ABSTRACT
Background
Atypical presentations of cutaneous leishmaniasis include sporotrichoid leishmaniasis (SL), which is clinically described as a primary ulcer combined with lymphangitis and nodules and/or ulcerated lesions along its pathway.
Aims
To assess the differences between patients with sporotrichoid leishmaniasis and typical cutaneous leishmaniasis (CL).
Methods
From January 2004 to December 2010, 23 cases of SL (4.7%) were detected among 494 CL patients diagnosed at a reference center for the disease in Rio de Janeiro State, Brazil. These 23 cases were compared with the remaining 471 patients presenting CL.
Results
SL predominated in female patients (60.9%, p = 0.024), with older age (p = 0.032) and with lesions in upper limbs (52.2%, p = 0.028). CL affected more men (64.5%), at younger age, and with a higher number of lesions exclusively in lower limbs (34.8%).
Conclusions
Differences in clinical and epidemiological presentation were found between SL patients as compared to CL ones, in a region with a known predominance of Leishmania (Viannia) braziliensis. The results are similar to the features of most of the sporotrichosis patients as described in literature, making the differential diagnosis between ATL and sporotrichosis more important in overlapping areas for both diseases, like in Rio de Janeiro State.
Keywords: Sporotrichoid leishmaniasis, American tegumentary leishmaniasis, Cutaneous leishmaniasis, Sporotrichosis
INTRODUCTION
The most characteristic presentation of cutaneous leishmaniasis is: one or a few painless ulcers with infiltrated borders in exposed body areas1. However, several other forms are also described2,3. This diversity could be related to the host immune response, skin area, and species of Leishmania4,5. In Rio de Janeiro State, Leishmania (Viannia) braziliensis is the almost exclusively prevalent species4, and different genotypes were not associated with the clinical variability6.
Sporotrichoid leishmaniasis (SL) presents itself as an ulcer, lymphangitis, and nodules or ulcerated lesions along its pathway; it resembles the typical presentation of sporotrichosis2,5,7. This study aimed to access the differences between SL and typical cutaneous leishmaniasis.
MATERIALS AND METHODS
Cross-sectional study with comparison of epidemiological and clinical parameters, laboratory findings at the time of diagnosis, as well as post-treatment follow-up, between sporotrichoid forms of leishmaniasis (SL) and typical forms of cutaneous leishmaniasis (CL).
We studied patients diagnosed with American tegumentary leishmaniasis (ATL) at a reference center for the disease in Rio de Janeiro, Brazil, between January 2004 and December 2010.
Patients who were included followed systematic research protocols, including epidemiological history, clinical examination and laboratory tests. Patients with concomitant involvement of skin and mucous membranes of the upper aerodigestive tract and those with diffuse anergic leishmaniasis were excluded.
Patients were evaluated by experienced dermatologists in the diagnosis and treatment of cutaneous leishmaniasis. Clinical diagnosis of SL was performed when there were ulcerated lesions accompanied by lymphangitis and gummas/nodes along the lymphatic path. Typical leishmaniasis lesions (CL) were not accompanied by this lymphatic pattern, and were generally comprised of ulcerated lesions with infiltrated borders, in most cases one lesion or a few; occasionally, an infiltrated plaque or, more rarely, verrucous lesions. Patients with ten or more lesions were also exceptions. Cases presenting with short lymphatic path around the lesion without gummas or nodules were not clinically classified as SL.
Diagnosis of leishmaniasis was confirmed by visualization and/or isolation of parasites by at least one of the following methods: Leishmania spp culture in NNN+ Schneider media8, or conventional histopathology and/or immunohistochemical examination9 of a skin lesion fragment collected through a biopsy procedure. Immunological tests included Montenegro skin test (MST)10 and serological tests such as the indirect immunofluorescence assay (IFA), and/or the enzyme-linked immunosorbent assay (ELISA)11.
The analysis considered clinical characteristics (gender, age, time of disease progression before diagnosis, affected area of the body, and response to treatment - cure or relapse of the disease), besides the results to the diagnostic exams listed above.
A database was constructed (SPSS16 software for Windows - SPSS Inc, Chicago, Illinois, USA), and kept under the responsibility of the authors, based on the records of the patients. Clinical features and the results of the laboratory exams of SL patients were compared with the same parameters of CL patients treated in the same period at this reference center. Measurements of central tendency and dispersion for quantitative variables (age in years, MST in millimeters, time of disease progression before diagnosis in months) were calculated and subsequently these parameters were transformed into categorical variables as follows:
Age: < 25 years, 25 to 44 years, > 44 years.
MST in millimeters: negative (0 to 4 mm); and positive (≥ 5 mm).
Time of disease progression before diagnosis: ≤ 3 months; and > 3 months.
Affected area of the body: lesions exclusively located in upper limbs; exclusively located in lower limbs; and in other or multiple locations.
The variables were analyzed using the Pearson Chi Squared and Fisher’s exact tests, as well as by logistic regression (SPSS16.0).
The species of Leishmania was determined in SL patients, whenever possible.
Missing data were not considered in the analysis, but they were made explicit in Table 1.
Table 1. - Clinical-epidemiological and laboratory characteristics, besides post-treatment follow-up- of patients with sporotrichoid leishmaniasis (SL) and typical cutaneous leishmaniasis (CL), 2004 - 2010.
| SL | CL | p-value | ||
|---|---|---|---|---|
| Gender | Male | 39.1% (09) | 64.5% (304) | |
| Female | 60.9% (14) | 35.5% (167) | 0.024a | |
| Missing data | 0 | 0 | ||
| Age (years) | Mean | 44.74 | 35.94 | |
| Standard deviation | 19.010 | 19.073 | 0.906b | |
| Minimum | 13 | 1 | ||
| Maximum | 80 | 92 | ||
| < 25 years | 13.0% (03) | 31.8% (150) | ||
| 25 – 44 years | 52.2% (12) | 36.3% (171) | 0.032c | |
| > 44 years | 34.8% (08) | 31.8% (150) | ||
| Missing data | 0 | 0 | ||
| Time of disease progression before diagnosis (months) | Mean | 2.55 | 3.11 | |
| Standard deviation | 2.283 | 3.769 | 0.987b | |
| Minimum | 1 | 1 | ||
| Maximum | 12 | 53 | ||
| ≤ 3 months | 90.9%* (20) | 75.3%* (342) | ||
| > 3 months | 9.1%* (02) | 24.7%* (112) | 0.125a | |
| Missing data | 01 | 17 | ||
| Affected site of the body | Exclusively upper limbs | 52.2% (12) | 27.2% (128) | |
| Exclusively lower limbs Other/multiple sites, with or without upper or lower limbs | 17.4% (4) 30.4% (7) | 34.8% (164) 38.0% (179) | 0.028b | |
| Montenegro skin test (millimeters) | Mean | 21.75 | 18.54 | |
| Standard deviation | 10.992 | 12.008 | 0.266b | |
| Minimum | 0 | 0 | ||
| Maximum | 50 | 75 | ||
| Negative (0-4 mm) | 5%* (01) | 6.3%* (26) | ||
| Positive (≥ 5 mm) | 95%* (19) | 93.7% (384) | 1.000a | |
| Missing data | 03 | 61 | ||
| Indirect immunofluorescence assay (IFA) | Negative | 41.2%* (07) | 29.5%* (101) | |
| Positive | 58.8%* (10) | 70.5%* (241) | 0.294a | |
| Missing data | 06 | 129 | ||
| Enzyme-linked immunosorbent assay (ELISA) | Negative | 21.1%* (04) | 11.6%* (35) | |
| Positive | 78.9%* (15) | 88.4%* (267) | 0.266a | |
| Missing data | 04 | 169 | ||
| Culture for Leishmania | Negative | 13.0% (03) | 14.2%* (57) | |
| Positive | 87.0% (20) | 85.8%* (345) | 1.000a | |
| Missing data | 0 | 69 | ||
| Histopathology | Without parasites | 52.2% (12) | 42.2%* (151) | |
| With parasites | 47.8% (11) | 57.8%* (207) | 0.389a | |
| Missing data | 0 | 113 | ||
| Post-treatment follow-up | Cure | 70.6%* (12) | 79.4%* (274) | |
| Re-activation | 29.4%* (05) | 20.3%* (70) | 0.394a | |
| Missing data | 06 | 127 |
*Valid percentages, missing data cases excluded. ( ) Number of cases. aFisher’s Exact Test. bPearson Chi Square. cLogistic regression. Bold p-value statistically significant.
Ethical considerations
Patients signed an informed consent at diagnosis. This study is a subproject of a larger research project approved by the Institutional Ethics in Research Committee under the Nº 0016.0.009-02, and it was re-submitted to the same Board and revalidated under the Nº 0056.0.009.000-10.
RESULTS
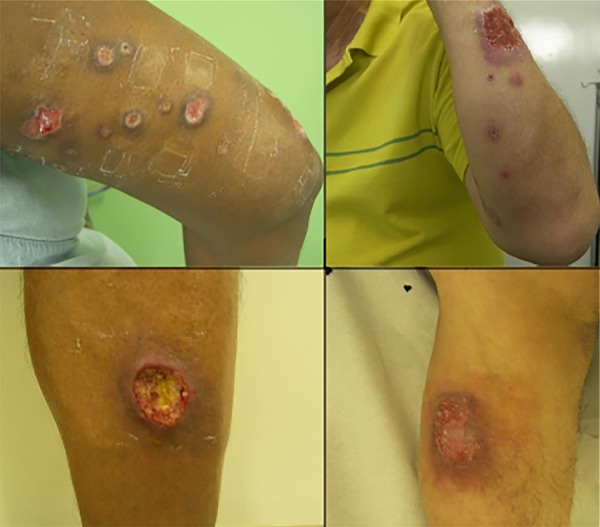
During the analyzed period, among 579 patients with ATL, 84 patients had mucosal involvement and one patient had diffuse anergic leishmaniasis and were therefore excluded, since they had a particular immunological behaviour; 494 patients with cutaneous leishmaniasis were studied, among them 23 (4.7%) presented SL. Figure 1 shows lesions of patients with SL in upper limbs (upside) and typical CL lesions in lower limbs (bottom).
Figure 1. - Sporotrichoid leishmaniasis in upper limbs (upside) and typical cutaneous leishmaniasis in lower limbs (bottom).
Twenty patients (86.9%) with SL had positive cultures for Leishmania spp, and 18 were characterized as L. (V.) braziliensis. From the three remaining patients, one had parasitologically confirmed SL through visualization of amastigotes in histopathology; the other two had SL diagnosis made through a compatible clinical and epidemiological history, aside from positive immunological tests (MST, IFA and/or ELISA), as well as a good response to treatment with meglumine antimoniate.
The results of the clinical and epidemiological features, laboratory tests performed for diagnosis, and follow-up after treatment for both groups of patients (SL and CL) are presented in Table 1.
DISCUSSION
There were significant differences when clinical and epidemiological characteristics of patients with SL and CL were compared, with more females and older age in SL. Patients with CL were predominantly males, in agreement with data from the Ministry of Health of Brazil, which reported 74% of cases of cutaneous leishmaniasis in men1. Although in both groups the lesions were mainly detected in exposed areas, we observed a predominance of lesions in the upper limbs in the SL group, as compared to the CL group, which presented a higher number of lower limb lesions, as classically described in the literature3,12. Interestingly, those features were are also present in sporotrichosis in Rio de Janeiro, with a predominance of older females and lesions in the upper limbs13. They are different pathogens showing similar clinical-epidemiological characteristics. Despite diverse explanations for their occurrence, in the case of sporotrichosis transmitted by cats, older women are more likely to care for and feed infected animals, which could also explain why lesions are presented mostly in the hands and forearms/arms due to the manipulation of cats13. Leishmaniasis, however, is transmitted by sand flies and this explanation does not elucidate the results obtained in SL.
We have generally observed that SL patients behaved similarly to CL patients in terms of laboratory exams, and did not present different post-treatment prognosis either.
MST is a tool for evaluating the delayed hypersensitivity cell response to Leishmania antigens inoculated intradermally, and it is widely used for diagnosis and in epidemiological surveys10,14. Despite the fact that it does not constitute a confirmatory parasitological test, it is usually the only available diagnostic test for cutaneous leishmaniasis in most primary health care facilities in Brazil. The test may be negative in the first four to six weeks from the onset of skin lesions1. Additionally, positivity to MST is independent of gender or age of patients15, and it is reported to have a high sensitivity: positivity close to 100% in confirmed ATL cases16. However, in individuals who do not have active leishmaniasis, lesions or scars suggestive of prior disease, or in those who reside in non-endemic areas for the disease, its positivity may vary between 20 and 30%1,10. The occurrence of positive MST in confirmed cases of sporotrichosis was previously detected in Rio de Janeiro17. Since endemic areas for sporotrichosis and leishmaniasis overlap extensively in Rio de Janeiro State, the presence of both agents could partly explain the positivity of MST in patients who do not have leishmaniasis, adding some difficulty to the differential diagnosis. However, in our study, the strong reactions found in almost all the patients with SL lead us to suggest that this test can be valuable in conditions of scarcity of exams for the parasitological diagnosis of leishmaniasis. There was a trend to higher values of MST in patients with SL than in patients with CL.
In patients with CL, there are also generally low to moderate levels of specific antibodies detected by both IFA and ELISA, when compared to the levels found in patients with the mucosal forms of the disease. However, there are individual variations11,18. In our study, both groups (SL and CL) showed moderate to high percentages of positivity in leishmaniasis serology by those methods.
Regarding parasite detection by culture of fragments of lesions collected through biopsy, considered as the gold standard for ATL diagnosis, the majority of our patients were diagnosed based on the parasite isolation (85% in both SL and CL groups). This positivity is higher than usually described12,19. In addition, both studied groups showed moderate sensitivity of parasite detection in histopathology. Sensitivity indices of histopathology in the literature show wide variation1,12; however, immunohistochemistry showed higher sensitivities than hematoxylin-eosin staining for the parasite detection9.
The analyzed patients in the present study came from a geographical area with a large predominance of Leishmania (V.) braziliensis, like most of Brazilian regions. This can be considered a limitation of the study. In the Amazon region, for example, the coexistence of different species of Leishmania could lead to other findings. Additional studies to verify clinical and epidemiological features of SL patients under conditions of greater diversity of the parasite population can add information to the SL characterization. However, as SL is reported in other countries20-22 where L. (V.) braziliensis has not been reported, it seems that this clinical presentation is mostly due to the characteristics of the patients. In addition, as sporotrichosis has been described in different regions all over the world23-26, differential diagnosis may become a real challenge elsewhere.
The results of the present study suggest that SL and CL patients have different characteristics, and they reflect mainly clinical and epidemiological variations between the groups. Since these differences could be produced by distinct immunological profiles, studies are now been performed in order to clarify in situ immunological distinctions between SL and CL.
ACKNOWLEDGEMENTS
The authors would like to thank the Instituto Nacional de Infectologia/Fundação Oswaldo Cruz; CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico); CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior); PROEP-CNPq (402557/2011-5) and FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa no Estado do Rio de Janeiro, process Nº E26/111.230/2014) for their financial support. The funders had no role in the study design, data collection and analysis, decision to publish or preparing the manuscript.
REFERENCES
- 1.1. Brasil. Ministério da Saúde. Manual de vigilância da leishmaniose Tegumentar Americana. 2ª ed. Brasília: Ministério da Saúde, 2010.; Brasil. Ministério da Saúde . Manual de vigilância da leishmaniose Tegumentar Americana. 2ª. Brasília: Ministério da Saúd; 2010. [Google Scholar]
- 2.2. Bari AU, Rahman SB. Many faces of cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol. 2008;74:23-7. [DOI] [PubMed]; Bari AU, Rahman SB. Many faces of cutaneous leishmaniasis. Indian J Dermatol Venereol Leprol. 2008;74:23–27. doi: 10.4103/0378-6323.38402. [DOI] [PubMed] [Google Scholar]
- 3.3. Guerra JA, Talhari S, Paes MG, Garrido M, Talhari JM. Aspectos clínicos e diagnósticos da leishmaniose tegumentar americana em militares simultaneamente expostos à infecção na Amazônia. Rev Soc Bras Med Trop. 2003;36:587-90. [PubMed]; Guerra JA, Talhari S, Paes MG, Garrido M, Talhari JM. Aspectos clínicos e diagnósticos da leishmaniose tegumentar americana em militares simultaneamente expostos à infecção na Amazônia. Rev Soc Bras Med Trop. 2003;36:587–590. [PubMed] [Google Scholar]
- 4.4. Azeredo-Coutinho RB, Conceição-Silva F, Schubach A, Cupolillo E, Quintella LP, Madeira MF, et al. First report of diffuse cutaneous leishmaniasis and Leishmania amazonensis infection in Rio de Janeiro State, Brazil. Trans R Soc Trop Med Hyg. 2007;101:735-7. [DOI] [PubMed]; Azeredo-Coutinho RB, Conceição-Silva F, Schubach A, Cupolillo E, Quintella LP, Madeira MF, et al. First report of diffuse cutaneous leishmaniasis and Leishmania amazonensis infection in Rio de Janeiro State, Brazil. Trans R Soc Trop Med Hyg. 2007;101:735–737. doi: 10.1016/j.trstmh.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 5.5. Iftikhar N, Bari I, Ejaz A. Rare variants of cutaneous leishmaniasis: whitlow, paronychia and sporotrichoid. Int J Dermatol. 2003;42:807-9. [DOI] [PubMed]; Iftikhar N, Bari I, Ejaz A. Rare variants of cutaneous leishmaniasis: whitlow, paronychia and sporotrichoid. Int J Dermatol. 2003;42:807–809. doi: 10.1046/j.1365-4362.2003.02015.x. [DOI] [PubMed] [Google Scholar]
- 6.6. Baptista C, Schubach AO, Madeira MF, Leal CA, Pires MQ, Oliveira FS, et al. Leishmania (Viannia) braziliensis genotypes identified in lesions of patients with atypical or typical manifestations of tegumentary leishmaniasis: evaluation by two molecular markers. Exp Parasitol. 2009;42:317-2. [DOI] [PubMed]; Baptista C, Schubach AO, Madeira MF, Leal CA, Pires MQ, Oliveira FS, et al. Leishmania (Viannia) braziliensis genotypes identified in lesions of patients with atypical or typical manifestations of tegumentary leishmaniasis: evaluation by two molecular markers. Exp Parasitol. 2009;42:317–312. doi: 10.1016/j.exppara.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 7.7. López-Escobar M, Drake-Monfort M, Salesa-Gutiérrez de Rozas R, Hermana-Ramírez S. Leishmaniose cutanea esporotricoide. Actas Dermosifiliogr. 2007;98:444-5. [PubMed]; López-Escobar M, Drake-Monfort M, Salesa-Gutiérrez de Rozas R, Hermana-Ramírez S. Leishmaniose cutanea esporotricoide. Actas Dermosifiliogr. 2007;98:444–445. [PubMed] [Google Scholar]
- 8.8. Almeida AB, Souza VR, Sorte EC, Figueiredo FB, Paula DA, Pimentel MF, et al. Use of parasitological culture to detect Leishmania (Leishmania) chagasi in naturally infected dogs. Vector Borne Zoonotic Dis. 2011;11:1555-60. [DOI] [PubMed]; Almeida AB, Souza VR, Sorte EC, Figueiredo FB, Paula DA, Pimentel MF, et al. Use of parasitological culture to detect Leishmania (Leishmania) chagasi in naturally infected dogs. Vector Borne Zoonotic Dis. 2011;11:1555–1560. doi: 10.1089/vbz.2011.0723. [DOI] [PubMed] [Google Scholar]
- 9.9. Quintella L, Cuzzi T, Madeira M, Okamoto T, Schubach AO. Immunoperoxidase technique using an anti-Leishmania (L.) chagasi hyperimmune serum in the diagnosis of culture-confirmed American tegumentary leishmaniasis. Rev Inst Med Trop Sao Paulo. 2009;51:83-6. [DOI] [PubMed]; Quintella L, Cuzzi T, Madeira M, Okamoto T, Schubach AO. Immunoperoxidase technique using an anti-Leishmania (L.) chagasi hyperimmune serum in the diagnosis of culture-confirmed American tegumentary leishmaniasis. Rev Inst Med Trop Sao Paulo. 2009;51:83–86. doi: 10.1590/s0036-46652009000200005. [DOI] [PubMed] [Google Scholar]
- 10.10. Fagundes A, Marzochi MC, Perez M, Schubach A, Ferreira A, Silva JP, et al. Skin reactivity to thimerosal and phenol-preserved Montenegro antigen in Brazil. Acta Trop. 2007;101:25-30. [DOI] [PubMed]; Fagundes A, Marzochi MC, Perez M, Schubach A, Ferreira A, Silva JP, et al. Skin reactivity to thimerosal and phenol-preserved Montenegro antigen in Brazil. Acta Trop. 2007;101:25–30. doi: 10.1016/j.actatropica.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11.11. Barroso-Freitas AP, Passos SR, Mouta-Confort E, Madeira MF, Schubach AO, Santos GP, et al. Accuracy of an ELISA and indirect immunofluorescence for the laboratory diagnosis of American tegumentary leishmaniasis. Trans R Soc Trop Med Hyg. 2009;103:383-9. [DOI] [PubMed]; Barroso-Freitas AP, Passos SR, Mouta-Confort E, Madeira MF, Schubach AO, Santos GP, et al. Accuracy of an ELISA and indirect immunofluorescence for the laboratory diagnosis of American tegumentary leishmaniasis. Trans R Soc Trop Med Hyg. 2009;103:383–389. doi: 10.1016/j.trstmh.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 12.12. Furtado T. Critérios para o diagnóstico da leishmaniose tegumentar americana. An Bras Dermatol. 1980;55:81-6.; Furtado T. Critérios para o diagnóstico da leishmaniose tegumentar americana. An Bras Dermatol. 1980;55:81–86. [Google Scholar]
- 13.13. Barros MB, Paes RA, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-54. [DOI] [PMC free article] [PubMed]; Barros MB, Paes RA, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633–654. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.14. Cuba-Cuba CA, Marsden PD, Barretto AC, Jones TC, Richards F. The use of different concentrations of leishmanial antigen in skin testing to evaluate delayed hypersensitivity in American cutaneous leishmaniasis. Rev Soc Bras Med Trop. 1985;18:231-6.; Cuba-Cuba CA, Marsden PD, Barretto AC, Jones TC, Richards F. The use of different concentrations of leishmanial antigen in skin testing to evaluate delayed hypersensitivity in American cutaneous leishmaniasis. Rev Soc Bras Med Trop. 1985;18:231–236. [Google Scholar]
- 15.15. Manzur A, Bari A. Sensitivity of leishmanin skin test in patients of acute cutaneous leishmaniasis. Dermatol Online J. 2006;12:2. [PubMed]; Manzur A, Bari A. Sensitivity of leishmanin skin test in patients of acute cutaneous leishmaniasis. 2Dermatol Online J. 2006;12 [PubMed] [Google Scholar]
- 16.16. Sadeghian G, Momeni A, Siadat AH, Yousefi P. Evaluation of leishmanin skin test and its relationship with the clinical form and duration of cutaneous leishmaniasis. Dermatol Online J. 2006;12:3. [PubMed]; Sadeghian G, Momeni A, Siadat AH, Yousefi P. Evaluation of leishmanin skin test and its relationship with the clinical form and duration of cutaneous leishmaniasis. 3Dermatol Online J. 2006;12 [PubMed] [Google Scholar]
- 17.17. De Lima Barros MB, Schubach A, Francesconi-do-Valle AC, Gutierrez-Galhardo MC, Schubach TM, Conceição-Silva F, et al. Positive Montenegro skin test among patients with sporotrichosis in Rio de Janeiro. Acta Trop. 2005;93:41-7. [DOI] [PubMed]; De Lima Barros MB, Schubach A, Francesconi-do-Valle AC, Gutierrez-Galhardo MC, Schubach TM, Conceição-Silva F, et al. Positive Montenegro skin test among patients with sporotrichosis in Rio de Janeiro. Acta Trop. 2005;93:41–47. doi: 10.1016/j.actatropica.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 18.18. Cataldo JI, Mello FC, Mouta-Confort E, Madeira MF, Schubach AO, Genestra MS, et al. Immunoenzymatic assay for the diagnosis of American tegumentary leishmaniasis using soluble and membrane-enriched fractions from infectious Leishmania (Viannia) braziliensis. J Clin Lab Anal. 2010;24:289-94. [DOI] [PMC free article] [PubMed]; Cataldo JI, Mello FC, Mouta-Confort E, Madeira MF, Schubach AO, Genestra MS, et al. Immunoenzymatic assay for the diagnosis of American tegumentary leishmaniasis using soluble and membrane-enriched fractions from infectious Leishmania (Viannia) braziliensis. J Clin Lab Anal. 2010;24:289–294. doi: 10.1002/jcla.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.19. Romero GA, Guerra MV, Paes MG, Macedo VO. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. Am J Trop Med Hyg. 2001;65:456-65. [DOI] [PubMed]; Romero GA, Guerra MV, Paes MG, Macedo VO. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. Am J Trop Med Hyg. 2001;65:456–465. doi: 10.4269/ajtmh.2001.65.456. [DOI] [PubMed] [Google Scholar]
- 20.20. Cozzani E, Satta R, Fausti V, Cottoni F, Parodi A. Cutaneous sporotrichoid leishmaniasis resistant to pentavalent antimonial therapy: complete resolution with itraconazole. Clin Exp Dermatol. 2011;36:49-51. [DOI] [PubMed]; Cozzani E, Satta R, Fausti V, Cottoni F, Parodi A. Cutaneous sporotrichoid leishmaniasis resistant to pentavalent antimonial therapy: complete resolution with itraconazole. Clin Exp Dermatol. 2011;36:49–51. doi: 10.1111/j.1365-2230.2010.03855.x. [DOI] [PubMed] [Google Scholar]
- 21.21. Masmoudi A, Ayadi N, Khabir A, Bouzid L, Bouassida S, Meziou T J, et al. Forme sporotrichoide de leishmaniose cutanée em Tunisie: étude clinique et histologique. Ann Dermatol Venereol. 2008;135:63-7. [DOI] [PubMed]; Masmoudi A, Ayadi N, Khabir A, Bouzid L, Bouassida S, Meziou T J, et al. Forme sporotrichoide de leishmaniose cutanée em Tunisie: étude clinique et histologique. Ann Dermatol Venereol. 2008;135:63–67. doi: 10.1016/j.annder.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 22.22. Talari S A, Talaei R, Shajari G, Vakili Z, Taghaviardakani A. Childhood cutaneous leishmaniasis: report of 117 cases from Iran. Korean J Parasitol. 2006;44:355-60. [DOI] [PMC free article] [PubMed]; Talari S A, Talaei R, Shajari G, Vakili Z, Taghaviardakani A. Childhood cutaneous leishmaniasis: report of 117 cases from Iran. Korean J Parasitol. 2006;44:355–360. doi: 10.3347/kjp.2006.44.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.23. de Oliveira MM, Veríssimo C, Sabino R, Aranha J, Zancopé-Oliveira RM, Sampaio P, et al. First autochthone case of sporotrichosis by Sporothrix globosa in Portugal. Diagn Microbiol Infect Dis. 2014;78:388-90. [DOI] [PubMed]; Oliveira MM, Veríssimo C, Sabino R, Aranha J, Zancopé-Oliveira RM, Sampaio P, et al. First autochthone case of sporotrichosis by Sporothrix globosa in Portugal. Diagn Microbiol Infect Dis. 2014;78:388–390. doi: 10.1016/j.diagmicrobio.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 24.24. Mata-Essayag S, Delgado A, Colella MT, Landaeta-Nezer ME, Rosello A, Perez de Salazar C, et al. Epidemiology of sporotrichosis in Venezuela. Int J Dermatol. 2013;52:974-80. [DOI] [PubMed]; Mata-Essayag S, Delgado A, Colella MT, Landaeta-Nezer ME, Rosello A, Perez de Salazar C, et al. Epidemiology of sporotrichosis in Venezuela. Int J Dermatol. 2013;52:974–980. doi: 10.1111/j.1365-4632.2012.05849.x. [DOI] [PubMed] [Google Scholar]
- 25.25. Subedi S, Kidd SE, Baird RW, Coatsworth N, Ralph AP. Sporotrichosis from the northern territory of Australia. Am J Trop Med Hyg. 2014;91:1263-8. [DOI] [PMC free article] [PubMed]; Subedi S, Kidd SE, Baird RW, Coatsworth N, Ralph AP. Sporotrichosis from the northern territory of Australia. Am J Trop Med Hyg. 2014;91:1263–1268. doi: 10.4269/ajtmh.14-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.26. Tobin EH, Jih WW. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician. 2001;63:326-32. [PubMed]; Tobin EH, Jih WW. Sporotrichoid lymphocutaneous infections: etiology, diagnosis and therapy. Am Fam Physician. 2001;63:326–332. [PubMed] [Google Scholar]


