ABSTRACT
The majority of HIV-infected patients develop Candida spp-associated clinical oral lesions. Studies have shown that asymptomatic oral colonization of Candida spp may lead to oral lesions or become a source of disseminated infections. The aim of this study was to verify the effects of periodontal conditions on Candida spp prevalence and Candida spp carriage in the oral cavity of HIV-infected patients compared to non-infected patients. Twenty-five patients not infected with HIV and 48 HIV-infected patients were classified according to periodontal conditions as being periodontal healthy or with periodontal disease. Candida spp carriage and classification were performed in oral rinse samples. Viral load and CD4+ T lymphocyte (CD4+L) counts were performed in blood samples from HIV-infected patients. No differences in Candida spp prevalence related to HIV status or periodontal condition were detected. However, Candida spp carriage was increased in periodontally affected HIV-infected patients when compared to periodontally healthy HIV-infected patients (p= 0.04). Periodontally healthy HIV-infected patients presented Candida spp carriage in similar levels as healthy or periodontally affected non-HIV-infected patients. Candida spp carriage was correlated with CD4+L counting in HIV-infected patients. We concluded that periodontal disease is associated with increased Candida spp carriage in HIV-infected patients and may be a predisposing factor to clinical manifestations of candidiasis.
Keywords: HIV infection, Oral Candida carriage, Periodontal disease, Candida spp
INTRODUCTION
In the last few decades, an increase in the prevalence of fungal oral infections was observed and they seem to be related to the growing antibiotic use and the immunosuppressive condition, especially in AIDS patients1. Most of the infections are related to Candida spp, which is generally a commensal in the oral cavity, but in immunologically-compromised patients it can assume pathogenic characteristics2. Some studies showed that candidiasis comes from commensal strains and that asymptomatic oral colonization can lead to oral lesions or become a source of disseminated infections3-5.
C. albicans is the most common species isolated in oral candidiasis6; however, other species, such as C. tropicalis, C. glabrata, C. parapsilosis, C. stellatoidea, C. guilliermondii, and C. krusei were also observed7.
In healthy individuals, the prevalence of Candida spp in the oral cavity is 40-60%8 while in HIV-infected patients, the prevalence increases to 62-93%9,10. Oral candidiasis is the most common lesion associated with HIV infection. It is considered one of the first clinical signs of HIV-related immunosuppression and it is highly predictable of the disease evolution11. Studies have shown that the prevalence of oral candidiasis varies between 20% and 70% in HIV-infected patients6,11, and although a decline in its prevalence has been observed after the antiretroviral therapy (ART) era12, it is estimated that 90% of HIV-infected patients will develop oral candidiasis during the evolution of HIV infection13, emphasizing the importance of studies on this topic. Among the factors that predispose to oral candidiasis are smoking14, age3,15 and oral hygiene16. According to many studies, the presence of yeasts in the biofilm, mainly C. albicans, contribute not only to oral candidiasis, but to dental caries16,17 and periodontal disease18-20.
Studies on the correlation between Candida spp and the etiology and aggravation of periodontal diseases are important, but it is also essential to verify the effects of periodontal conditions on Candida spp carriage, either due to the possible correlation with the development of oral lesions or systemic disseminations3-5. Thus, due to the importance and prevalence of Candida spp associated with oral diseases in HIV-infected patients, the aim of this study was to identify different species of Candida in oral rinses from patients with and without periodontal disease in two population groups, with and without HIV infection. Furthermore, to quantify the number of Candida ssp colonies in the oral rinses between not infected and HIV-infected patients with and without periodontal disease.
MATERIALS AND METHODS
The study protocol was approved by the Ethics Committee of the Clinical Hospital of the School of Medicine of Ribeirão Preto, University of São Paulo, Ribeirão Preto (protocol Nº 3621/2011). The procedures were performed in accordance with the ethical standards on human experimentation and the Helsinki Declaration of 1975 and 1983 revision. Prior to selection, oral and written explanations about the research protocol were given to eligible participants. All patients provided a written informed consent before their participation in this study.
Twenty-five patients not infected with HIV and 48 HIV-infected patients were selected from the Clinical Hospital of the School of Medicine of Ribeirão Preto, University of São Paulo. Patients were over 18 years old, had more than 19 teeth, and had not received any periodontal treatment, antibiotic therapy, anti-inflammatory or antifungal therapy in the three months before the study participation.
All patients had no clinical signs of candidiasis and no history of complete or partial removable prosthesis use with palatal coverage. Participants with other oral lesions associated with HIV were not excluded from the study. Only patients who met the following criteria were selected: no clinical sign of periodontal disease, no probing depth greater than 4 mm, no loss of attachment and less than 20% of sites with bleeding. Patients who presented more than 20% of sites with bleeding and/ or at least 4 sites with loss of clinical attachment and more than 3 mm and/or 4 sites with probing depths than 4 mm were also included.
HIV-infected patients presented HIV seropositivity confirmed by ELISA and Western Blot tests, as well as by a recent viral load and CD-4+ T lymphocyte (CD4+L) count exams (at least three months before the beginning of the study).
Patients’ Clinical Evaluation
Patients underwent examination and collection of an oral rinse sample. The periodontal exam was performed after the collection of the oral rinse sample, so bleeding could not possibly interfere with the quality of collected samples. For the periodontal exam, we used a millimeter periodontal probe and all teeth (except third molars) were probed at six sites: mesiobuccal, buccal, distobuccal, mesiolingual, lingual and distolingual. In each site, we performed three different measures: deep probing, loss of attachment and bleeding.
Related to the periodontal condition, patients were classified into periodontally healthy or with periodontal disease. The periodontally healthy were patients who had no clinical sign of periodontal disease, no probing depth greater than 4 mm, no loss of attachment and less than 20% of sites with bleeding. The patients who were periodontally affected presented more than 20% of sites with bleeding and/ or at least 4 sites with loss of clinical attachment and more than 3 mm and/ or 4 sites with probing depths than 4 mm21.
Biological Sample Collection
Oral Rinse Sample
One day before collection, sterilized bottles with 10 ml of PBS were prepared in autoclaves (pH 7.3, 0.1 M) and conditioned at 4 °C until use. Before oral rinse collection, the subjects were instructed not to eat, drink, smoke or brush their teeth for one hour prior to sample collection to minimize the risk of contamination. Participants were instructed to rinse with 10 ml of PBS for 60 seconds and spit inside a universal collector that was immediately centrifuged (2,000 g per 10 minutes). After centrifugation, supernatant was discarded and solid residue was diluted in 1 ml of PBS. In the obtained solution, Candida spp was counted and identified. The count and identification of Candida spp were performed in the oral PBS rinse sample by the Samaranayke’s technique, described in 198622.
Blood Collection
For blood exams, 10 mL of blood were collected in vacuteiner tubes with ethylenediamine tetra-acetic acid (EDTA). Plasma was obtained by centrifugation of total blood (800 x g per 10 min.), divided in tubes and stored at –80 ºC in a maximum period of six months, until its use.
Laboratorial Analysis
Viral load and CD4+L count
The HIV-Monitor kit (Roche Diagnostic Systems, Branchburg, NJ, USA) was used to quantify the HIV RNA (viral load) in -blood plasma. The RNA was extracted from the samples using a modified silica protocol (QIAmp RNA viral kit; Qiagen, Chatsworth, CA, USA), and polymerase chain reaction was performed using the HIV-Monitor kit (Roche Diagnostic Systems, Branchburg, NJ, USA). The CD4+L counts in the blood plasma were determined by flow cytometry (FACS), which was performed within three months after sample collection.
Candida spp quantification
The oral rinse samples were diluted in PBS to obtain three different concentrations: pure sample, 10x PBS-diluted sample and 100x PBS-diluted sample. One hundred microliters of each concentration was plated in duplicate on petri dishes with Agar Sabouraud-Dextrose and chloramphenicol and incubated at 37 ºC for 48 hours. The colony forming units per milliliter (CFU/ ml) was obtained by the mean between duplicates of positive culture. Depending on the number of CFU/ mL, counts were categorized into 0 (none), 1 (isolated, <10 CFU/mL), 2 (moderate, 10-102CFU/ mL), 3 (many, 102-103 CFU/mL) and 4 (massive, >103 CFU/ mL)23.
Candida spp identification
To identify Candida spp, 100 microliters of oral rinse 10x PBS-diluted were plated on petri dishes with CHROMagar Candida. Later, colonies were plated in tubes with Sabouraud Dextrose Agar (Himedia, India) and incubated at 37 °C per 24 hours. From such inocula in the exponential growth state, the following were performed: fungal microcultivated test24, germ tube formation25; hypertonic Sabouraud broth26; reduction of trifeniltetrazole chloride; zimogram; API (ID 32 C); formation of germinate tube, chlamydoconidia production, carbohydrates fermentation and assimilation24. The species identification was obtained based on the positivity or negativity of Sandven (1990) tests27.
Statistical Analyses
Statistical analysis was performed using the Graph Pad Prism software (San Diego, CA, USA). Data were not normally distributed. Fisher‘s exact test and the Chi-squared test were used to compare the number of patients using ART, the number of patients who smoked and the prevalence of different Candida species in the groups. Differences between groups were assessed using Chi-squared test. The Mann-Whitney U test was used to compare two means, and the one-way Kruskal Wallis test was used to compare three or more means. Data are presented as the mean ± standard deviation, and for the analysis, a confidence interval of 95% was used, and p values were considered to be significant when they were equal to or less than 0.05.
RESULTS
Seventy-three patients participated in this study: 25 who were not HIV-infected and 48 who were HIV-infected. Patients were divided into four groups according to serum status and periodontal condition. Group A: 12 not HIV-infected and periodontally healthy patients; Group B: 13 not HIV-infected and periodontally affected patients; Group C: 19 HIV-infected and periodontally healthy patients; Group D: 29 HIV-infected and periodontally affected patients.
The participants of all groups were homogeneous -regarding epidemiologic and clinical characteristics, as described in Table 1.
Table 1. Demographic Data and Clinical Parameters of Subjects Stratified by HIV Status and Oral Condition.
| Group | |||||
|---|---|---|---|---|---|
|
| |||||
| A | B | C | D | P value | |
| HIV status | Negative | Negative | Positive | Positive | - |
| Oral conditions | Healthy | Gingivitis/Periodontitis | Healthy | Gingivitis/Periodontitis | - |
| Number of subjects | 12 | 13 | 19 | 29 | - |
| Mean age (years) | 35 (± 9) | 37 (±9) | 38 (±9) | 39 (±5) | 0.411a |
| Males (%) | 50 | 54 | 32 | 55 | 0.4157b |
| Smokers (%) | 8 | 23 | 15 | 34 | 0.2473b |
| Mean number of teeth | 27 (±4) | 27 (±5) | 27 (±5) | 26 (±5) | 0.6106a |
| Sites with BOP (%) | 9 (±7) | 35 (±21) | 5 (±6) | 30 (±13) | <0.0001a |
| Sites with PPD> 4 mm (%) | 0 | 1,2 (±2) | 0 | 1,3 (±2) | <0.0001a |
| Use of ART (%) | 84 | 79 | 1.000c | ||
| Mean CD-4+ cells | 514 (±312) | 387 (±276) | 0.2254d | ||
| Median viral load (cop/ml) | <50 | <50 | 0.2365d | ||
aKruskal Wallis test; bChi-squared test; cFisher’s exact test; dMann Whitney test. Group A: Not HIV-infected and periodontally healthy patients. Group B: Not HIV-infected and periodontally affected patients. Group C: HIV-infected and periodontally healthy patients. Group D: HIV-infected and periodontally affected patients. BOP: bleeding on probing. PPD: probing pocket depth. ART: Antiretroviral therapy.
Oral Candida spp counting and identification related to HIV-infected and non-infected patients’ periodontal conditions
In general, we identified oral Candida spp to have an equivalent distribution in all groups, as described in Table 2.
Table 2. Identification of the Oral Candida spp in Studied Groups.
| Group A | Group B | p valuea | Group C | Group D | p valuea | |
|---|---|---|---|---|---|---|
| Candida spp | 8 (67%) | 10 (77%) | 0.6728 | 11 (58%) | 24 (83%) | 0.0959 |
| Candida albicans | 7 (58%) | 8 (61%) | 1.000 | 11 (58%) | 23 (79%) | 0.1931 |
| Candida krusei | 0 (0%) | 0 (0%) | - | 0 (0%) | 2 (7%) | 0.5115 |
| Candida parapisilosis | 3 (25%) | 2 (15%) | 0.6447 | 1 (5%) | 2 (7%) | 1.000 |
| Candida tropicalis | 0 (0%) | 0 (0%) | - | 1 (5%) | 2 (7%) | 1.000 |
| Candida dubliniensis | 0 (0%) | 0 (0%) | - | 0 (0%) | 1 (3%) | 1.000 |
| Candida glabrata | 0 (0%) | 0 (0%) | - | 0 (0%) | 3 (10%) | 0.2673 |
aFisher’s exact test. Group A: Not HIV-infected and periodontally healthy patients. Group B: Not HIV-infected and periodontally affected patients. Group C: HIV-infected and periodontally healthy patients. Group D: HIV-infected and periodontally affected patients
C. albicans and C. parapisilosis were identified in all studied groups. C. tropicalis was identified in the C and D groups and C. dubliniensis, and C. glabrata, in the D group exclusively. Although a major Candida spp variety had been verified in the D group, its prevalence was not statistically significant (Table 2).
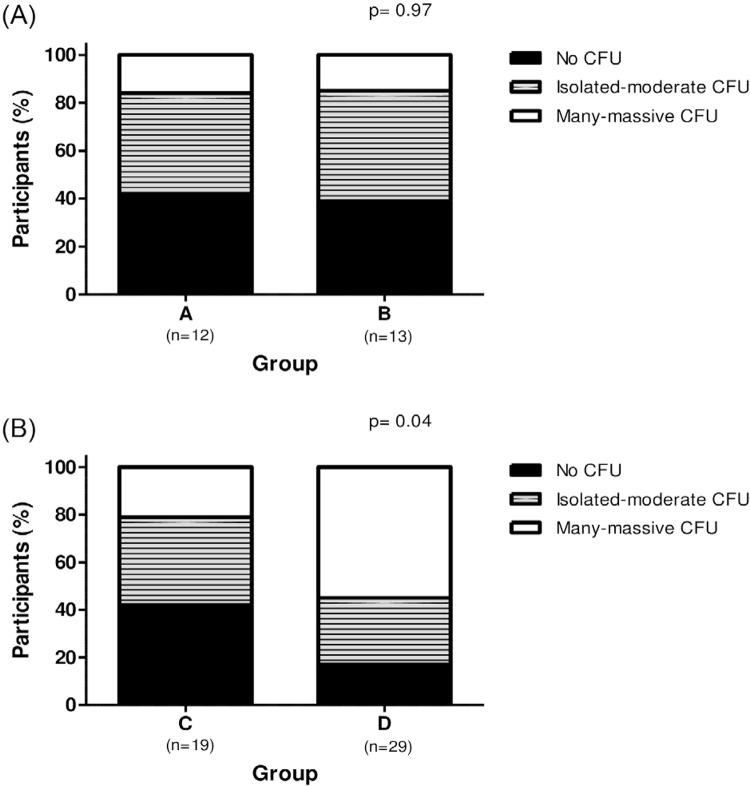
Candida spp were distributed in a homogeneous way among groups, but despite this, Candida spp counting was increased in - patients from the D group. Among non-HIV-infected patients, Candida spp counting did not correlate with the periodontal state. In the group A, analysis of the oral rinse revealed that 58.3% (n=7) of the samples were Candida carriers, 41.6% (n=5) of the patients showed isolated to moderate numbers of CFU/ mL, whereas 16.6% (n=2) presented many to massive CFU/ mL. Similar results were found in group B: 63.5% (n=8) of the samples were Candida carriers; 48.1% (n=6) of the patients showed isolated to moderate numbers of CFU/ mL, whereas 15.4% (n=2) presented many to massive CFU/ mL (p= 0.97), as described in Figure 1A. Differently, among HIV-infected patients, group C presented 36.8% (n=7) of the samples with isolated to moderate numbers of CFU/ mL and 21.1% (n=4) showed many to massive CFU/ mL. High CFU/ mL was noted in the group D, 27.6% (n=8) and 55.2% (n=16) showed isolated to moderate, and many to massive numbers of CFU/ mL, respectively (p= 0.04), as described in Figure 1B.
Figure 1. (A) Relative Candida spp carriage in oral rinses of non-HIV-infected patients without (Group A) and with (Group B) periodontal diseases; p = 0.97, Chi-squared test; (B) Relative Candida spp carriage in HIV-infected patients without (Group C) and with (Group D) periodontal diseases; p = 0.04, Chi-squared test.
It is important to note that Candida spp counting was similar between non-HIV-infected and infected patients who were periodontally healthy, groups A and C (p=0.94).
Influence of viral load and CD4+L count on the Candida spp carriage of HIV-infected patients
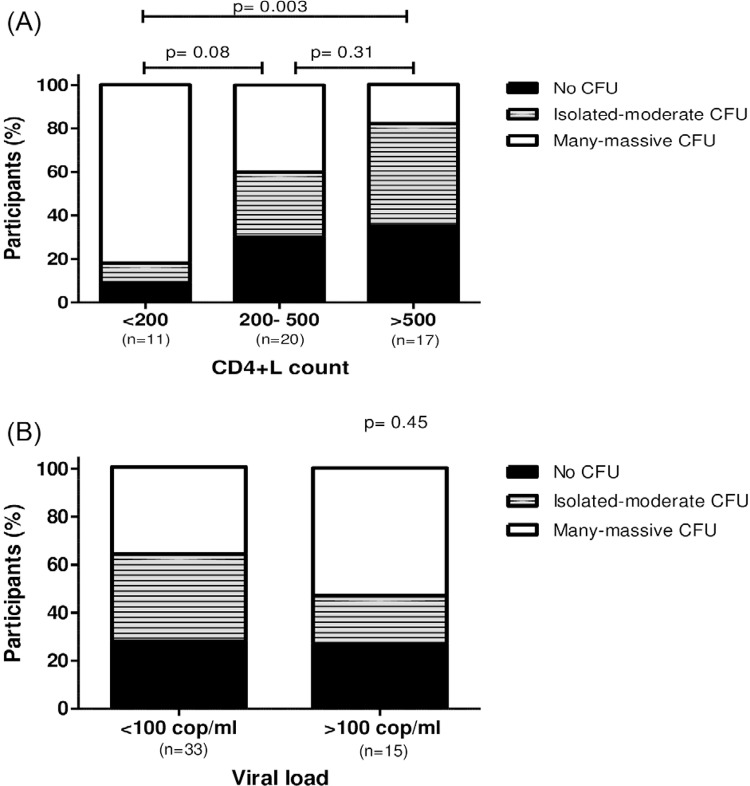
Among HIV-infected patients, we verified that Candida spp carriage was associated with viral load and CD4+L count. The 11 patients who presented less than 200 cells/mm3 CD4+L presented 9% (n=1) with none CFU count; 9% (n=1) and 82% (n=9) showed isolated to moderate and many to massive numbers of CFU, respectively. The 20 patients who presented between 200 and 500 CD4+L/mm3 presented 30% (n=6) with none CFU count, 30% presented isolated to moderate CFU count and 40% (n=8) showed many to massive CFU count. Candida spp carriage was reduced in the 17 patients with more than 500 CD4+T/ mm3, when compared to the patients who presented less than 200 cells/mm3, 35.3% (n=6) presented none CFU count, 47% (n=8) and 17.7% (n=3) showed isolated to moderate and many to massive numbers of CFU, respectively (p= 0,003), as demonstrated in Figure 2A.
Figure 2. (A) Relative Candida spp carriage in HIV-infected patients with different CD4+T cells count (< 200, 200-500 and > 500 cells/ mm3). A significant difference was found between patients with less than 200 CD4+T cells/ mm3 and patients with more than 500 CD4+ T cells/ mm3; p= 0.003, Chi-squared test; (B) Relative Candida spp carriage in HIV-infected patients with different viral loads (< 100 and > 100 copies/ mL); p=0.45, Chi-squared test.
No association between viral load and Candida spp carriage was detected. Thirty-three patients with viral load less than 100 copies/mL presented 27% (n=9) no CFU count, 36.5% (n=12) with isolated to moderate and 36.5% with many to massive CFU/ mL commensal Candida spp, while 15 patients presented a viral load greater than 100 copies/mL showed 26.7% (n=4) of the participants with none CFU/mL count, 20% (n=3) and 53.3% (n=8) with isolated to moderate and many to massive numbers of CFU/mL, respectively (p=0.45), as demonstrated in Figure 2B.
DISCUSSION
The majority of HIV-infected patients develops Candida spp-associated clinical lesions. These lesions can increase in frequency and severity according to the progression of HIV infection11. Although the clinical manifestations of candidiasis depend on mucosal fungus adhesion and colonization28, the asymptomatic Candida spp in HIV-infected patients with low CD4+L count is also associated with clinical disease29. This is the reason why it is of great importance to know the predisposing factors of increased prevalence of commensal Candida spp in the oral cavity, especially HIV-positive patients.
In this study, we found similar prevalences of different commensal Candida species in the oral rinse from non-HIV-infected and infected patients, regardless of their periodontal state.
The prevalence found in our study was higher than that in other studies. Kamtane et al.30, in 2003, verified salivary Candida spp in 15% of non-HIV-infected patients and 55% in HIV-infected patients. Other studies reported fungus colonization in HIV-positive patients between 44% and 62%9,31-33. In the present study, we verified the presence of different Candida spp in 72% of non-HIV-infected patients and 73% of HIV-infected patients. The variation among the different studies may be due the collection methodology, such as the use of swabs, total saliva collection and oral rinse. According to Samaranayake et al.22, oral rinse is the most sensitive technique for quantifying and detecting Candida spp, as used in the present study. No differences in Candida spp prevalences were detected in these study groups.
Candida spp carriage was increased in patients with less than 200 CD4+L cells when compared to patients presenting more than 500 cells/ mm3 (p= 0.003). These results agree with previous studies that verified more colonization in inferior CD4+L counting, 200 cells/ mm3 33-35. On the other hand, some studies have not found this association32-36.
The highlight of our work is the high commensal Candida spp count in HIV-infected and periodontally affected patients, when compared to HIV-infected patients not affected by periodontal disease. These results address the importance of oral health in oral candidiasis prevention because Candida spp density is associated with a higher risk for candidiasis development29. On the other hand, periodontal conditions were not observed as an important factor for Candida spp carriage in non-HIV-infected patients. Non-HIV-infected patients presented similar Candida spp prevalence and counting, regardless of the periodontal state, and these results agree with those of Darwazeh et al.37, who verified a similar prevalence and counting in 149 orally healthy patients. One limitation of this study was the absence of the sample size calculation, therefore, the study may not have enough power to detect the difference between groups.
Among HIV-infected patients, the periodontal state did not influence Candida spp presence or absence, but it was responsible for the 4.8 fold increase in counting. This increase is epidemiologically important as demonstrated by Fong et al.29, in 1997, who showed that candidiasis only developed in patients with persistent asymptomatic carriage of C. albicans.
The predisposal of HIV-infected patients to more oral C. albicans carriage, associated with the influence of oral hygiene on Candida spp carriage, are responsible for the high values of Candida spp in HIV-infected patients who have periodontal disease, as shown in this present study. It is important to emphasize that HIV-infected patients who presented good periodontal conditions had Candida spp levels similar to those of non-HIV-infected patients, renforcing the affirmation that the periodontal condition may be considered a factor -of increases in the count of Candida spp in HIV-infected patients.
Many studies have shown C. albicans to be an etiologic and worsening factor of periodontal disease19,20,38. This study shows that periodontal disease increases with Candida spp carriage, acting as a vicious cycle in HIV-infected patients because in this condition, periodontal disease increases Candida spp counts, which worsen periodontal conditions.
In conclusion, periodontal disease may be a factor responsible for the increase in commensal Candida spp count in HIV-infected patients, and based on current medical literature, it is reasonable to affirm that this increase may predispose the patient to the development of the clinical manifestations of candidiasis.
ACKNOWLEDGEMENTS
The authors thank Prof. L.P. Samaranayake for the discussions about the design of the study. This work was supported by funds from the National Counsel of Technological and Scientific Development (CNPq), Process Nº 477754/2009-0, and the Coordination of Improvement of Higher Level Personnel (CAPES).
REFERENCES
- 1.1. Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunossupressed patients. Lancet Infect Dis. 2003;3:685-702. [DOI] [PubMed]; Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunossupressed patients. Lancet Infect Dis. 2003;3:685–702. doi: 10.1016/s1473-3099(03)00801-6. [DOI] [PubMed] [Google Scholar]
- 2.2. Li YY, Chen WY, Li X, Li HB, Li HQ, Wang L, et al. Asymptomatic oral yeast carriage and antifungal susceptibility profile of HIV-infected patients in Kunming, Yunnan Province of China. BMC Infect Dis. 2013;13:46. [DOI] [PMC free article] [PubMed]; Li YY, Chen WY, Li X, Li HB, Li HQ, Wang L, et al. Asymptomatic oral yeast carriage and antifungal susceptibility profile of HIV-infected patients in Kunming, Yunnan Province of China. 46BMC Infect Dis. 2013;13 doi: 10.1186/1471-2334-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.3. Lockhart SR, Joly S, Vargas K, Swails-Wenger J, Enger L, Sool DR. Natural defenses against Candida colonization breakdown in the oral cavities of the elderly. J Dent Res. 1999;78:857-68. [DOI] [PubMed]; Lockhart SR, Joly S, Vargas K, Swails-Wenger J, Enger L, Sool DR. Natural defenses against Candida colonization breakdown in the oral cavities of the elderly. J Dent Res. 1999;78:857–868. doi: 10.1177/00220345990780040601. [DOI] [PubMed] [Google Scholar]
- 4.4. Vargas KG, Joly S. Carriage frequency, intensity of carriage, and strains of oral yeast species vary in the progression to oral candidiasis in human immunodeficiency virus-positive individuals. J Clin Microbiol. 2002;40:341-50. [DOI] [PMC free article] [PubMed]; Vargas KG, Joly S. Carriage frequency, intensity of carriage, and strains of oral yeast species vary in the progression to oral candidiasis in human immunodeficiency virus-positive individuals. J Clin Microbiol. 2002;40:341–350. doi: 10.1128/JCM.40.2.341-350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.5. Fidel PL Jr. Candida-host interactions in HIV disease: relationships in oropharyngeal candidiasis. Adv Dent Res. 2006;19:80-4. [DOI] [PubMed]; Fidel PL., Jr Candida-host interactions in HIV disease: relationships in oropharyngeal candidiasis. Adv Dent Res. 2006;19:80–84. doi: 10.1177/154407370601900116. [DOI] [PubMed] [Google Scholar]
- 6.6. Ramos-Gomez FJ, Flaitz C, Catapano F, Murray P, Milnes AR, Dorenbaum A. Classification, diagnostic criteria, and treatment recommendations for orofacial manifestations in HIV-infected pediatric patients. Collaborative Workgroup on Oral Manifestations of Pediatric HIV Infection. J Clin Pediatr Dent. 1999;23:85-96. [PubMed]; Ramos-Gomez FJ, Flaitz C, Catapano F, Murray P, Milnes AR, Dorenbaum A. Classification, diagnostic criteria, and treatment recommendations for orofacial manifestations in HIV-infected pediatric patients. Collaborative Workgroup on Oral Manifestations of Pediatric HIV Infection. J Clin Pediatr Dent. 1999;23:85–96. [PubMed] [Google Scholar]
- 7.7. Scully C, el-Kabir M, Samaranayke LP. Candida and oral candidosis: a review. Crit Rev Oral Biol Med. 1994;5:125-57. [DOI] [PubMed]; Scully C, el-Kabir M, Samaranayke LP. Candida and oral candidosis: a review. Crit Rev Oral Biol Med. 1994;5:125–157. doi: 10.1177/10454411940050020101. [DOI] [PubMed] [Google Scholar]
- 8.8. Samaranayake L. Commensal oral Candida in Asian cohorts. Int J Oral Sci. 2009;1:2-5. [DOI] [PMC free article] [PubMed]; Samaranayake L. Commensal oral Candida in Asian cohorts. Int J Oral Sci. 2009;1:2–5. doi: 10.4248/ijos.08006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.9. Costa CR, Cohen AJ, Fernandes OF, Miranda KC, Passos XS, Souza LK, et al. Asymptomatic oral carriage of Candida species in HIV-infected patients in the highly active antiretroviral therapy era. Rev Inst Med Trop São Paulo. 2006;48:257-61. [DOI] [PubMed]; Costa CR, Cohen AJ, Fernandes OF, Miranda KC, Passos XS, Souza LK, et al. Asymptomatic oral carriage of Candida species in HIV-infected patients in the highly active antiretroviral therapy era. Rev Inst Med Trop São Paulo. 2006;48:257–261. doi: 10.1590/s0036-46652006000500004. [DOI] [PubMed] [Google Scholar]
- 10.10. Felix DH, Wray D. The prevalence of oral candidiasis in HIV-infected individuals and dental attenders in Edinburgh. J Oral Pathol Med. 1993;22:418-20. [DOI] [PubMed]; Felix DH, Wray D. The prevalence of oral candidiasis in HIV-infected individuals and dental attenders in Edinburgh. J Oral Pathol Med. 1993;22:418–420. doi: 10.1111/j.1600-0714.1993.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 11.11. Lourenço AG, Figueiredo LT. Oral lesions in HIV infected individuals from Ribeirão Preto, Brazil. Med Oral Patol Oral Cir Bucal. 2008;13:E281-6. [PubMed]; Lourenço AG, Figueiredo LT. Oral lesions in HIV infected individuals from Ribeirão Preto, Brazil. Med Oral Patol Oral Cir Bucal. 2008;13:E281–E286. [PubMed] [Google Scholar]
- 12.12. Lourenço AG, Motta AC, Figueiredo LT, Machado AA, Komesu MC. Oral lesions associated with HIV infection before and during the antiretroviral therapy era in Ribeirão Preto, Brazil. J Oral Sci. 2011;53:379-85. [DOI] [PubMed]; Lourenço AG, Motta AC, Figueiredo LT, Machado AA, Komesu MC. Oral lesions associated with HIV infection before and during the antiretroviral therapy era in Ribeirão Preto, Brazil. J Oral Sci. 2011;53:379–385. doi: 10.2334/josnusd.53.379. [DOI] [PubMed] [Google Scholar]
- 13.13. de Repentigny L, Lewandowski D, Jolicoeur P. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev. 2004;17:729-59. [DOI] [PMC free article] [PubMed]; de Repentigny L, Lewandowski D, Jolicoeur P. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev. 2004;17:729–759. doi: 10.1128/CMR.17.4.729-759.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.14. Soysa NS, Ellepola AN. The impact of cigarette/tobacco smoking on oral candidosis: an overview. Oral Dis. 2005;11:268-73. [DOI] [PubMed]; Soysa NS, Ellepola AN. The impact of cigarette/tobacco smoking on oral candidosis: an overview. Oral Dis. 2005;11:268–273. doi: 10.1111/j.1601-0825.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 15.15. Kleinegger CL, Lockhart SR, Vargas K, Soll DR. Frequency, intensity, species, and strains of oral Candida vary as a function of host age. J Clin Microbiol. 1996; 34:2246-54. [DOI] [PMC free article] [PubMed]; Kleinegger CL, Lockhart SR, Vargas K, Soll DR. Frequency, intensity, species, and strains of oral Candida vary as a function of host age. J Clin Microbiol. 1996;34:2246–2254. doi: 10.1128/jcm.34.9.2246-2254.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.16. Starr JR, White TC, Leroux BG, Luis HS, Bernardo M, Leitao J, et al. Persistence of oral Candida albicans carriage in healthy Portuguese schoolchildren followed for 3 years. Oral Microbiol Immunol. 2002;17:304-10. [DOI] [PubMed]; Starr JR, White TC, Leroux BG, Luis HS, Bernardo M, Leitao J, et al. Persistence of oral Candida albicans carriage in healthy Portuguese schoolchildren followed for 3 years. Oral Microbiol Immunol. 2002;17:304–310. doi: 10.1034/j.1399-302x.2002.170507.x. [DOI] [PubMed] [Google Scholar]
- 17.17. Nikawa H, Hamada T, Yamamoto T. Denture plaque-past and recent concerns. J Dent. 1998;26:299-304. [DOI] [PubMed]; Nikawa H, Hamada T, Yamamoto T. Denture plaque-past and recent concerns. J Dent. 1998;26:299–304. doi: 10.1016/s0300-5712(97)00026-2. [DOI] [PubMed] [Google Scholar]
- 18.18. Hannula J, Dogan B, Slots J, Okte E, Asikainen S. Subgingival strains of Candida albicans in relation to geographical origin and occurrence of periodontal pathogenic bacteria. Oral Microbiol Immunol. 2001;16:113-8. [DOI] [PubMed]; Hannula J, Dogan B, Slots J, Okte E, Asikainen S. Subgingival strains of Candida albicans in relation to geographical origin and occurrence of periodontal pathogenic bacteria. Oral Microbiol Immunol. 2001;16:113–118. doi: 10.1034/j.1399-302x.2001.016002113.x. [DOI] [PubMed] [Google Scholar]
- 19.19. Järvensivu A, Hietanen J, Rautemaa R, Sorsa T, Richardson M. Candida yeasts in chronic periodontitis tissues and subgingival microbial biofilms in vivo. Oral Dis. 2004;10:106-12. [DOI] [PubMed]; Järvensivu A, Hietanen J, Rautemaa R, Sorsa T, Richardson M. Candida yeasts in chronic periodontitis tissues and subgingival microbial biofilms in vivo. Oral Dis. 2004;10:106–112. doi: 10.1046/j.1354-523x.2003.00978.x. [DOI] [PubMed] [Google Scholar]
- 20.20. Canabarro A, Valle C, Farias MR, Santos FB, Lazera M, Wanke B. Association of subgingival colonization of Candida albicans and other yeasts with severity of chronic periodontitis. J Periodontal Res. 2013;48:428-32. [DOI] [PubMed]; Canabarro A, Valle C, Farias MR, Santos FB, Lazera M, Wanke B. Association of subgingival colonization of Candida albicans and other yeasts with severity of chronic periodontitis. J Periodontal Res. 2013;48:428–432. doi: 10.1111/jre.12022. [DOI] [PubMed] [Google Scholar]
- 21.21. Ramseier CA, Kinney JS, Herr AE, Sugai JV, Shelburne CA, Rayburn LA, et al. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. 2009;80:436-46. [DOI] [PMC free article] [PubMed]; Ramseier CA, Kinney JS, Herr AE, Sugai JV, Shelburne CA, Rayburn LA, et al. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. 2009;80:436–446. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.22. Samaranayake LP, MacFarlane TW, Lamey PJ, Ferguson MM. A comparison of oral rinse and imprint sampling techniques for the detection of yeast, coliform and Staphylococcus aureus carriage in the oral cavity. J Oral Pathol. 1986;15:386-88. [DOI] [PubMed]; Samaranayake LP, MacFarlane TW, Lamey PJ, Ferguson MM. A comparison of oral rinse and imprint sampling techniques for the detection of yeast, coliform and Staphylococcus aureus carriage in the oral cavity. J Oral Pathol. 1986;15:386–388. doi: 10.1111/j.1600-0714.1986.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 23.23. Klaus K, Eichenauer J, Sprenger R, Ruf S. Oral microbiota carriage in patients with multibracket appliance in relation to the quality of oral hygiene. Head Face Med. 2016;12:28. [DOI] [PMC free article] [PubMed]; Klaus K, Eichenauer J, Sprenger R, Ruf S. Oral microbiota carriage in patients with multibracket appliance in relation to the quality of oral hygiene. 28Head Face Med. 2016;12 doi: 10.1186/s13005-016-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.24. Lacaz CS, Porto E, Martins JE. Micologia médica: fungos actinomicetos e algas de interesse médico. 8ª ed. São Paulo: Sarvier; 1991.; Lacaz CS, Porto E, Martins JE. Micologia médica: fungos actinomicetos e algas de interesse médico. 8ª. São Paulo: Sarvier; 1991. [Google Scholar]
- 25.25. Sindrin JJ, Rocha MF. Micologia médica à luz dos autores contemporâneos. Rio de Janeiro: Guanabara Koogan; 2004.; Sindrin JJ, Rocha MF. Micologia médica à luz dos autores contemporâneos. Rio de Janeiro: Guanabara Koogan; 2004. [Google Scholar]
- 26.26. Alves SH, Milan EP, de Laet Sant’Ana P, Oliveira LO, Santurio JM, Colombo AL. Hypertonic sabouraud broth as a simple and powerful test for Candida dubliniensis screening. Diagn Microbiol Infect Dis. 2002;43:85-6. [DOI] [PubMed]; Alves SH, Milan EP, de Laet Sant’Ana P, Oliveira LO, Santurio JM, Colombo AL. Hypertonic sabouraud broth as a simple and powerful test for Candida dubliniensis screening. Diagn Microbiol Infect Dis. 2002;43:85–86. doi: 10.1016/s0732-8893(02)00368-1. [DOI] [PubMed] [Google Scholar]
- 27.27. Sandven P. Laboratory identification and sensitivity testing of yeast isolates. Acta Odontol Scand. 1990;48:27-36. [DOI] [PubMed]; Sandven P. Laboratory identification and sensitivity testing of yeast isolates. Acta Odontol Scand. 1990;48:27–36. doi: 10.3109/00016359009012731. [DOI] [PubMed] [Google Scholar]
- 28.28. Hisajima T, Ishibashi H, Yamada T, Nishiyama Y, Yamaguchi, Funakoshi K, et al. Invasion process of Candida albicans to tongue surface in early stages of experimental murine oral candidiasis. Med Mycol. 2008;46:697–704. [DOI] [PubMed]; Hisajima T, Ishibashi H, Yamada T, Nishiyama Y, Yamaguchi, Funakoshi K, et al. Invasion process of Candida albicans to tongue surface in early stages of experimental murine oral candidiasis. Med Mycol. 2008;46:697–704. doi: 10.1080/13693780802039919. [DOI] [PubMed] [Google Scholar]
- 29.29. Fong IW, Laurel M, Burford-Mason A. Asymptomatic oral carriage of Candida albicans in patients with HIV infection. Clin Invest Med. 1997;20:85–93. [PubMed]; Fong IW, Laurel M, Burford-Mason A. Asymptomatic oral carriage of Candida albicans in patients with HIV infection. Clin Invest Med. 1997;20:85–93. [PubMed] [Google Scholar]
- 30.30. Kamtane S, Subramaniam A, Survarna P. A comparative study of oral candidal carriage and its association with CD4 count between HIV-positive and healthy individuals. J Int Assoc Provid AIDS Care. 2013;12:39-43. [DOI] [PubMed]; Kamtane S, Subramaniam A, Survarna P. A comparative study of oral candidal carriage and its association with CD4 count between HIV-positive and healthy individuals. J Int Assoc Provid AIDS Care. 2013;12:39–43. doi: 10.1177/1545109711423444. [DOI] [PubMed] [Google Scholar]
- 31.31. Hung CC, Yang YL, Lauderdale TL, McDonald LC, Hsiao CF, Cheng HH, et al. Colonization of human immunodeficiency virus-infected outpatients in Taiwan with Candida species. J Clin Microbiol. 2005;43:1600-3. [DOI] [PMC free article] [PubMed]; Hung CC, Yang YL, Lauderdale TL, McDonald LC, Hsiao CF, Cheng HH, et al. Colonization of human immunodeficiency virus-infected outpatients in Taiwan with Candida species. J Clin Microbiol. 2005;43:1600–1603. doi: 10.1128/JCM.43.4.1600-1603.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.32. Campisi G, Pizzo G, Milici ME, Mancuso S, Margiotta V. Candidal carriage in the oral cavity of human immunodeficiency virus infected subjects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:281-6. [DOI] [PubMed]; Campisi G, Pizzo G, Milici ME, Mancuso S, Margiotta V. Candidal carriage in the oral cavity of human immunodeficiency virus infected subjects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:281–286. doi: 10.1067/moe.2002.120804. [DOI] [PubMed] [Google Scholar]
- 33.33. Delgado AC, de Jesus Pedro R, Aoki FH, Resende MR, Trabasso P, Colombo AL, et al. Clinical and microbiological assessment of patients with a long-term diagnosis of human immunodeficiency virus infection and Candida oral colonization. Clin Microbiol Infect. 2009;15:364-71. [DOI] [PubMed]; Delgado AC, de Jesus Pedro R, Aoki FH, Resende MR, Trabasso P, Colombo AL, et al. Clinical and microbiological assessment of patients with a long-term diagnosis of human immunodeficiency virus infection and Candida oral colonization. Clin Microbiol Infect. 2009;15:364–371. doi: 10.1111/j.1469-0691.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 34.34. Lin JN, Lin CC, Lai CH, Yang YL, Chen HT, Weng HC, et al. Predisposing factors for oropharyngeal colonization of yeasts in human immunodeficiency virus-infected patients: a prospective cross-sectional study. J Microbiol Immunol Infect. 2013;46:129-35. [DOI] [PubMed]; Lin JN, Lin CC, Lai CH, Yang YL, Chen HT, Weng HC, et al. Predisposing factors for oropharyngeal colonization of yeasts in human immunodeficiency virus-infected patients: a prospective cross-sectional study. J Microbiol Immunol Infect. 2013;46:129–135. doi: 10.1016/j.jmii.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 35.35. Sangeorzan JA, Bradley SF, He X, Zarins LT, Ridenour GL, Tiballi RN, et al. Epidemiology of oral candidiasis in HIVinfected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med. 1994;97:339-46. [DOI] [PubMed]; Sangeorzan JA, Bradley SF, He X, Zarins LT, Ridenour GL, Tiballi RN, et al. Epidemiology of oral candidiasis in HIVinfected patients: colonization, infection, treatment, and emergence of fluconazole resistance. Am J Med. 1994;97:339–346. doi: 10.1016/0002-9343(94)90300-x. [DOI] [PubMed] [Google Scholar]
- 36.36. Gottfredson M, Cox GM, Indridason OS, Almeida GM, Heald AE, Perfect JR. Association of plasma levels of human immunodeficiency virus type 1 RNA and oropharyngeal Candida colonization. J Infect Dis. 1999;180:534-7. [DOI] [PubMed]; Gottfredson M, Cox GM, Indridason OS, Almeida GM, Heald AE, Perfect JR. Association of plasma levels of human immunodeficiency virus type 1 RNA and oropharyngeal Candida colonization. J Infect Dis. 1999;180:534–537. doi: 10.1086/314887. [DOI] [PubMed] [Google Scholar]
- 37.37. Darwazeh AM, Hammad MM, Al-Jamaei AA.The relationship between oral hygiene and oral colonization with Candida species in healthy adult subjects. Int J Dent Hyg. 2010;8:128–33. [DOI] [PubMed]; Darwazeh AM, Hammad MM, Al-Jamaei AA. The relationship between oral hygiene and oral colonization with Candida species in healthy adult subjects. Int J Dent Hyg. 2010;8:128–133. doi: 10.1111/j.1601-5037.2009.00407.x. [DOI] [PubMed] [Google Scholar]
- 38.38. Odden K, Schenck K, Kooppang H, Hurlen B. Candidal infection of the gingiva in HIV-infected persons. J Oral Pathol Med. 1994;23:178-83. [DOI] [PubMed]; Odden K, Schenck K, Kooppang H, Hurlen B. Candidal infection of the gingiva in HIV-infected persons. J Oral Pathol Med. 1994;23:178–183. doi: 10.1111/j.1600-0714.1994.tb01109.x. [DOI] [PubMed] [Google Scholar]