was used in immunofluorescence histology as a marker of AECs in the lungs of dox-treated triple transgenic (GRepi−) mice (homozygous for floxed GR alleles; Figure E3) and in control littermates (have a WT GR allele) at E18.5. Cre recombinase could be detected by Western blot on whole lung protein only in those dox-treated mice that carried both rtTA and (tet-O)7-Cre (Figure E4). After dox treatment, there was extensive epithelial colocalization of GR immunoreactivity with the Cre in the controls, but no epithelial colocalization was seen in the GRepi− mice (Figure 1), indicating widespread and substantial suppression of GR protein in the AECs of GRepi− mice. Consistent with this finding, the GR mRNA levels in whole lungs of GRepi− mice was lower than in controls (Figure 2).
Figure 1.
Epithelial-specific suppression of glucocorticoid receptor (GR) in lungs of triple transgenic (GRepi−) mice. Immunofluorescence for Cre (A and D; green), GR (B and E; red) and both (C and F). All lungs from E18.5 fetuses of dox-treated females. (A–C) show tissue sections of lungs from fetuses that express wild-type (WT) GR. (D–F) show tissue sections of lungs from fetuses that express floxed, but not WT, GR. All express Cre exclusively in alveolar epithelial cells (AECs). Cre and GR colocalize (yellow) in C (control), but there is no colocalization in F (GRepi−), reflecting widespread suppression of GR expression in AECs.
Figure 2.
GR mRNA levels are reduced in lungs of Embryonic Day (E)18.5 GRepi− fetuses. Values normalized relative to 18S rRNA. Mean ± SEM; n = 10 in each group. *P < 0.05, t test.
Increased Neonatal Mortality
Because GC-responsive genes are known to have a major impact upon lung development, we speculated that suppression of GR beginning before the onset of lung formation and continuing throughout gestation would delay lung development and thus compromise neonatal respiratory status and viability. Indeed, compared with littermate controls, GRepi− mice had a significantly increased mortality at PN0.5 (Table 1), due to respiratory insufficiency.
TABLE 1.
NEONATAL VIABILITY
|
GRepi− |
||||
|---|---|---|---|---|
| All dox-Treated |
Littermate Controls |
Observed |
(Expected) |
|
| Dead | 2 | 24 | (7) | |
| Alive |
11 |
20 |
(37) |
|
GRepi− newborns (triple transgenic Postnatal Day 0.5 pups of dox-treated females) had significantly decreased viability compared with littermates that were heterozygote for wild-type and floxed glucocorticoid receptor while expressing both surfactant protein C–rtTA and (tetO)7-Cre. P = 0024, Fisher Exact test.
Delayed Lung Development—Increased Lung Cellularity
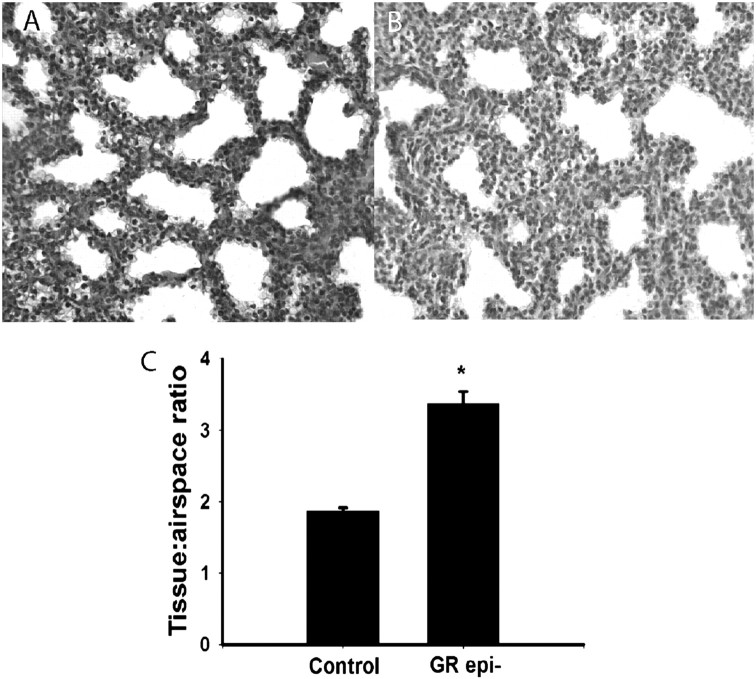
We examined a variety of aspects of lung development to assess the implications of GR deficiency in AECs. Increased lung cellularity at term is a hallmark of delayed lung development found in GR knockout (KO) mice (7, 10, 16) due to impaired glucocorticoid regulation (11). To determine whether GR activity in AECs is required for the normal reduction in cellularity during late gestation, we evaluated the ratio of tissue to airspace in lung sections at E18.5. GRepi− lungs had more tissue than littermate controls (Figure 3), confirming a requirement for GR in AECs.
Figure 3.
Epithelial GR suppression is associated with a less mature histologic appearance than controls. Lung cellularity and septal thickness were increased in GRepi− mice (B) versus Control (A). Tissue to airspace ratio (a measure of tissue cellularity inversely related to histological maturity) is higher in GRepi− than in Control mice (C). *P < 0.05, n = 4.
Delayed Lung Development—Reduced Epithelial Differentiation
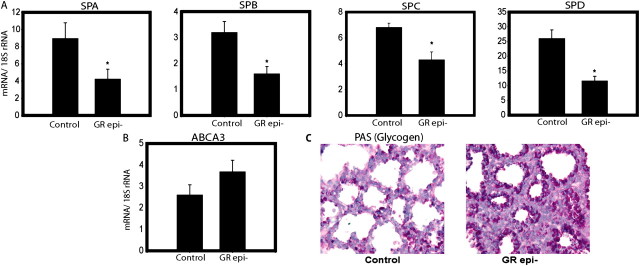
Critical components of late gestation lung development are features of AEC differentiation, including (type II cells) production of SPs, metabolism of glycogen, and absorption of airway liquid electrolyte (especially Na+) and (type I cells) water. SP genes are GC- responsive (3, 17). Consistent with delayed AEC differentiation in GRepi− mice, we observed an approximately 2-fold decrease in mRNA for SP-A, SP-B, and SP-D and a 1.5-fold decrease in SP-C (Figure 4A; n = 10 in each group, P < 0.05). No significant change was detected in mRNA levels of ABCA3 (involved in lamellar body formation and surfactant homeostasis; Figure 4B).
Figure 4.
Surfactant protein mRNA levels are reduced and metabolism of glycogen stores is delayed in GRepi− mice. Lungs of E18.5 GRepi− mice, compared with littermate controls, have (A) reduced mRNA levels of surfactant proteins (SPs)-A, -B, -C, and -D, but not (B) ABCA3; and (C) increased histochemical staining for Periodic Acid Schiff (glycogen, dark purple). All mRNA levels normalized to18S rRNA levels, in arbitrary units. Mean ± SEM, n = 10 in each group. *P < 0.05, Student's t test or Mann-Whitney U-test as appropriate.
Undifferentiated lung epithelium is characterized by abundant pools of glycogen (reviewed in Ref. 18). Sections of whole lung from E18.5 GRepi− mice showed increased PAS (and thus glycogen) staining in the epithelium, further reflecting impaired AEC differentiation (Figure 4C).
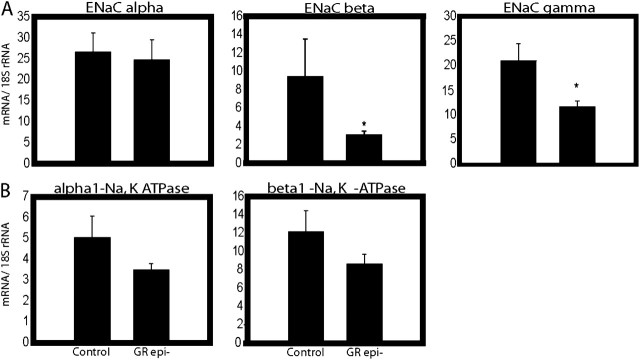
At birth, GCs play a role in the switch of the mammalian lung from secretion of Cl− and fluid to absorption of Na+ and fluid (19). Transepithelial absorption of Na+ from the airspace is mediated by the apical epithelial sodium channel (ENaC), a complex of multiple subunits. In the lungs of GRepi− mice, there was a statistically significant (2-fold) decrease in ENaC β and γ, but not α, subunits (Figure 5A). There was no significant difference in mRNA levels of either of the α1- or β1-components of the basolateral Na+-K+-ATPase (Figure 5B).
Figure 5.
Electrolyte clearance: epithelial Na channel (ENaC) subunits are reduced in GRepi− mice. Lungs of E18.5 GRepi− mice, compared with controls, had (A) reduced levels of mRNA encoding ENaC β and γ, but not α, subunits; (B) no differences in mRNA for Na+, K+ ATPase α and β subunits. All mRNA levels normalized to 18S rRNA levels, in arbitrary units. Mean ± SEM, n = 10 in each group. *P < 0.05, Student's t test or Mann-Whitney U-test as appropriate.
Reduced Markers of Epithelial Type I Cells and Proximal AECs
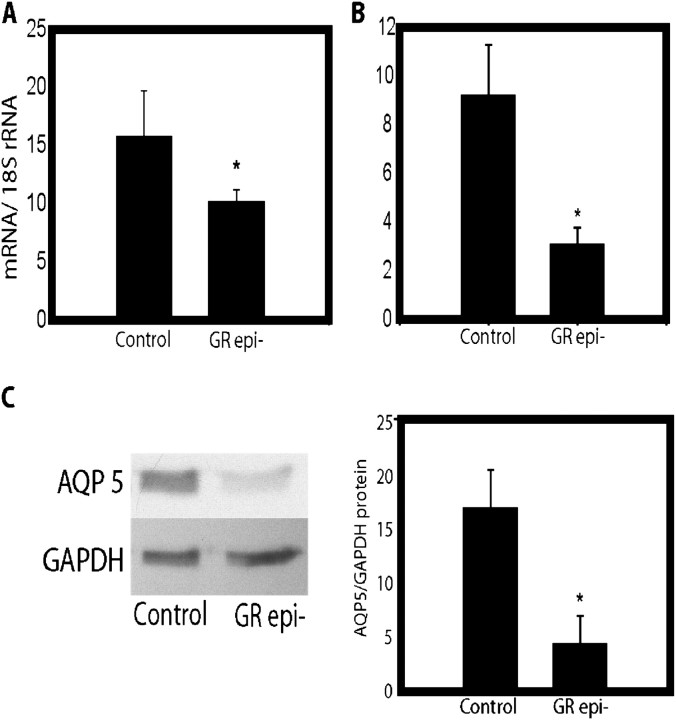
Given that type I AECs represent the final stage of epithelial cell differentiation, we evaluated whether GRepi− mice have impaired type I cell differentiation. mRNA levels of the type I cell marker T1α (20) were reduced by approximately 1.5-fold (Figure 6A), and there was also a decrease in protein and mRNA levels of another type I cell marker, the water channel AQP5 (21) (Figure 6B). However, at PN0.5 no differences were detected between GRepi− mice and littermate controls in lung wet:dry weight ratio, a measure of tissue water content (data not shown).
Figure 6.
Epithelial cell markers are reduced in GRepi− mice. Lungs of E18.5 GRepi− mice had reduced mRNA levels for the epithelial type I cell markers (A) T1 α and (B) the water channel aquaporin (AQP) 5. (C) AQP5 protein by Western blot was also reduced. All mRNA levels normalized to18S rRNA levels, in arbitrary units. Mean ± SEM, n = 10 in each group. *P < 0.05, Student's t test or Mann-Whitney U-test as appropriate.
There was also about a 2-fold reduction in mRNA levels for the predominantly bronchiolar (proximal) epithelium Clara cell marker CCSP (22) (data not shown).
Epithelial Transcription Factors
AECs, among other cell types in the developing lung, secrete transcription factors that regulate lung development (reviewed in Ref. 23). No difference was detected in the mRNA levels of two important epithelial transcription factors, Ttf1 and Foxa2, in GRepi− versus control mice (data not shown).
Products of GC-responsive genes that are not epithelial-specific.
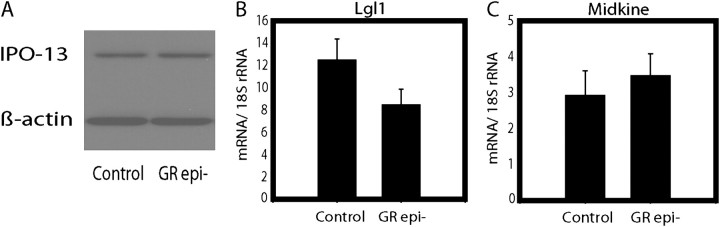
We assessed fetal lung for the levels of products of three GC-responsive genes that are abundantly expressed in nonepithelial tissues: the GR transport protein IPO-13 (24), the mesenchymal regulator of AECs Lgl1, and the retinoid-responsive midkine (which we demonstrated in earlier work is inhibited by GC [25]). We found significant differences neither in IPO13 protein nor in mRNA levels of LGL1 or midkine in GRepi− mice compared with littermate controls (Figure 7).
Figure 7.
The levels of products of three GC-responsive genes that are not epithelial-specific are unaltered in whole lungs of GRepi− mice. (A) Levels of protein (IPO13) by Western blot or mRNA ([B] late gestation lung 1 (Lgl1) and [C] midkine) were not altered in newborn GRepi− mice whole lung. P not significant, n = 9.
DISCUSSION
To assess the requirement of the developing lung for GC-GR in AECs in vivo, we bred a new line of triple transgenic mice that exhibit an AEC-specific suppression of GR expression after exposure to dox (GRepi− mice). In late-gestation GRepi− mice, whole lung GR mRNA levels are lowered and the levels of GR protein in AECs are suppressed to below the limits of detection by immunofluorescence. At birth, GRepi− mice have physical signs of respiratory insufficiency associated with an increased mortality (∼ 50%) compared with identically treated littermate controls. Our findings demonstrate that there is a specific need in lung development for GR in antenatal AECs.
Bi-directional mesenchymal–epithelial cross-talk has been implicated in mediating GC-GR stimulation in mammalian lung development (4), but to date most attention has been paid to the GC-responsive influence of mesenchyme upon epithelium (9). Indeed, it has been suggested that the important regulation of lung development resides in the mesenchyme, not the epithelium (26). To our knowledge, our present model presents the first opportunity to assess the effect on lung development of knocking out GC-GR signaling specifically in the epithelial cells while maintaining normal GR expression in all other tissues. As such, it should simplify the analysis of a highly complex set of developmental regulatory interactions in vivo.
Elimination of either GC or GR effectively disrupts GC-GR signaling. Mice carrying a targeted disruption of the corticotrophin-releasing hormone (CRH) gene (6) that are born of CRH KO mothers (no trans-placental GC stimulation) lack GC-GR signaling due to the absence of endogenous GC and die of respiratory insufficiency at birth (2). Mice have been reported with distinct GR (Nr3c1) gene disruptions in all cell types of all organs. Insertion of a neomycin cassette into the second exon created a hypomorphic (partial loss of function, hypo) allele. GRhypo mice, homozygous for the hypo allele, express an mRNA species encoding a truncated protein (27, 28) that retains the capacity to regulate gene expression both positively and negatively in a ligand-responsive manner (29). Another GR gene disruption reported by Tronche and colleagues consists of a deletion of the third exon that removes the first Zn-finger of the DNA-binding domain, which is thought to lead to a complete inactivation of the gene (8). A GR KO mouse caused by targeted disruption of exons 1C and 2 (7) has an apparently identical phenotype, dying within 24 hours of birth of respiratory insufficiency with histologic evidence of delayed lung maturation and no detectable GR protein. All GR KO mice and most GRhypo mice (12, 27, 28) also display respiratory distress and die within hours of birth. Our present observation of a 50% mortality rate in newborn GRepi− mice pups, despite having intact mesenchymal GR expression, indicates that mesenchymal GC-GR signaling alone is not sufficient for normal fetal lung development.
Evidence suggesting that administration of dox to mice expressing rtTA may in certain strains have nonspecific toxic effects leading to suppression of mRNA levels for a variety of genes has been reviewed recently (30). The authors have argued for caution in the interpretation of the results of studies using the SPC-rtTA, (tetO)7-Cre system to selectively suppress epithelial expression of floxed genes. In our present study, no evidence was found of any difference between control groups with or without rtTA expression in levels of mRNA for GR or for the epithelial genes AQP5 and SP-B, indicating the observed differences in these mRNA levels between controls and GRepi− may be attributed to selective suppression of GR expression in lung epithelial cells rather than to nonspecific rtTA toxicity. Indeed, the viability of newborn GRepi− mice was significantly reduced when compared with littermate controls that also express rtTA under the influence of an SP-C promoter, demonstrating that this difference, too, cannot be attributed to nonspecific rtTA toxicity.
The exact cause of lowered neonatal viability in GR KO and CRH KO is not completely understood, although it could be due to a combination of factors. Theoretical possibilities include impaired epithelial cell differentiation leading to decreases in surfactant lipids, reduction in SPs, impairment of fluid transport, and increased barriers to diffusion and thus gas exchange. The undifferentiated epithelium is characterized by large pools of glycogen, thought to be used a carbon source for surfactant lipids (31) as well as a form of stored energy for rapid surfactant phospholipid synthesis close to term. Before the commencement of surfactant lipid synthesis, glycogen stores increase dramatically (32) and peak before term, and then rapidly fall to low levels at birth, coincident with an increase in pulmonary phospholipid synthesis and maturation of the pulmonary epithelium (32).
The augmented production of surfactant by late gestation lung in response to GCs has been recognized for many years (33). Production of the essential phospholipid components of surfactant by epithelial type II cells does not respond to GCs directly, but rather through paracrine stimulation by soluble factors secreted by the mesenchyme (33, 34). Thus a deficiency of surfactant phospholipids is unlikely to explain the neonatal mortality of our present mice. On the other hand, epithelial production of the important SPs is responsive to direct GC-GR stimulation in vitro (3). Although the transcription factors Ttf-1 (reviewed in Ref. 35) and FoxA2 (36) have been implicated in the regulation of SPs, their mRNA levels were not changed in GRepi− mice, consistent with the suggestion that the observed changes in SP mRNA levels were due to direct effects of GC. In GRepi− mice there was no change in mRNA levels of ABCA3, a protein important in lamellar body (LB) formation (37, 38). SP-B levels were significantly reduced in GRepi− mice, consistent with reports by one group that found decreases in SP-B mRNA in E18.5 CRH KO mice (39), while others saw a decrease at E17.5 but not E18.5 (2). Mice with a targeted mutation in the gene for SP-A, SP-D, or SP-C, resulting in complete protein deficiency without other abnormalities in lung morphology, do not develop respiratory distress in the neonatal period under basal conditions (40–42). SP-B KO mice on the other hand do succumb to respiratory insufficiency in the neonatal period (43). Given the established importance of the SPs to overall surfactant function and hence neonatal viability, it is conceivable that modest reduction in all SPs and especially SP-B may in part reduce neonatal viability similar to SP-B KO mice.
The secretion of lung liquid plays a critical role in the development of the fetal lung (44). Withdrawal of this liquid from the developing airways permits the transition to breathing air after birth (45), and fluid retention is a characteristic feature of neonatal respiratory distress syndrome (46). The epithelial sodium channel (ENaC), composed of α, β, and γ protein subunits, contributes to the induction of an absorptive phenotype in the perinatal lung epithelium that is crucial to the successful transition to air-breathing at birth. Normally the rate-limiting step in transepithelial Na+ transport, ENaC activity is modulated by GC (47). In GR KO mice, mRNA levels for the ENaC α subunit did not differ from controls, but mRNA levels for the β and γ subunit did (10). Given the epithelial localization of ENaC, it was not surprising that we also detected significant decreases in mRNA levels of both the β and γ, but not α, subunits of ENaC in the lungs of GRepi− mice. In addition, there were reductions in protein and mRNA levels of AQP5 (a water channel that may play a role in the high water permeability of ATI cells [48]).
We were unable to demonstrate a statistically significant difference in lung wet weight:dry weight ratios between control and GRepi− newborn mice, but the relatively low statistical power of our study suggests that this negative finding should be interpreted with caution. Na+-K+-ATPases are also a vital part of the fluid removal mechanism in the lung. The mRNA levels of these channels did not differ significantly between control and GRepi− mice.
We (12, 16) and others (10) have found delayed histologic maturation in GR KO and nonsurviving GRhypo mice. Studies of CRH KO (2) and GR KO (11) mice indicate that hypercellularity, failure of septal thinning, and immature structural formation of the airways are the result of abnormally prolonged periods of cell proliferation rather than a failure of timely apoptosis. Bird and coworkers (11) pointed out that our report of transgenic expression of GR in AECs on a GRhypo background, reducing lung cellularity to levels comparable to WT littermates (12), suggests that GC-stimulated signaling between the epithelium and the mesenchyme is likely to be necessary for reduced cell proliferation. This concept is not surprising, given that it has been recognized for some time that respiratory epithelium can regulate the function of a number of cell types (49). Like the hypercellularity reported in CRH KO, GR KO, or even GRhypo mice, our GRepi− mice show increased lung cellularity (as measured by tissue to airspace ratio). However, the degree of the increase in our present mice appears to be somewhat less dramatic. This could be due in part to an incomplete suppression of AEC GR or, alternatively, GC-GR signaling capable of exerting a biologically significant effect upon the proliferation may be located in nonepithelial as well as epithelial cell types, which could account for both our previous (12) and present findings.
GRepi− mice had reductions in the mRNA levels of ATI (AQP5 and T1α) cell markers, consistent with the findings of Cole and colleagues (10) that the lungs of GR KO mice exhibit a decrease in the number of type I AECs. Our findings suggest that AEC differentiation may be affected by a lack of GR in lung epithelial cells. Cole and coworkers suggested that GC-GR signaling facilitates the differentiation of epithelial cells into type I cells, but is not obligatory for type II cell differentiation (10). This is consistent with E18.5 GRhypo mice, where there is an increase in ATII and undifferentiated AEC, and a decrease in ATI cells, indicating that GCs are not required for ATII differentiation but are essential for ATI differentiation (10).
Levels of mRNA for CCSP were decreased in GRepi− mice. Although there can be some overlap in the distribution (proximal versus distal) of CCSP and the distal AEC marker SP-C, the degree of overlap is not large, suggesting that an indirect, perhaps paracrine, relationship may exist between distal and proximal AECs that modulates the activity of the proximal phenotype in response to GC. Alternatively, administration of dox as early in gestation as E6.5 may suppress GR expression before even proximal airway epithelial cells develop.
We tested for GC-GR–responsive AEC modulation of mesenchymal gene expression. We have previously reported the cloning, preliminary characterization, and distribution of expression of Lgl1, a highly GC-responsive gene. The LGL1 protein product is synthesized in mesenchyme, secreted, associates with AECs, and modifies various epithelial functions at different stages in lung development (50–52). Since Lgl1 mRNA levels were not altered in GRepi− mice, we could not support the possibility that GC-GR signaling in AECs may modulate Lgl1 expression. Although we have previously reported an inhibition by GC of the retinoid-responsive midkine in fetal lung (25), there was no significant difference between in GRepi− and control mice, thus failing to support the possibility of AEC modulation of midkine expression.
In summary, we provide evidence of a specific need for GR in AECs during murine lung development. We observed the elimination of GR exclusively in AECs of triple transgenic mice pups at E18.5 following in utero exposure to dox (GRepi− mice), resulting in a marked reduction in newborn viability due to respiratory insufficiency. Multiple findings of delayed lung maturation in perinatal GRepi− mice included increased lung tissue cellularity and epithelial glycogen coincident with reduced mRNA levels for surfactant proteins, type I cell markers, and major channels of transepithelial salt and water transport. These results point to a greater importance of the role of epithelial GC-responsive genes in lung development than has been previously appreciated.
This work was supported by operating grants from the Canadian Institutes of Health Research to N.B.S. and F.K.; and by graduate scholarships to N.M. from the Natural Sciences and Engineering Research Council of Canada and the Research Training Centre, Hospital for Sick Children, Toronto, and to S.C. from the Montreal Children's Hospital Research Institute.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rccm.2009-0263OC on December 30, 2009
Author Disclosure: L.M. received a research grant from Pfizer for more than $100,001 and a sponsored grant from the National Institutes of Health for more than $100,001. F.K. received a sponsored grant from Canadian Institute of Health Research for more than $100,001. N.B.S. received more than $100,001 from both the Canadian Institutes of Health Research and the Canadian Cystic Fibrosis Foundation as an investigator. He has a spouse/life partner employed by the Canadian Cystic Fibrosis Foundation. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Guettari N, Dufour ME, Marin L. Effects of the antiglucocorticoid RU 486 on the initiation of ultrastructural type-II cell differentiation in fetal rat lung. Biol Neonate 1990;58:173–180. [DOI] [PubMed] [Google Scholar]
- 2.Muglia LJ, Bae DS, Brown TT, Vogt SK, Alvarez JG, Sunday ME, Majzoub JA. Proliferation and differentiation defects during lung development in corticotropin-releasing hormone-deficient mice. Am J Respir Cell Mol Biol 1999;20:181–188. [DOI] [PubMed] [Google Scholar]
- 3.Oshika E, Liu S, Ung LP, Singh G, Shinozuka H, Michalopoulos G, Katyal SL. Glucocorticoid-induced effects on pattern formation and epithelial cell differentiation in early embryonic rat lungs. Pediatr Res 1998;43:305–314. [DOI] [PubMed] [Google Scholar]
- 4.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev 2000;92:55–81. [DOI] [PubMed] [Google Scholar]
- 5.Tschanz SA, Damke BM, Burri PH. Influence of postnatally administered glucocorticoids on rat lung growth. Biol Neonate 1995;68:229–245. [DOI] [PubMed] [Google Scholar]
- 6.Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature 1995;373:427–432. [DOI] [PubMed] [Google Scholar]
- 7.Brewer JA, Kanagawa O, Sleckman BP, Muglia LJ. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J Immunol 2002;169:1837–1843. [DOI] [PubMed] [Google Scholar]
- 8.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 1999;23:99–103. [DOI] [PubMed] [Google Scholar]
- 9.Whitsett JA, Matsuzaki Y. Transcriptional regulation of perinatal lung maturation. Pediatr Clin North Am 2006;53:873–887. (viii.). [DOI] [PubMed] [Google Scholar]
- 10.Cole TJ, Solomon NM, Van Driel R, Monk JA, Bird D, Richardson SJ, Dilley RJ, Hooper SB. Altered epithelial cell proportions in the fetal lung of glucocorticoid receptor null mice. Am J Respir Cell Mol Biol 2004;30:613–619. [DOI] [PubMed] [Google Scholar]
- 11.Bird AD, Tan KH, Olsson PF, Zieba M, Flecknoe SJ, Liddicoat DR, Mollard R, Hooper SB, Cole TJ. Identification of glucocorticoid-regulated genes that control cell proliferation during murine respiratory development. J Physiol 2007;585:187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gagnon S, Atmodjo W, Humes D, McKerlie C, Kaplan F, Sweezey NB. Transgenic glucocorticoid receptor expression driven by the SP-C promoter reduces neonatal lung cellularity and midkine expression in GRhypo mice. Biol Neonate 2006;90:46–57. [DOI] [PubMed] [Google Scholar]
- 13.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res 2002;11:21–29. [DOI] [PubMed] [Google Scholar]
- 14.Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res 2005;33:e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewer JA, Khor B, Vogt SK, Muglia LM, Fujiwara H, Haegele KE, Sleckman BP, Muglia LJ. T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat Med 2003;9:1318–1322. [DOI] [PubMed] [Google Scholar]
- 16.Nemati B, Atmodjo W, Gagnon S, Humes D, McKerlie C, Kaplan F, Sweezey NB. Glucocorticoid receptor disruption delays structural maturation in the lungs of newborn mice. Pediatr Pulmonol 2008;43:125–133. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 18.Copland I, Post M. Lung development and fetal lung growth. Paediatr Respir Rev 2004;5:S259–S264. [DOI] [PubMed] [Google Scholar]
- 19.Wallace MJ, Hooper SB, Harding R. Effects of elevated fetal cortisol concentrations on the volume, secretion, and reabsorption of lung liquid. Am J Physiol 1995;269:R881–R887. [DOI] [PubMed] [Google Scholar]
- 20.Williams MC, Cao Y, Hinds A, Rishi AK, Wetterwald A. T1 alpha protein is developmentally regulated and expressed by alveolar type I cells, choroid plexus, and ciliary epithelia of adult rats. Am J Respir Cell Mol Biol 1996;14:577–585. [DOI] [PubMed] [Google Scholar]
- 21.Ben Y, Chen J, Zhu R, Gao L, Bai C. Upregulation of AQP3 and AQP5 induced by dexamethasone and ambroxol in A549 cells. Respir Physiol Neurobiol 2008;161:111–118. [DOI] [PubMed] [Google Scholar]
- 22.McDowell EM. Patterns of proliferation and differentiation during fetal development of the airway epithelium. Anat Rec 1993;236:11–13. [DOI] [PubMed] [Google Scholar]
- 23.Whitsett J. A lungful of transcription factors. Nat Genet 1998;20:7–8. [DOI] [PubMed] [Google Scholar]
- 24.Tao T, Lan J, Presley JF, Sweezey NB, Kaplan F. Nucleocytoplasmic shuttling of Lgl2 is developmentally regulated in fetal lung. Am J Respir Cell Mol Biol 2004;30:350–359. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan F, Comber J, Sladek R, Hudson TJ, Muglia LJ, MacRae T, Gagnon S, Asada M, Brewer JA, Sweezey NB. The growth factor midkine is modulated by both glucocorticoid and retinoid in fetal lung development. Am J Respir Cell Mol Biol 2003;28:33–41. [DOI] [PubMed] [Google Scholar]
- 26.Odom MW, Ballard PL Developmental and hormonal regulation of the surfactant system. In: MacDonald JA, editor. Lung growth and development. New York: Marcel Dekker; 1997. pp. 495–575.
- 27.Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 1995;9:1608–1621. [DOI] [PubMed] [Google Scholar]
- 28.Cole TJ, Myles K, Purton JF, Brereton PS, Solomon NM, Godfrey DI, Funder JW. GRKO mice express an aberrant dexamethasone-binding glucocorticoid receptor, but are profoundly glucocorticoid resistant. Mol Cell Endocrinol 2001;173:193–202. [DOI] [PubMed] [Google Scholar]
- 29.Mittelstadt PR, Ashwell JD. Disruption of glucocorticoid receptor exon 2 yields a ligand-responsive C-terminal fragment that regulates gene expression. Mol Endocrinol 2003;17:1534–1542. [DOI] [PubMed] [Google Scholar]
- 30.Perl AK, Zhang L, Whitsett JA. Conditional expression of genes in the respiratory epithelium in transgenic mice: cautionary notes and toward building a better mouse trap. Am J Respir Cell Mol Biol 2009;40:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourbon JR, Rieutort M, Engle MJ, Farrell PM. Utilization of glycogen for phospholipid synthesis in fetal rat lung. Biochim Biophys Acta 1982;712:382–389. [DOI] [PubMed] [Google Scholar]
- 32.Ridsdale R, Post M. Surfactant lipid synthesis and lamellar body formation in glycogen-laden type II cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L743–L751. [DOI] [PubMed] [Google Scholar]
- 33.Smith BT, Post M. Fibroblast-pneumocyte factor. Am J Physiol 1989;257:L174–L178. [DOI] [PubMed] [Google Scholar]
- 34.Post M, Torday JS, Smith BT. Alveolar type II cells isolated from fetal rat lung organotypic cultures synthesize and secrete surfactant-associated phospholipids and respond to fibroblast-pneumonocyte factor. Exp Lung Res 1984;7:53–65. [DOI] [PubMed] [Google Scholar]
- 35.Boggaram V. Regulation of lung surfactant protein gene expression. Front Biosci 2003;8:d751–d764. [DOI] [PubMed] [Google Scholar]
- 36.Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem 2005;280:13809–13816. [DOI] [PubMed] [Google Scholar]
- 37.Stahlman MT, Besnard V, Wert SE, Weaver TE, Dingle S, Xu Y, von Zychlin K, Olson SJ, Whitsett JA. Expression of ABCA3 in developing lung and other tissues. J Histochem Cytochem 2007;55:71–83. [DOI] [PubMed] [Google Scholar]
- 38.Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem 2002;277:22147–22155. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Stokes JB, McCray PB Jr. Endogenous and exogenous glucocorticoid regulation of ENaC mRNA expression in developing kidney and lung. Am J Physiol Cell Physiol 2002;283:C762–C772. [DOI] [PubMed] [Google Scholar]
- 40.Korfhagen TR, Bruno MD, Ross GF, Huelsman KM, Ikegami M, Jobe AH, Wert SE, Stripp BR, Morris RE, Glasser SW, et al. Altered surfactant function and structure in SP-A gene targeted mice. Proc Natl Acad Sci USA 1996;93:9594–9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Botas C, Poulain F, Akiyama J, Brown C, Allen L, Goerke J, Clements J, Carlson E, Gillespie AM, Epstein C, et al. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci USA 1998;95:11869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glasser SW, Burhans MS, Korfhagen TR, Na CL, Sly PD, Ross GF, Ikegami M, Whitsett JA. Altered stability of pulmonary surfactant in SP-C-deficient mice. Proc Natl Acad Sci USA 2001;98:6366–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, Weaver TE, Whitsett JA. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci USA 1995;92:7794–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alcorn D, Adamson TM, Lambert TF, Maloney JE, Ritchie BC, Robinson PM. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat 1977;123:649–660. [PMC free article] [PubMed] [Google Scholar]
- 45.Bland RD, Nielson DW. Developmental changes in lung epithelial ion transport and liquid movement. Annu Rev Physiol 1992;54:373–394. [DOI] [PubMed] [Google Scholar]
- 46.O'Brodovich H. Immature epithelial Na+ channel depression is one of the pathogenetic mechanisms leading to human respiratory distress syndrome. Proc Assoc Am Phys 1996;108:1–12. [PubMed] [Google Scholar]
- 47.Otulakowski G, Rafii B, Harris M, O'Brodovich H. Oxygen and glucocorticoids modulate alphaENaC mRNA translation in fetal distal lung epithelium. Am J Respir Cell Mol Biol 2006;34:204–212. [DOI] [PubMed] [Google Scholar]
- 48.Bai C, Fukuda N, Song Y, Ma T, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J Clin Invest 1999;103:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spina D. Epithelium smooth muscle regulation and interactions. Am J Respir Crit Care Med 1998;158:S141–S145. [DOI] [PubMed] [Google Scholar]
- 50.Kaplan F, Ledoux P, Kassamali FQ, Gagnon S, Post M, Koehler D, Deimling J, Sweezey NB. A novel developmentally regulated gene in lung mesenchyme: homology to a tumor-derived trypsin inhibitor. Am J Physiol 1999;276:L1027–L1036. [DOI] [PubMed] [Google Scholar]
- 51.Oyewumi L, Kaplan F, Sweezey NB. Lgl1, a mesenchymal modulator of early lung branching morphogenesis, is a secreted glycoprotein imported by late gestation lung epithelial cells. Biochem J 2003;376:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oyewumi L, Kaplan F, Gagnon S, Sweezey NB. Antisense oligodeoxynucleotides decrease LGL1 mRNA and protein levels and inhibit branching morphogenesis in fetal rat lung. Am J Respir Cell Mol Biol 2003;28:232–240. [DOI] [PubMed] [Google Scholar]