Abstract
The airway epithelium in asthma displays altered repair and incomplete barrier formation. Basal cells are the progenitor cells of the airway epithelium, and can repopulate other cell types after injury. We previously reported increased numbers of basal cells expressing the transcription factor p63 in the airway epithelium of patients with asthma. Here we sought to determine the molecular consequences of p63 expression in basal human airway epithelial cells during wound repair. Because at least six isoforms of p63 exist (N-terminally truncated [ΔN] versus transcriptional activation promoter variants and α, β, or γ 3′ splice variants), the expression of all isoforms was investigated in primary human airway epithelial cells (pHAECs). We modulated p63 expression, using small interfering RNA (siRNA) and adenoviral constructs to determine the effects of p63 on 21 candidate target genes by RT-PCR, and on repair using a scratch wound assay. We found that basal pHAECs from asthmatic and nonasthmatic donors predominantly expressed the N-terminally truncated p63α variant (ΔNp63α) isoform, with no disease-specific differences in expression. The knockdown of ΔNp63, using specific siRNA, decreased the expression of 11 out of 21 genes associated with epithelial repair and differentiation, including β-catenin, epidermal growth factor receptor, and Jagged1. The loss of ΔNp63 significantly inhibited wound closure (which was associated with the decreased expression of β-catenin and Jagged1), reduced epithelial proliferation as measured by Ki-67 staining, and increased E-cadherin expression, potentially preventing cytokinesis. In conclusion, ΔNp63α is the major isoform expressed in basal pHAECs, and is essential for epithelial wound repair. The role of ΔNp63α in epithelial barrier integrity requires further study to understand its role in health and disease.
Keywords: p63, airway epithelium, wound repair, asthma
The airway epithelium is the first structural barrier to the inhaled environment. The epithelium of patients with asthma displays several structural and functional abnormalities, including goblet cell metaplasia, a loss of ciliated epithelial cells, increased susceptibility to oxidant-induced stress, abnormal cytokine and extracellular matrix release, and mitotic dyssynchrony (1–5). Our group (6, 7) has also described a marked expansion of the population of basal cells expressing the transcription factor p63 in the epithelium of patients with asthma. Importantly, this phenotype is conserved when these cells are grown in vitro under air–liquid interface (ALI) culture conditions (7), indicating that this expansion is an intrinsic response and not solely a product of an in vivo inflammatory milieu.
The transcription factor p63 is a homologue of the p53 tumor suppressor, and is absolutely required for the appropriate development of stratified epithelial tissues. The genetic deletion of p63 in mice results in the absence of stratified epithelia, most notably in the epidermis (8, 9). The subsequent deficiency of barrier function results in perinatal lethality because of dehydration. Similarly, the airway epithelium is the first structural barrier to the inhaled environment, and its ability to repair and regenerate after injury is crucial to maintaining normal tissue function. The basal cells of the airway epithelium exhibit progenitor capacity, and are able to repopulate other cell types, including ciliated and mucus-producing cells, during cellular differentiation (6, 10) and in response to injury (11). In contrast to the normal pseudostratified airway epithelium consisting of basal, ciliated, and mucous cells, the tracheobronchial epithelium of newborn p63−/− mice displays only a single layer of columnar ciliated cells and a complete absence of basal and mucous cells (12). Thus, the lack of stratified epithelium in p63−/− mice has led to two hypotheses, namely, (1) p63 may play a role in maintaining stem-cell populations (8, 13), or (2) p63 may regulate the commitment to specific epithelial lineages (9).
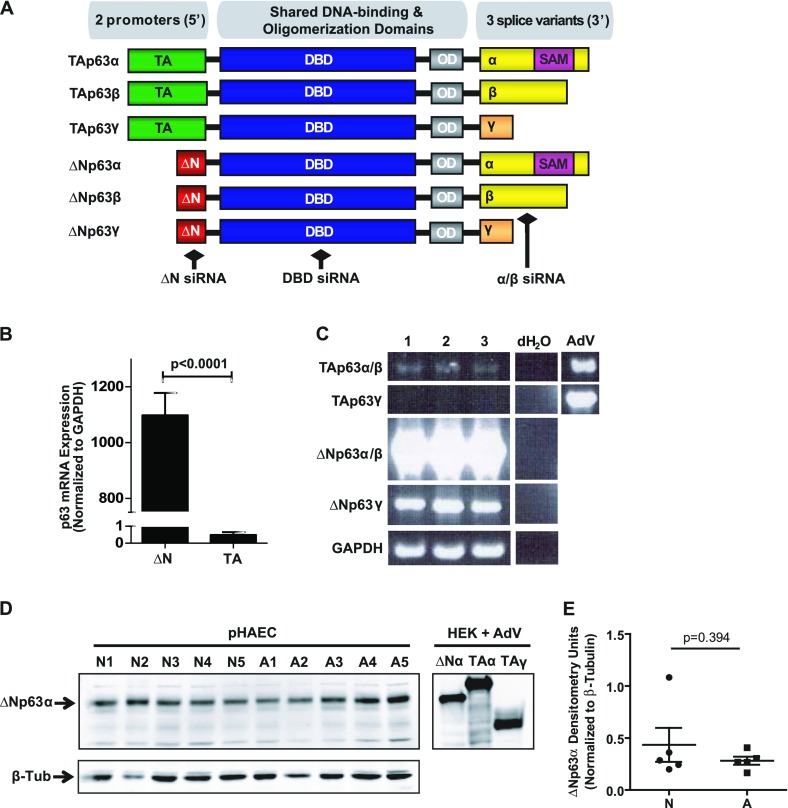
Studies of p63 have demonstrated that the gene can be expressed in at least six different mRNA/protein isoforms (14). The initiation of transcription at the canonical promoter gives rise to isoforms containing the full-length transcriptional activation (TA) domain, whereas initiation from an alternative promoter within intron three produces the N-terminally truncated (ΔN) isoforms (Figure 1A). All isoforms share a common DNA-binding domain (DBD) and an oligomerization domain, which are required for appropriate function (15). Alternative splicing at the 3′ end produces transcripts of varying length, termed α, β, and γ. The α and β splice variants are highly similar. The α variant contains only one additional exon, which encodes a sterile alpha motif and a transcription inhibition domain. The γ variant is much shorter and contains a novel 3′ end. Two additional 3′ variants, δ and ε, have been reported, which function similarly to the N-terminally truncated p63α variant (ΔNp63α) (16), although most current work remains focused on the α, β, and γ variants. ΔNp63α is by far the most predominant endogenous isoform in basal cells of stratified epithelial tissues such as the skin (14), cornea (17), and prostate (18), and thus has been the most frequent subject of functional studies. To date, the expression and role of specific p63 isoforms in the maintenance and repair of the human airway epithelium have not been explored.
Figure 1.
The N-terminally truncated p63 α variant (ΔNp63α) is the most abundant isoform in human airway epithelial cells in vitro. (A) Schematic of p63 isoforms arising from the TP63 gene locus, indicating the two possible promoter variants (transcriptional activation [TA] versus N-terminally truncated [ΔN]), a shared DNA-binding (DBD) and oligomerization (OD) domain, and at least three 3′ splice variants (α, β, and γ). The α isoforms contain a sterile α motif (SAM). Approximate binding sites of small interfering (siRNA) to the ΔN, DBD, and α/β domains are indicated. (B) Quantitative RT-PCR for ΔN versus TAp63 mRNA in cultured primary human airway epithelial cells (pHAECs) indicates robust expression of the ΔN but not the TA isoforms (n = 7). (C) Semiquantitative PCR for full-length p63 isoforms in cultured pHAECs from n = 3 donors indicates that ΔNp63α/β are the predominant mRNA isoforms. Adenoviral (AdV) overexpression provides a positive control for TAp63α and TAp63γ, which were not endogenously detected. Distilled water (dH2O) was used as a no-template control. (D) Immunoblot for total p63 in monolayer pHAEC cultures (from n = 5 each) nonasthmatic (N) and asthmatic (A) donors (left). Adenoviral overexpression of ΔNp63α, TAp63α, and TAp63γ in human embryonic kidney (HEK) cells (right), included as positive controls. β-tubulin (β-Tub) is included as a loading control. (E) Densitometry analysis of ΔNp63α protein, followed by a two-tailed, unpaired t test, indicates that ΔNp63α expression is not significantly different between disease groups (P = 0.394). GAPDH, glyceraldehyde 3–phosphate dehydrogenase.
In this study, we hypothesized that an overabundance of p63 contributes to the observed abnormalities in cellular function and repair observed in the asthmatic epithelium. We sought to determine which specific isoforms of p63 are expressed in the human airway epithelial basal cell, and how these isoforms contribute to the regulation of key genes involved in processes such as proliferation and repair.
Materials and Methods
Human Airway Epithelial Cell Culture
This study was approved by the Research Ethics Board of the University of British Columbia. Primary human airway epithelial cells (pHAECs) were isolated from nonasthmatic and asthmatic donor lungs deemed unsuitable for transplantation, as described previously (6). In addition, pHAECS were obtained by bronchial brushings of nonasthmatic and asthmatic subjects (19) or from commercial sources (normal human bronchial epithelial cells; Lonza, Basel, Switzerland). pHAECs were maintained in bronchial epithelial growth media (Lonza) and supplemented with antibiotics/antimycotics (Gibco, Burlington, ON, Canada). Experiments were performed on pHAECs at Passage 2 or 3. A549 cells were cultured in Dulbecco’s Modified Eagle’s Medium, supplemented with 10% FBS and antibiotics (all from Gibco). Minimally immortalized bronchial epithelial cells (HBEC6-KTs) were generously provided by Dr. John Minna (20) and maintained in keratinocyte serum-free media (Invitrogen, Burlington, ON, Canada) with growth supplements and antibiotics. Experiments were performed on HBEC6-KTs at Passages 12 and 13. For full culture conditions, see the online supplement.
Small Interfering RNAs and Adenoviruses
Small interfering RNAs (siRNAs) were designed to target specific domains of p63. Sequences can be found in the online supplement. pHAECs were transfected with 50 nM siRNA using HiPerfect (5% vol/vol; Qiagen, Valencia, CA), according to the manufacturer’s instructions.
Adenoviruses encoding ΔNp63α (21), TAp63α, TAp63γ, and b-galactosidase (LacZ) (22) were kindly provided by Dr. Wendy Weinberg. pHAECs were infected at 70% confluence at a multiplicity of infection (MOI) of 0.15 plaque-forming units (pfu) per cell. A549 cells were infected at 30% confluence with an MOI of 1.0 or 3.0 pfu/cell. Samples were collected after 48 hours (RNA) or 72 hours (protein).
Gene Expression Analysis
RNA was extracted using an RNeasy Mini Kit (Qiagen) and reverse-transcribed. Gene expression was determined by TaqMan quantitative RT-PCR, according to the manufacturer’s instructions (Applied Biosystems, Burlington, ON, Canada) or by semiquantitative PCR. Refer to the online supplement for primers and conditions.
Epithelial Gene Expression Array
Five hundred nanograms of total RNA were used to synthesize cDNA for use on custom-designed, preloaded RT2 Profiler Arrays (SABiosciences, QIAGEN, Valenica, CA) containing quantitative RT-PCR primers for 21 potential p63-regulated genes. Gene expression was normalized to glyceraldehyde 3–phosphate dehydrogenase, and subsequently to untreated control values. Data are expressed as means ± SEMs between all experiments.
Wound Repair
HBEC6-KT cells were seeded in four-well chamber slides and transfected at 70% confluence with 25 nM siRNA and 3% vol/vol HiPerfect. After 24 hours, cells were scratch-wounded in a cross pattern, rinsed, and retransfected. Wounds were photographed at 0, 4, 8, and 24 hours. Repair was quantified using ImagePro Plus software (Media Cybernetics, Rockville, MD). Samples were then either lysed for quantitative RT-PCR, the immunoblotting of proteins of interest, or fixed for 20 minutes in 4% paraformaldehyde to determine proliferation. A full description is presented in the online supplement.
Statistical Analysis
One-way ANOVA followed by the Dunnett post hoc test was used to assess statistical significance of treatment conditions versus control conditions (Prism version 5.0; GraphPad Software, La Jolla, CA) for multiple conditions. A two-tailed, unpaired t test was used for two-category comparisons. P < 0.05 was considered statistically significant.
Results
ΔNp63α Is the Most Abundant p63 Isoform in Human Airway Epithelial Basal Cells
When submerged in culture, airway epithelial cells form a relatively homogenous cuboidal monolayer that is representative of basal cells within the airway epithelium. We have shown that p63 is only expressed in basal cells identified by positive staining for cytokeratin-5, CD151, and tissue factor (6). Given the multiple possible isoforms of p63, we first needed to determine the relative contributions of the ΔN and TA isoforms in pHAECs. Using quantitative PCR, we show abundant expression of ΔNp63 in pHAECs, whereas TAp63 expression is virtually undetectable (Figure 1B). We then used semiquantitative PCR to detect the presence of full-length mRNA isoforms of p63 in pHAECs (Figure 1C). As shown in Figure 1A, to design primers that are specific for the p63β isoform is not possible, so we used primers that were specific for both the p63α/β isoforms and γ isoforms. Using this strategy, we determined that the most predominant isoforms expressed were ΔNp63α/β, followed by ΔNp63γ. Although TAp63α/β was detected, it was at a very low level even after 40 cycles of amplification, which is in agreement with our quantitative PCR data for the TA isoform. However, endogenous TAp63γ mRNA was not detected. As TAp63 isoforms were expressed at such low levels, we used the adenovirus-mediated overexpression of TAp63α or TAp63γ in pHAECs as positive controls, and were able to detect robust expression, indicating that endogenous levels of TAp63 isoforms are indeed extremely low in pHAECs.
We then used immunoblotting to confirm our mRNA findings and demonstrate that the ΔNp63α/β isoform is the most abundantly expressed protein isoform in basal pHAECs (Figure 1D, left). The adenovirus-mediated overexpression of ΔNp63α, TAp63α, and TAp63γ in human embryonic kidney cells are included as positive controls and for molecular weight comparisons (Figure 1D, right), indicating that although our system was also able to detect TAp63, the endogenous levels were too low to detect in pHAECs.
We have previously shown that the number of basal epithelial cells expressing p63 is elevated in asthmatic compared with nonasthmatic donors, both in the airway and in ALI cultures (7). To determine whether the expression of p63 was altered in a homogenous basal culture system, we compared the expression of ΔNp63α protein in monolayer cultures of pHAECs derived from nonasthmatic (N) and asthmatic (A) donors (Figure 1D; see Table 1 for patient data). In contrast to our findings in differentiated airway epithelium, no significant difference in the expression of ΔNp63α protein was evident between basal pHAECs derived from asthmatic and nonasthmatic donors, as measured by densitometry (Figure 1E; P = 0.394).
TABLE 1.
DONOR DEMOGRAPHICS OF PHAEC CULTURES USED FOR IMMUNOBLOTTING OF p63 ISOFORMS
| Donor | Sex | Age (yr)* | Asthma Status† | Sample Type | Cause of Death |
|---|---|---|---|---|---|
| N1 | F | 26 | None; PC20 > 8.0 | Bronchial brushing | |
| N2 | M | 20 | None | Donor lung | Head trauma |
| N3 | F | 23 | None; PC20 > 8.0 | Bronchial brushing | |
| N4 | F | 47 | None | Donor lung | Head trauma |
| N5 | M | 29 | None; PC20 > 8.0 | Bronchial brushing | |
| A1 | F | 23 | PC20 = 0.757 | Bronchial brushing | |
| A2 | F | 23 | PC20 = 0.25 | Bronchial brushing | |
| A3 | M | 11 | Diagnosed; age 2 years | Donor Lung | Anoxia; asthma |
| A4 | F | 29 | PC20 = 0.42 | Bronchial brushing | |
| A5 | M | 25 | Diagnosed; age 11 years | Donor lung | Anoxia; asthma |
Definition of abbreviations: A, asthmatic; F, female; M, male; N, nonasthmatic; PC20, provocative concentration of methacholine causing a 20% fall in FEV1; PHAEC, primary human airway epithelial cell.
Unpaired, two-tailed t test confirmed no significant difference in mean age between disease groups (P = 0.267).
For cells obtained by bronchial brushing, methacholine challenge data either confirmed (PC20 < 1.0 mg/ml) or excluded (PC20 > 8.0 mg/ml) a diagnosis of asthma.
Knockdown of ΔNp63α Decreases the Expression of Genes Involved in Epithelial Differentiation and Repair
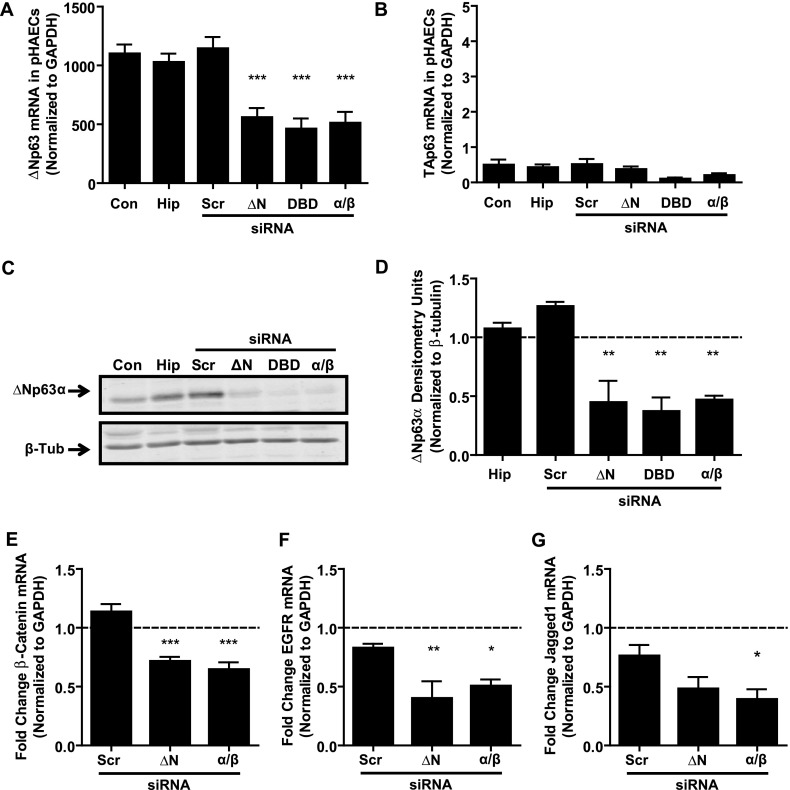
To understand the effects of loss of function of ΔNp63α in pHAECs, we designed siRNAs to target different regions of this mRNA, including the 5′ (ΔN), DNA-binding (DBD), and 3′ α/β domains of this isoform. The knockdowns using ΔN, DBD, or α/β siRNA each significantly decreased total ΔNp63 mRNA expression in pHAECs (P < 0.001; Figure 2A), but did not significantly affect TAp63 mRNA expression (Figure 2B). We next determined the impact of ΔNp63 mRNA knockdown on ΔNp63α protein expression by immunoblotting (Figure 2C). The densitometry analysis of three independent experiments showed a significant reduction in ΔNp63α protein expression after transfection with each of the siRNAs (P < 0.01; Figure 2D). Given that ΔN and α/β are most specific for the ΔNp63α isoform, we used these siRNA constructs to understand which candidate target genes are regulated by ΔNp63α in pHAECs from nonasthmatic donors (n = 5; see Table 2 for patient data).
Figure 2.
Loss of p63 significantly down-regulates genes involved in epithelial function. (A) Quantitative RT-PCR of ΔNp63 mRNA in pHAECs, 48 hours after transfection with siRNA to N-terminally truncated (ΔN), DNA binding (DBD), or α/β domains of p63, indicates significantly decreased expression of ΔNp63 with each of the p63-specific siRNAs, but no change with HiPerfect (Hip) transfection reagent or scrambled siRNA (Scr) construct. Con, control. (B) Quantitative RT-PCR of TAp63 mRNA in pHAECs indicates no change at 48 hours after transfection with any siRNA constructs. (C) Representative immunoblot of ΔNp63α protein, 72 hours after transfection with 50 nM siRNA to ΔN, DBD, or α/β domains of p63. (D) Densitometry analysis of ΔNp63α expression in n = 3 pHAEC experiments. Values were normalized to β-tubulin as a loading control, and are shown as fold change over untreated control values (denoted with dashed line). (E–G) Quantitative RT-PCR of candidate p63 target genes by epithelial gene expression array, 48 hours after the knockdown of ΔN or α/β isoforms of p63 in n = 5 pHAECs. (E) β-catenin. (F) Epidermal growth factor receptor (EGFR). (G) Jagged1. Gene expression (relative to glyceraldehyde 3–phosphate dehydrogenase [GAPDH]) is shown as fold change over untreated control condition (dashed line). One-way ANOVA with the Dunnett post hoc test was used to assess statistical significance between treatments and scrambled siRNA control. *P < 0.05. **P < 0.01. ***P < 0.001.
TABLE 2.
DONOR DEMOGRAPHICS OF NONASTHMATIC PHAEC CULTURES USED FOR EPITHELIAL GENE EXPRESSION ARRAY
| Donor | Sex | Age (yr) | Sample Type* | Cause of Death |
|---|---|---|---|---|
| N1 | M | 20 | Donor lung | Head trauma |
| N2 | F | 20 | Donor lung | Head trauma |
| N3 | M | 21 | NHBEs (Lonza, Basel, Switzerland) | |
| N4 | F | 23 | Bronchial brushing | |
| N5 | M | 47 | Donor lung | Head trauma |
Definition of abbreviations: F, female; M, male; N, nonasthmatic; NHBEs, normal human bronchial epithelial cells; PHAEC, primary human airway epithelial cell.
Airway epithelial cells were obtained from the upper airways of human lungs deemed unsuitable for transplantation, from bronchial brushing or commercially available NHBEs. All donors lacked doctor-diagnosed asthma.
To determine the impact of ΔNp63 knockdown on genes involved in key epithelial differentiation and repair functions, we designed an array of PCR primers for 21 genes (Table 3) that have been shown to be regulated by p63 in other epithelial tissues and/or that contain a p63 response element in their promoter region (according to the p53 FamTaG database) (23). We found that 11 of 21 candidate target genes were significantly down-regulated by the knockdown of ΔNp63α with either the ΔN or α/β siRNA constructs (Table 3), compared with the scrambled control samples. Interestingly, we found no genes that showed increased mRNA expression after the knockdown of ΔNp63α. As demonstrated by three of the 11 p63-regulated genes, CTNNB1 (β-catenin; Figure 2E), epidermal growth factor receptor (EGFR; Figure 2F), and JAG1 (Jagged1; Figure 2G), both the ΔN and α/β siRNA constructs induced similar mRNA down-regulation, indicating that ΔNp63α/β is the predominant isoform of ΔNp63 inducing this phenotype. These three genes in particular were selected according to their potential contributions to epithelial differentiation and repair in asthma. β-catenin is a subunit of the adherens junction that anchors the actin cytoskeleton to regulate cell growth and adhesion between cells, and can also function as a transcriptional coactivator (24, 25). EGFR regulates responses to the epithelial mitogen epidermal growth factor, and is crucially involved in the process of repair in the epithelium (26, 27). JAG1 encodes the developmental gene Jagged1, a ligand for the Notch family of proteins that is involved in cell-fate determination in tissue-resident progenitor cells (28).
TABLE 3.
EPITHELIAL GENE EXPRESSION ARRAY OVERVIEW: FOLD CHANGE IN GENE EXPRESSION AFTER 48-HOUR TREATMENT WITH SIRNA TO ΔN OR α/β p63 ISOFORMS
| Gene | ΔN siRNA |
α/β siRNA |
||
|---|---|---|---|---|
| Fold Change ± SEM* | P Value† | Fold Change ± SEM | P Value | |
| CTNNB1 | 0.71 ± 0.04 | < 0.001 | 0.64 ± 0.06 | < 0.001 |
| LAMC2 | 0.46 ± 0.07 | < 0.001 | 0.68 ± 0.05 | < 0.01 |
| TJP1 | 0.62 ± 0.02 | < 0.001 | 0.62 ± 0.07 | < 0.001 |
| CD44 | 0.68 ± 0.05 | < 0.01 | 0.64 ± 0.08 | < 0.001 |
| EGFR | 0.40 ± 0.15 | < 0.01 | 0.50 ± 0.06 | < 0.05 |
| EP300 | 0.66 ± 0.06 | < 0.01 | 0.54 ± 0.07 | < 0.001 |
| F11R/JAM1 | 0.57 ± 0.07 | < 0.01 | 0.56 ± 0.07 | < 0.001 |
| GSK3B | 0.74 ± 0.05 | < 0.01 | 0.81 ± 0.06 | < 0.01 |
| PLEC1 | 0.50 ± 0.08 | < 0.05 | 0.50 ± 0.10 | < 0.05 |
| CCND1 | 0.84 ± 0.06 | < 0.05 | 0.77 ± 0.11 | < 0.01 |
| JAG1 | 0.48 ± 0.10 | NS | 0.39 ± 0.09 | < 0.05 |
| CDKN1A | 0.86 ± 0.07 | NS | 0.91 ± 0.08 | NS |
| CEBP | 1.14 ± 0.18 | NS | 0.92 ± 0.09 | NS |
| KRT18 | 0.68 ± 0.07 | NS | 0.69 ± 0.10 | NS |
| KRT5 | 0.57 ± 0.07 | NS | 0.52 ± 0.13 | NS |
| CLDN1‡ | 0.95 ± 0.05 | < 0.001 | 0.66 ± 0.08 | P < 0.001 |
| NOTCH2‡ | 0.83 ± 0.04 | < 0.001 | 0.75 ± 0.06 | P < 0.001 |
| BMP7 | Low expression | Low expression | ||
| EGF | Low expression | Low expression | ||
| FGFR2 | Low expression | Low expression | ||
| WNT1 | Low expression | Low expression | ||
Definition of abbreviations: ΔN, N-terminally truncated; NS, no significance; siRNA, small interfering RNA.
Values expressed represent fold change ± SEM, relative to untreated control samples (n = 5 donors).
One-way ANOVA, followed by the Dunnett post hoc test, was used to assess statistical significance versus scrambled siRNA construct.
Indicates genes that were significantly altered by scrambled siRNA treatment versus untreated control samples.
Overexpression of ΔNp63α Alters the Expression of Genes Involved in Epithelial Function
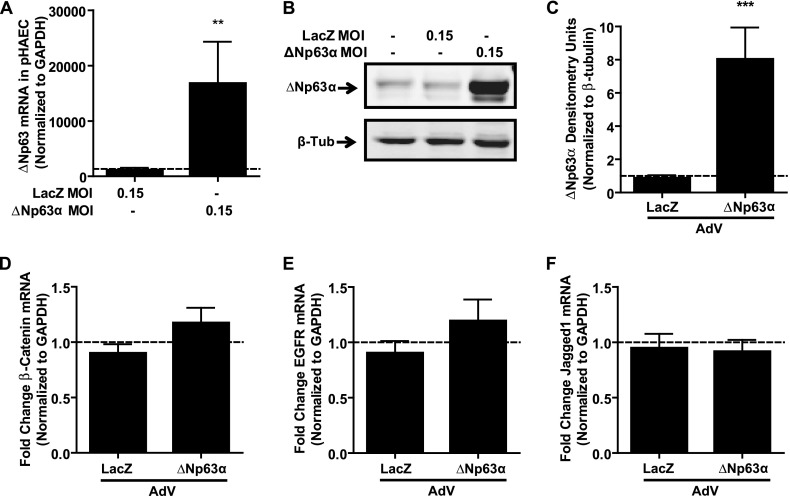
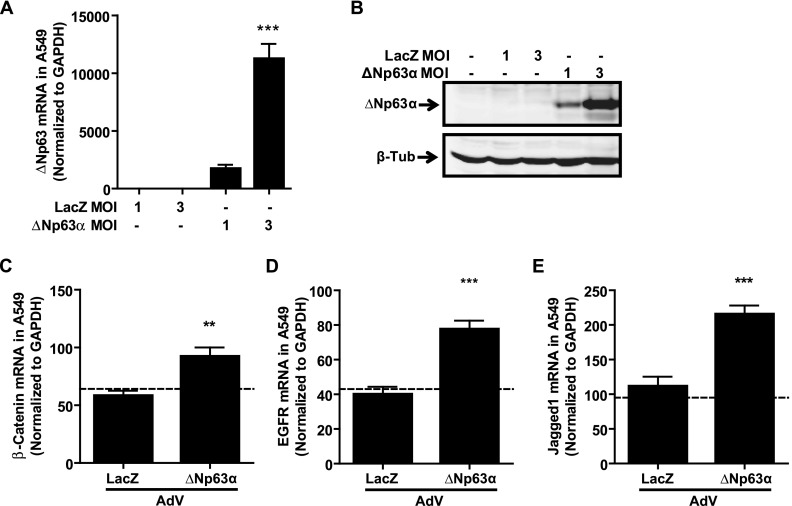
Having shown that the knockdown of ΔNp63α/β reduces the mRNA expression of β-catenin, EGFR, and Jagged1, we investigated the effects of overexpressing ΔNp63α using adenoviral vectors (21). Infection with adenovirus encoding ΔNp63α but not LacZ (MOI = 0.15 pfu/cell) led to a statistically significant increase in ΔNp63 mRNA after 48 hours (Figure 3A; P < 0.01) and an up-regulation of ΔNp63α protein, as shown by immunoblot after 72 hours (Figure 3B). When quantified by densitometry, ΔNp63α adenovirus infection resulted in a statistically significant eightfold increase in ΔNp63α protein expression (Figure 3C; P < 0.001). Surprisingly, the analysis of mRNA expression for any of the three candidate genes (β-catenin, EGFR, or Jagged1) showed no significant changes with ΔNp63α overexpression (Figures 3D–3F). Because endogenous ΔNp63α is highly expressed within basal pHAECs, we hypothesized that the lack of effect on target gene expression was attributable to the saturation of ΔNp63α signaling, despite further exogenous ΔNp63α expression. To test this hypothesis, we infected an alveolar Type II cell line (A549) that lacks endogenous ΔNp63α with ΔNp63α-encoding adenovirus. Infection with ΔNp63α-encoding adenovirus at an MOI of 3.0 pfu/cell significantly induced ΔNp63 mRNA expression in A549 cells (Figure 4A; P < 0.001), to a level comparable to that observed after infection with an MOI of 0.15 pfu/cell in pHAECs (as seen in Figure 3A). ΔNp63α protein expression (Figure 4B) was also induced by ΔNp63α adenovirus in A549 cells. Importantly, the adenovirus-mediated overexpression of ΔNp63α (MOI 3.0 pfu/cell) in A549 cells significantly increased the expression of β-catenin (Figure 4C; P < 0.01), EGFR (Figure 4D; P < 0.001), and Jagged1 (Figure 4E; P < 0.001) mRNA, compared with LacZ-infected conditions, supporting our hypothesis that ΔNp63α is indeed a positive regulator of these three target genes in human pulmonary epithelial cells.
Figure 3.
Exogenous ΔNp63α does not induce target gene expression in pHAECs. (A) Quantitative RT-PCR of ΔNp63, 48 hours after transduction with adenovirus expressing either LacZ or ΔNp63α (multiplicity of infection [MOI] = 0.15 plaque-forming units [pfu]/cell), indicates robust induction of ΔNp63 mRNA expression. (B) Representative immunoblot of ΔNp63α protein, 72 hours after transduction with adenovirus and (C) densitometry analysis of ΔNp63α protein expression in n = 3 experiments, indicates a significant up-regulation of ΔNp63α protein. Values were normalized to β-tubulin as a loading control, and are shown as fold change over untreated control values (dashed line). (D–F) Quantitative RT-PCR of candidate downstream target genes, 48 hours after adenoviral transduction in n = 4 pHAECs. (D) β-catenin. (E) Epidermal growth factor receptor. (F) Jagged1. Statistical significance was assessed using an unpaired two-tailed t test between LacZ and ΔNp63α adenovirus treatment. Dashed line represents values in untreated control samples. **P < 0.01. ***P < 0.001.
Figure 4.
Exogenous ΔNp63α significantly induces expression of candidate gene expression in p63-null A549 cells. (A) Quantitative RT-PCR analysis of ΔNp63 expression, 48 hours after transduction with adenovirus expressing LacZ or ΔNp63α at MOI = 1.0 or 3.0 pfu/cell in p63-null A549 cells (n = 6), demonstrates negligible endogenous ΔNp63 mRNA, but a significant up-regulation of ΔNp63 mRNA after treatment with ΔNp63α adenovirus, as assessed by one-way ANOVA with the Dunnett post hoc test. (B) Representative immunoblot for total p63 protein, 72 hours after adenoviral infection, indicates expression of ΔNp63α protein only in the presence of ΔNp63α adenovirus. β-tubulin is included as loading control. (C–E) Quantitative RT-PCR for target gene mRNA, 48 hours after the adenoviral (AdV) transduction of A549 cells with LacZ or ΔNp63α (MOI = 3.0 pfu/cell), indicates a significant up-regulation of all target genes by ΔNp63α. (C) β-catenin. (D) EGFR. (E) Jagged1. Expression data were normalized to GAPDH for n = 6 experiments. Dashed line represents values in untreated control samples. Statistical significance was assessed using an unpaired, two-tailed t test between LacZ and ΔNp63α adenovirus treatment. **P < 0.01. ***P < 0.001.
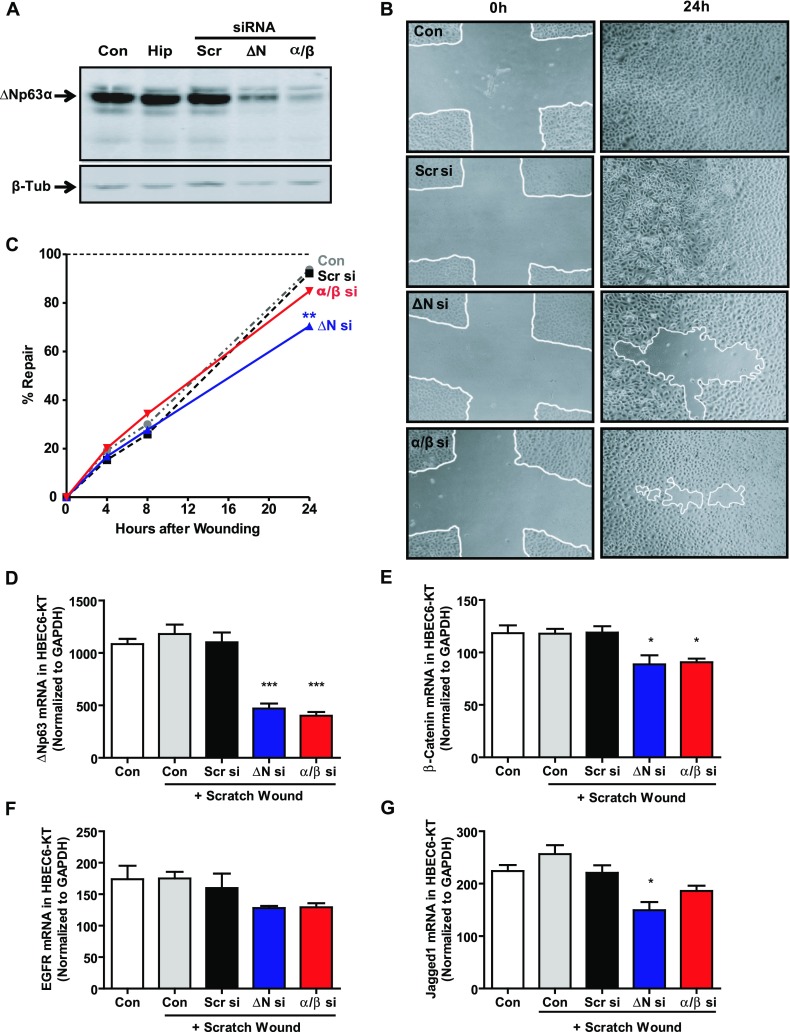
Loss of ΔNp63 Significantly Impairs Epithelial Cell Wound Repair
Having shown that ΔNp63α regulates several genes known to affect epithelial function, we wanted to determine whether the knockdown of ΔNp63α/β isoforms affects epithelial wound repair. For these experiments, we used minimally immortalized HBEC6-KT cells, which, like pHAECs, express predominantly ΔNp63α, which is responsive to the knockdown of protein expression using either the ΔN or α/β siRNA constructs (Figure 5A). We used a scratch wound repair model and, as shown by representative light microscopy images (Figure 5B) of HBEC6-KT cultures at time 0 and 24 hours after wounding, ΔNp63 siRNA constructs noticeably slowed wound repair. Further, by quantifying repair over time, we demonstrate that although control or scrambled siRNA-treated cultures were equally able to reconstitute more than 90% of the wound area within 24 hours, the knockdown of total ΔNp63 inhibited wound closure to only 71% ± 5% (Figure 5C; P < 0.01). In contrast, specific knockdown with the α/β siRNA construct did not significantly alter wound repair. To confirm that the knockdown of ΔNp63α in wounded HBEC6-KT cells still down-regulated the candidate genes identified in Figure 2, we performed quantitative RT-PCR. We demonstrated that the knockdown of ΔNp63α, using either ΔNp63 or α/β siRNA constructs, maintained the down-regulation of ΔNp63 (Figure 4D), β-catenin (Figure 4E), and Jagged1 (Figure 4G) mRNA 24 hours after wounding. EGFR mRNA was reproducibly down-regulated by ΔN or α/β siRNA constructs (Figure 4F), but this did not reach statistical significance.
Figure 5.
Loss of ΔNp63 significantly impairs repair in scratch-wounded monolayer cultures of human bronchial epithelial cells. (A) Representative immunoblot of monolayer culture of minimally immortalized bronchial epithelial cells (HBEC6-KT) cells, 72 hours after transfection with 25 nM p63-specific siRNA, indicates decreased ΔNp63α protein expression. (B) Phase-contrast images of confluent monolayer cultures of HBEC6-KT cells at time of scratch wounding (0 h) and after 24 hours (24h) indicate repair progress in the presence of scrambled, N-terminally truncated (ΔN), or α/β siRNA constructs. White lines denote edges of wound areas. Images are representative of n = 6 independent experiments. (C) Analysis of wound repair in n = 6 independent experiments at 0, 4, 8, and 24 hours after scratch wounding. Wound repair was calculated as percent area reconstituted compared with the initial wound area (time 0), where the horizontal dashed line indicates 100% closure. Statistical significance was assessed at each time point using one-way ANOVA with the Dunnett post hoc test versus Scr siRNA (Scr si) conditions. ΔN si, ΔN siRNA. (D–G) Quantitative RT-PCR of target gene mRNA, 24 hours after media change or scratch wounding in the presence of scrambled, ΔN, or α/β siRNA, indicates sustained down-regulation of (D) ΔNp63, (E) β-catenin, (F) EGFR, and (G) Jagged1 mRNA expression. Statistical significance was assessed at each time point, using one-way ANOVA with the Dunnett post hoc test versus Scr siRNA conditions. *P < 0.05. **P < 0.01. ***P < 0.001.
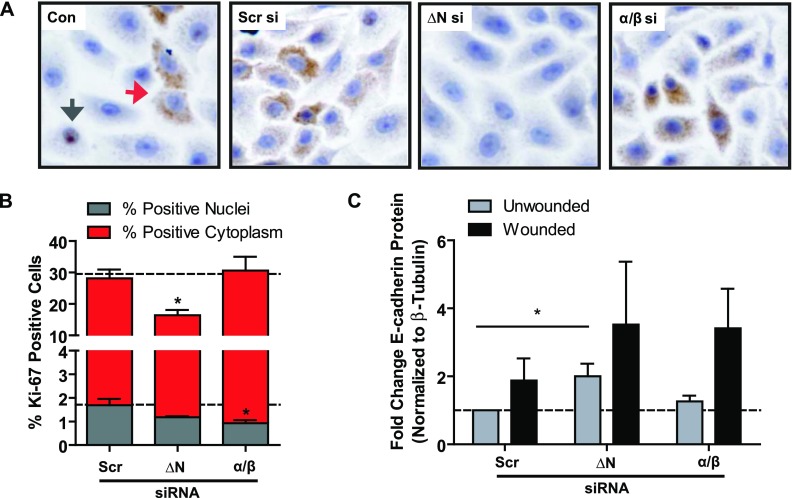
To determine whether the decreased repair induced by decreased ΔNp63 expression was attributable to effects on proliferation, we examined the expression of the proliferation marker Ki-67 (Figure 6A), 24 hours after wounding. The knockdown of ΔNp63 significantly reduced the number of Ki-67–positive cells (Figure 6B; P < 0.05), but the use of the α/β siRNA construct did not. Interestingly, although Ki-67 expression is generally thought to be restricted to the nucleus (Figure 6B, gray arrow), we observed abundant cytoplasmic staining (Figure 6B, red arrow). As such, we separately quantified the expression of nuclear (Figure 6B, gray bars) and cytoplasmic (Figure 6B, red bars) Ki-67 expression, and demonstrated that knockdown with the ΔNp63 siRNA construct primarily reduced cytoplasmic staining compared with control samples (P < 0.05), and this accounted for the significant loss of Ki-67.
Figure 6.
Loss of ΔNp63 significantly impairs epithelial proliferation in scratch-wounded monolayers of human bronchial epithelial cells. (A) Immunocytochemistry for proliferation marker Ki-67 (brown) indicates both nuclear (gray arrow) and cytoplasmic (red arrow) staining. Nuclei are counterstained with Harris hematoxylin (blue staining). (B) Quantification by manual point-counting of Ki-67 nuclear (gray bars) and cytoplasmic (red bars) staining was performed in five images from each of n = 3 independent experiments, 24 hours after wounding, and indicates significantly decreased Ki-67 expression with ΔNp63 siRNA treatment. Dashed lines represent untreated control conditions. (C) Densitometry quantification of n = 3 immunoblots for E-cadherin protein, 24 hours after media change or scratch-wounding of confluent HBEC6-KT monolayers pretreated with N-terminally truncated (ΔN), DBD, or α/β siRNA constructs, indicates a significant up-regulation of E-cadherin protein with the ΔNp63 siRNA construct. Statistical significance was assessed using one-way ANOVA with the Dunnett post hoc test versus Scr si conditions. *P < 0.05.
Because both proliferation and migration occur during wound repair, and have been shown to be linked to cell adhesion, we quantified the expression of the adherens junction protein E-cadherin according to immunoblotting (Figure 6C), and found that ΔNp63 siRNA significantly increased E-cadherin protein expression compared with scrambled control samples (P < 0.05), suggesting that ΔNp63 is a negative regulator of E-cadherin. After scratch wounding, E-cadherin protein expression was up-regulated, but this was not statistically significant. We propose that, because E-cadherin contains a p63 response element and its loss and redistribution are essential for cytokinesis during epithelial cell proliferation and for the dissolution of adherens junctions during migration, a loss of ΔNp63 leads to the increased expression of E-cadherin, and as a consequence, a less migratory and proliferative cell phenotype.
Discussion
We and others have previously shown that the expression of the transcription factor p63 is confined to the basal cells of the airway epithelium (6, 12). Here we demonstrate that ΔNp63 is the exclusive promoter isoform expressed, and more specifically that the ΔNp63α splice variant is the most abundantly expressed isoform in human airway epithelial basal cells. We show that the loss of function of ΔNp63α, using targeted siRNA, induces a significant decrease in the expression of a number of genes involved in epithelial differentiation and repair. Further, we demonstrate a strong role for ΔNp63 in epithelial repair through the regulation of epithelial proliferation and E-cadherin expression during wound closure in vitro. These data support our hypothesis that ΔNp63α is an important regulatory protein in the human airway epithelial basal cell.
This finding of the predominance of ΔNp63α in airway epithelial basal cells, which we and others have shown to be the progenitor cells of the airway epithelium (6, 10), is supported by studies demonstrating the expression of ΔNp63α in the basal cells of several nonpulmonary epithelial tissues (specifically skin, prostate, cornea, and breast) (8, 17, 18, 29). The role of ΔNp63 in maintaining the basal-cell population within epithelial tissues has been supported by several homozygous null p63 murine studies that demonstrated a striking lack of epithelial tissues and, in particular, the epidermis (8, 9). Further, Candi and colleagues found that complementation of the p63 null background with ΔNp63 was able to rescue the basal-cell layer of the epidermis (30). Finally, the recent study from Romano and colleagues, in which the specific knockout of ΔNp63 was generated, found that these mice phenocopied the epidermal abnormalities of the total p63 knockout, indicating the crucial role of ΔNp63 in the development and maintenance of epithelial tissues (31). Interestingly, we have shown that the number of basal cells expressing ΔNp63 is significantly greater in the epithelium of patients with asthma in vivo (7), which may indicate a defect in the normal mechanism of epithelial repair and/or differentiation. We therefore wanted to determine the expression levels of each p63 isoform in pHAECs from asthmatic and nonasthmatic donors. When cultured as a submerged monolayer culture, pHAECs form a cuboidal epithelium consisting of relatively undifferentiated basal cells (32). When we measured the expression of ΔNp63 isoforms, we found that ΔNp63α is the predominant isoform in basal pHAECs, with no differences in expression between asthmatic and nonasthmatic subjects. These data indicate that although the number of basal cells expressing ΔNp63α is elevated in patients asthma compared with nonasthmatic subjects, the total protein levels of ΔNp63α do not differ in basal cells from diseased donors. In addition, our finding that TAp63 mRNA expression is exceptionally low (∼ 2,000-fold less than the ΔN promoter variant, whereas protein expression was not detectable) is consistent with previous studies demonstrating that TAp63 is not expressed in noncancerous adult epithelial basal cells (33, 34). Thus, ΔNp63α is the most abundant isoform expressed in human airway epithelial basal cells.
We then further investigated the role of ΔNp63α in epithelial repair by modulating its expression, using pHAECs from nonasthmatic donors. Epithelial repair is a highly integrated series of processes that includes migration, proliferation, and differentiation. As such, we selected 21 candidate target genes involved in these processes, based on the knowledge that they have been shown to be regulated by p63 in other epithelial tissues (21, 33, 35–38) and/or to contain a p63-response element in their promoter region (23). We found that 11 of 21 candidate genes were significantly down-regulated by the knockdown of either ΔN or α/β p63 isoforms (Table 3; CTNNB1, LAMC2, TJP1, CD44, EGFR, EP300, F11R/JAM1, GSK3B, PLEC1, CCND1, and JAG1), in pHAECs. Further, four genes showed no significant differences after siRNA treatment (CDKN1A, CEBPA, KRT18, and KRT5). Interestingly, of these, CDKN1A (p21WAF1) is known to be directly repressed by ΔNp63α in keratinocytes (39), and thus we would have expected increased expression with the loss of ΔNp63α. This mechanism of regulation may not be predominant in airway epithelial cells, or may be a consequence of pro-proliferative growth conditions. Our model also involved adult-derived pHAECs. This may partly explain why we found four genes involved in development (BMP7, EGF, FGFR2, and WNT1) to be expressed at very low levels.
Because the asthmatic epithelium is widely accepted to display abnormal differentiation and repair, we subsequently focused our efforts on three candidate genes involved in these processes: CTNNB1 (β-catenin), EGFR, and JAG1 (Jagged1). Although β-catenin has been shown to be dispensable in the repair of mouse airway epithelium after naphthalene-induced injury (40), the inhibition of β-catenin in cytokeratin 14–expressing basal cells significantly delayed normal epithelial repair processes after chemical injury (41), and the activation of β-catenin in these cells increased proliferation. The expression of EGFR has been shown to be increased in the airway epithelium of patients with asthma (26), suggesting an important role for this receptor in epithelial repair. In addition, ΔNp63α has been shown to be a direct transcriptional activator of EGFR expression in pancreatic cancer cell lines (43) and keratinocytes (43). Reciprocally, p63 may also be regulated by EGFR (44), providing a further rationale for its inclusion in this analysis. Jagged ligands are involved in epithelial differentiation and repair through signaling through Notch family ligands. Jagged1 has also been shown to be directly transcriptionally regulated by p63 in keratinocytes (43). Jagged1 is also a β-catenin–dependent gene. Therefore, its upstream regulation by p63 may have implications for dysregulated epithelial wound repair responses in asthma (3).
The adenoviral-mediated expression of ΔNp63α in pHAECs exerted no effect on target gene expression, despite a significant increase in the expression of ΔNp63α itself. This surprising finding led us to postulate that endogenous levels of ΔNp63α were constitutively high or not modifiable. Indeed, this turned out to be the case, because infection of the alveolar Type II cell line (A549) that lacks p63 expression induced a significant increase in all target genes, verifying that p63 indeed regulates these targets. In support of this, King and colleagues demonstrated that the overexpression of ΔNp63α was able to maintain primary murine keratinocytes in a basal phenotype (38), and the ΔN domain appeared to be sufficient to maintain cells in a proliferative state. These findings highlight the idea that endogenous levels of ΔNp63α in pHAECs in monolayer culture may in fact be sufficient for target gene induction, but further increases in expression may exert no additive effect. Interestingly, a loss of p63 expression in pHAECs did not completely abrogate the expression of β-catenin, EGFR, and Jagged1, indicating the existence of other transcriptional regulators of these genes. β-catenin is known to be regulated by the Wnt ligands at the protein level and at the transcriptional level by the TCF4 transcription factor and by β-catenin protein itself, as has been shown during the migration of cancer cells (45). EGFR is known to be regulated by the mitogen EGF, and also by multiple other growth factors. Jagged1 expression is regulated by Notch signaling, and interestingly is also significantly regulated by Wnt/β-catenin/TCF signaling (46). Chromatin immunoprecipitation experiments in human keratinocytes previously demonstrated that p63 can directly bind to the EGFR and Jagged1 promoters (43). In contrast, the mechanism of action by p63 on β-catenin expression is somewhat less clear, because it may function either by forming protein complexes with β-catenin, suggesting that ΔNp63 acts as a regulator of β-catenin signaling through nonpromoter binding properties (47), or by complexing with the TCF/LEF family of Wnt-related transcription factors, to recruit transcriptional modifiers to Wnt-responsive genes, such as β-catenin, as shown in urinary bladder carcinoma cells (24). Many factors can contribute to the ability of a transcription factor to bind a given promoter, including epigenetic mechanisms such as promoter methylation, histone modifications, microRNAs, and additional transcription factors. Although the elucidation of specific regulatory mechanisms of ΔNp63α in pHAECs would be of interest, these experiments are beyond the scope of the present study.
To examine the functional involvement of ΔNp63α in epithelial repair, we scratch-wounded minimally immortalized human bronchial epithelial cell (HBEC6-KT) monolayers after the knockdown of ΔNp63, and found that this significantly compromised the capacity of airway epithelial cells to undergo repair. In contrast, in a scratch-wound model using immortalized keratinocytes, the loss of ΔNp63α by siRNA treatment increased the migration of cells into the wounded area, independent of proliferation (48), a finding that has also been reported in vivo (49). However, because p63 has been shown to exhibit distinct cell-type specificity, we believe that ΔNp63α is in fact necessary for repair in airway epithelial basal cells, primarily by regulating cellular proliferation, although specific targeting of the α/β isoforms did not significantly affect wound repair. Two explanations may account for this: first, TAp63α may be induced upon wounding, and may exert an inhibitory effect on repair, as has been shown in a murine deep-skin wound model (50). However, we did not observe any induction of TAp63 mRNA after wounding (data not shown). The second possibility states that ΔNp63γ may be involved in wound repair although its role appears to be cell-type specific. Indeed, in mammary epithelial cells, the loss of ΔNp63α/β allowed ΔNp63γ to confer a more migratory phenotype, leading to epithelial–mesenchymal transition (EMT) (51). However, in human epidermal keratinocytes, the overexpression of ΔNp63α itself resulted in enhanced transforming growth factor (TGF)–β1–induced EMT (52). We have previously shown that dNp63-expressing airway epithelial basal cells are highly susceptible to TGF-β1–induced EMT (7). In this scenario, the expression of ΔNp63γ may confer a degree of cell plasticity, enabling repair via epithelial migration or EMT-like effects. However, because we did not observe enhanced repair beyond or even to the same degree as that under control conditions, ΔNp63γ alone is unlikely able to compensate for the ΔNp63α-dependent loss of proliferation.
We tested the effects of ΔNp63 knockdown on wound repair by examining the expression of Ki-67, which has been shown to be a specific marker of proliferation. Interestingly, we saw the vast majority of Ki-67 staining within the cytoplasm, with lesser amounts in the nuclei of cells. The cytoplasmic expression of Ki-67 has been documented previously, although the functional effect of this expression remains unclear. For example, cytoplasmic Ki-67 in breast cancer biopsies has been found to correlate with worse prognoses (53), but it has also been described as a disease-independent phenomenon in rat atrial cells undergoing postnatal remodeling (54). Whether our finding is physiological or pathological in nature remains unclear at this time, but it is noteworthy that we observed dramatically decreased cytoplasmic Ki-67 expression in response to a loss of ΔNp63, indicating that wound closure was inhibited because of decreased proliferation.
The process of wound repair requires epithelial cells to both proliferate and migrate. E-cadherin is known to be involved in the maintenance of adherens junctions that tether the actin cytoskeleton between adjacent cells. Thus the loss of E-cadherin expression is required for cytokinesis during proliferation (55) and to permit cell migration (56). We found that ΔNp63 siRNA significantly up-regulated E-cadherin protein expression, but α/β siRNA did not. These data support our finding that the knockdown of ΔNp63 was able to prevent wound repair significantly, which we propose could be attributable to enhanced E-cadherin expression. Also in agreement with this finding, we have previously shown both increased ΔNp63 (57) and decreased E-cadherin (58) in the asthmatic airway epithelium in vivo and in ALI culture in vitro, indicating the potential for a negative regulation of E-cadherin by ΔNp63α.
In this study, we have attempted to elucidate the roles of ΔNp63α in pHAECs. We have focused here on the use of epithelial cells in a monolayer culture, which results in a relatively homogenous model consisting of cells with a basal-like phenotype, rather than on the use of differentiated cell culture models. Although this may seem a limitation with regard to in vivo comparisons, we believe that a monolayer culture offers the most appropriate model, because ΔNp63α is known to be expressed primarily in basal cells in vivo. In addition, studies of isoform-specific functions are somewhat limited by the high degree of shared domains between various isoforms. As such, targeting a specific region of the p63 molecule with siRNA will affect a subset of isoforms rather than one specific isoform. Here we targeted either the ΔNp63 domain, knocking down all ΔNp63 isoforms, or a region shared by both the α and β isoforms, which leaves only the γ isoform expressed. We chose this as a two-pronged approach to target ΔNp63α, based on the abundance of this isoform under our study conditions. To minimize the ambiguity of this approach, we have described reciprocal regulatory effects using the adenovirus expressing ΔNp63α, further showing that ΔNp63α is the primary isoform responsible for target-gene regulation.
In conclusion, we have demonstrated that of the six potential p63 isoforms, ΔNp63α is the predominant isoform expressed in pHAECs. This isoform appears to be active in regulating several epithelial genes, and plays a key role in epithelial repair in basal cells in monolayer culture. Further elucidation of the role of ΔNp63α, as well as its upstream regulators, will have important implications in our understanding of epithelial differentiation and regeneration, and in our understanding of how the role of ΔNp63α pertains to the pathophysiology of epithelial injury in asthma.
Acknowledgments
Acknowledgments
The authors thank Dr. Wendy Weinberg for generously providing us with the adenoviruses used in this study. In addition, the authors thank Drs. Tokino and Yamashita, who generated the β-galactosidase (LacZ), TAp63α, and TAp63γ adenoviruses, and permitted us to use them. We kindly thank Dr. John Minna for providing us with HBEC6-KT cells.
Footnotes
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0447OC on July 9, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bucchieri F, Puddicombe SM, Lordan JL, Richter A, Buchanan D, Wilson SJ, Ward J, Zummo G, Howarth PH, Djukanovic R, et al. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol. 2002;27:179–185. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- 2.Freishtat RJ, Watson AM, Benton AS, Iqbal SF, Pillai DK, Rose MC, Hoffman EP. Asthmatic airway epithelium is intrinsically inflammatory and mitotically dyssynchronous. Am J Respir Cell Mol Biol. 2011;44:863–869. doi: 10.1165/rcmb.2010-0029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kicic A, Hallstrand TS, Sutanto EN, Stevens PT, Kobor MS, Taplin C, Pare PD, Beyer RP, Stick SM, Knight DA. Decreased fibronectin production significantly contributes to dysregulated repair of asthmatic epithelium. Am J Respir Crit Care Med. 2010;181:889–898. doi: 10.1164/rccm.200907-1071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kicic A, Sutanto EN, Stevens PT, Knight DA, Stick SM. Intrinsic biochemical and functional differences in bronchial epithelial cells of children with asthma. Am J Respir Crit Care Med. 2006;174:1110–1118. doi: 10.1164/rccm.200603-392OC. [DOI] [PubMed] [Google Scholar]
- 5.Ge Q, Moir LM, Black JL, Oliver BG, Burgess JK. TGFβ1 induces IL-6 and inhibits IL-8 release in human bronchial epithelial cells: the role of Smad2/3. J Cell Physiol. 2010;225:846–854. doi: 10.1002/jcp.22295. [DOI] [PubMed] [Google Scholar]
- 6.Hackett TL, Shaheen F, Johnson A, Wadsworth S, Pechkovsky DV, Jacoby DB, Kicic A, Stick SM, Knight DA. Characterization of side population cells from human airway epithelium. Stem Cells. 2008;26:2576–2585. doi: 10.1634/stemcells.2008-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, et al. Induction of epithelial–mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor–beta1. Am J Respir Crit Care Med. 2009;180:122–133. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 8.Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 9.Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 10.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musah S, Chen J, Hoyle GW. Repair of tracheal epithelium by basal cells after chlorine-induced injury. Respir Res. 2012;13:107. doi: 10.1186/1465-9921-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–C181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- 13.Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 15.Natan E, Joerger AC. Structure and kinetic stability of the p63 tetramerization domain. J Mol Biol. 2012;415:503–513. doi: 10.1016/j.jmb.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangiulli M, Valletti A, Caratozzolo MF, Tullo A, Sbisa E, Pesole G, D’Erchia AM. Identification and functional characterization of two new transcriptional variants of the human p63 gene. Nucleic Acids Res. 2009;37:6092–6104. doi: 10.1093/nar/gkp674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki S, Tanioka H, Yamasaki K, Connon CJ, Kinoshita S. Expression and tissue distribution of p63 isoforms in human ocular surface epithelia. Exp Eye Res. 2006;82:293–299. doi: 10.1016/j.exer.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F, Loda M. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157:1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallstrand TS, Wurfel MM, Lai Y, Ni Z, Gelb MH, Altemeier WA, Beyer RP, Aitken ML, Henderson WR. Transglutaminase 2, a novel regulator of eicosanoid production in asthma revealed by genome-wide expression profiling of distinct asthma phenotypes. PLoS ONE. 2010;5:e8583. doi: 10.1371/journal.pone.0008583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 21.King KE, Ponnamperuma RM, Yamashita T, Tokino T, Lee LA, Young MF, Weinberg WC. DeltaNp63alpha functions as both a positive and a negative transcriptional regulator and blocks in vitro differentiation of murine keratinocytes. Oncogene. 2003;22:3635–3644. doi: 10.1038/sj.onc.1206536. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki Y, Morimoto I, Ishida S, Yamashita T, Imai K, Tokino T. Adenovirus-mediated transfer of the p53 family genes, p73 and p51/p63 induces cell cycle arrest and apoptosis in colorectal cancer cell lines: potential application to gene therapy of colorectal cancer. Gene Ther. 2001;8:1401–1408. doi: 10.1038/sj.gt.3301538. [DOI] [PubMed] [Google Scholar]
- 23.Sbisa E, Catalano D, Grillo G, Licciulli F, Turi A, Liuni S, Pesole G, De Grassi A, Caratozzolo MF, D’Erchia AM, et al. p53FamTag: a database resource of human p53, p63 and p73 direct target genes combining in silico prediction and microarray data. BMC Bioinformatics. 2007;8:S20. doi: 10.1186/1471-2105-8-S1-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drewelus I, Gopfert C, Hippel C, Dickmanns A, Damianitsch K, Pieler T, Dobbelstein M. p63 antagonizes Wnt-induced transcription. Cell Cycle. 2010;9:580–587. doi: 10.4161/cc.9.3.10593. [DOI] [PubMed] [Google Scholar]
- 25.Talos F, Schulz R, Moll UM. p63 and canonical Wnt signaling. Cell Cycle. 2010;9:648–649. [PubMed] [Google Scholar]
- 26.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 27.Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:813–820. doi: 10.1167/iovs.03-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu N, Rollin J, Masse I, Lamartine J, Gidrol X. p63 regulates human keratinocyte proliferation via Myc-regulated gene network and differentiation commitment through cell adhesion–related gene network. J Biol Chem. 2012;287:5627–5638. doi: 10.1074/jbc.M111.328120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yalcin-Ozuysal O, Fiche M, Guitierrez M, Wagner KU, Raffoul W, Brisken C. Antagonistic roles of Notch and p63 in controlling mammary epithelial cell fates. Cell Death Differ. 2010;17:1600–1612. doi: 10.1038/cdd.2010.37. [DOI] [PubMed] [Google Scholar]
- 30.Candi E, Rufini A, Terrinoni A, Dinsdale D, Ranalli M, Paradisi A, De Laurenzi V, Spagnoli LG, Catani MV, Ramadan S, et al. Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice. Cell Death Differ. 2006;13:1037–1047. doi: 10.1038/sj.cdd.4401926. [DOI] [PubMed] [Google Scholar]
- 31.Romano RA, Smalley K, Magraw C, Serna VA, Kurita T, Raghavan S, Sinha S. {Delta}Np63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development. 2012;139:772–782. doi: 10.1242/dev.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hackett NR, Shaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, Witover B, Salit J, Crystal RG. The human airway epithelial basal cell transcriptome. PLoS ONE. 2011;6:e18378. doi: 10.1371/journal.pone.0018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT. Differential expression of p63 isoforms in female reproductive organs. Mech Dev. 2005;122:1043–1055. doi: 10.1016/j.mod.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Birkaya B, Ortt K, Sinha S. Novel in vivo targets of DeltaNp63 in keratinocytes identified by a modified chromatin immunoprecipitation approach. BMC Mol Biol. 2007;8:43. doi: 10.1186/1471-2199-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll DK, Brugge JS, Attardi LD. p63, cell adhesion and survival. Cell Cycle. 2007;6:255–261. doi: 10.4161/cc.6.3.3799. [DOI] [PubMed] [Google Scholar]
- 37.Carroll DK, Carroll JS, Leong CO, Cheng F, Brown M, Mills AA, Brugge JS, Ellisen LW. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol. 2006;8:551–561. doi: 10.1038/ncb1420. [DOI] [PubMed] [Google Scholar]
- 38.King KE, Ponnamperuma RM, Gerdes MJ, Tokino T, Yamashita T, Baker CC, Weinberg WC. Unique domain functions of p63 isotypes that differentially regulate distinct aspects of epidermal homeostasis. Carcinogenesis. 2006;27:53–63. doi: 10.1093/carcin/bgi200. [DOI] [PubMed] [Google Scholar]
- 39.Westfall MD, Mays DJ, Sniezek JC, Pietenpol JA. The Delta Np63 alpha phosphoprotein binds the p21 and 14–3-3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome–derived mutations. Mol Cell Biol. 2003;23:2264–2276. doi: 10.1128/MCB.23.7.2264-2276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zemke AC, Teisanu RM, Giangreco A, Drake JA, Brockway BL, Reynolds SD, Stripp BR. Beta-catenin is not necessary for maintenance or repair of the bronchiolar epithelium. Am J Respir Cell Mol Biol. 2009;41:535–543. doi: 10.1165/rcmb.2008-0407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giangreco A, Lu L, Vickers C, Teixeira VH, Groot KR, Butler CR, Ilieva EV, George PJ, Nicholson AG, Sage EK, et al. Beta-catenin determines upper airway progenitor cell fate and preinvasive squamous lung cancer progression by modulating epithelial–mesenchymal transition. J Pathol. 2012;226:575–587. doi: 10.1002/path.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danilov AV, Neupane D, Nagaraja AS, Feofanova EV, Humphries LA, DiRenzo J, Korc M. DeltaNp63alpha-mediated induction of epidermal growth factor receptor promotes pancreatic cancer cell growth and chemoresistance. PLoS ONE. 2011;6:e26815. doi: 10.1371/journal.pone.0026815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Testoni B, Borrelli S, Tenedini E, Alotto D, Castagnoli C, Piccolo S, Tagliafico E, Ferrari S, Vigano MA, Mantovani R. Identification of new p63 targets in human keratinocytes. Cell Cycle. 2006;5:2805–2811. doi: 10.4161/cc.5.23.3525. [DOI] [PubMed] [Google Scholar]
- 44.Matheny KE, Barbieri CE, Sniezek JC, Arteaga CL, Pietenpol JA. Inhibition of epidermal growth factor receptor signaling decreases p63 expression in head and neck squamous carcinoma cells. Laryngoscope. 2003;113:936–939. doi: 10.1097/00005537-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Bandapalli OR, Dihlmann S, Helwa R, Macher-Goeppinger S, Weitz J, Schirmacher P, Brand K. Transcriptional activation of the beta-catenin gene at the invasion front of colorectal liver metastases. J Pathol. 2009;218:370–379. doi: 10.1002/path.2539. [DOI] [PubMed] [Google Scholar]
- 46.Rodilla V, Villanueva A, Obrador-Hevia A, Robert-Moreno A, Fernandez-Majada V, Grilli A, Lopez-Bigas N, Bellora N, Alba MM, Torres F, et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci USA. 2009;106:6315–6320. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patturajan M, Nomoto S, Sommer M, Fomenkov A, Hibi K, Zangen R, Poliak N, Califano J, Trink B, Ratovitski E, et al. DeltaNp63 induces beta-catenin nuclear accumulation and signaling. Cancer Cell. 2002;1:369–379. doi: 10.1016/s1535-6108(02)00057-0. [DOI] [PubMed] [Google Scholar]
- 48.Ichikawa T, Suenaga Y, Koda T, Ozaki T, Nakagawara A. DeltaNp63/BMP-7–dependent expression of matrilin-2 is involved in keratinocyte migration in response to wounding. Biochem Biophys Res Commun. 2008;369:994–1000. doi: 10.1016/j.bbrc.2008.02.128. [DOI] [PubMed] [Google Scholar]
- 49.Barbieri CE, Tang LJ, Brown KA, Pietenpol JA. Loss of p63 leads to increased cell migration and up-regulation of genes involved in invasion and metastasis. Cancer Res. 2006;66:7589–7597. doi: 10.1158/0008-5472.CAN-06-2020. [DOI] [PubMed] [Google Scholar]
- 50.Bamberger C, Hafner A, Schmale H, Werner S. Expression of different p63 variants in healing skin wounds suggests a role of p63 in reepithelialization and muscle repair. Wound Repair Regen. 2005;13:41–50. doi: 10.1111/j.1067-1927.2005.130106.x. [DOI] [PubMed] [Google Scholar]
- 51.Lindsay J, McDade SS, Pickard A, McCloskey KD, McCance DJ. Role of DeltaNp63gamma in epithelial to mesenchymal transition. J Biol Chem. 2011;286:3915–3924. doi: 10.1074/jbc.M110.162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh JE, Kim RH, Shin KH, Park NH, Kang MK. DeltaNp63alpha protein triggers epithelial–mesenchymal transition and confers stem cell properties in normal human keratinocytes. J Biol Chem. 2011;286:38757–38767. doi: 10.1074/jbc.M111.244939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faratian D, Munro A, Twelves C, Bartlett JM. Membranous and cytoplasmic staining of Ki67 is associated with HER2 and ER status in invasive breast carcinoma. Histopathology. 2009;54:254–257. doi: 10.1111/j.1365-2559.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 54.Ciulla MM, Acquistapace G, Toffetti L, Paliotti R, Ferrero S, Magrini F, Braidotti P. Ki67 cytoplasmic expression: observations in normal tissue from heart atrial appendages of healthy rats. Cell Cycle. 2009;8:2125. doi: 10.4161/cc.8.13.8785. [DOI] [PubMed] [Google Scholar]
- 55.Bauer T, Motosugi N, Miura K, Sabe H, Hiiragi T. Dynamic rearrangement of surface proteins is essential for cytokinesis. Genesis. 2008;46:152–162. doi: 10.1002/dvg.20377. [DOI] [PubMed] [Google Scholar]
- 56.Masterson JC, Molloy EL, Gilbert JL, McCormack N, Adams A, O’Dea S. Bone morphogenetic protein signalling in airway epithelial cells during regeneration. Cell Signal. 2011;23:398–406. doi: 10.1016/j.cellsig.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Hackett TL, Warner SM, Stefanowicz D, Shaheen F, Pechkovsky DV, Murray LA, Argentieri R, Kicic A, Stick SM, Bai TR, et al. Induction of epithelial–mesenchymal transition in primary airway epithelial cells from asthmatic patients by TGF{beta}1. Am J Respir Crit Care Med. 2009;180:122–133. doi: 10.1164/rccm.200811-1730OC. [DOI] [PubMed] [Google Scholar]
- 58.Hackett TL, Singhera GK, Shaheen F, Hayden P, Jackson GR, Hegele RG, Van Eeden S, Bai TR, Dorscheid DR, Knight DA. Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to respiratory syncytial virus and air pollution. Am J Respir Cell Mol Biol. 2011;45:1090–1100. doi: 10.1165/rcmb.2011-0031OC. [DOI] [PubMed] [Google Scholar]