Highlights
-
•
Clear cell sarcomas are rare malignancies of the soft tissue.
-
•
Clear cell sarcoma share characteristics with malignant melanoma, but modern techniques are able to distinguish the tumours.
-
•
Timely and accurate primary diagnosis is important, as tumour size larger than 5 cm is correlated to a more sinister prognosis.
-
•
Surgical treatment is imperative, the role of radiotherapy and chemotherapy remain unknown.
-
•
Follow-up in a multidisciplinary setting is recommended.
Keywords: Clear cell sarcoma, Sarcoma, Malignant melanoma of the soft tissue, Surgery, Plastic surgery, Clear cell sarcoma of tendons and aponeuroses, Clear cell sarcoma of the soft tissue
Abstract
Introduction
Clear cell sarcoma (CCS) is a rare tumour of the soft tissue often misdiagnosed, as it shares characteristics with malignant melanoma (MM). Previously, CCS has been characterised, as malignant melanoma of the soft tissue, contemporary immunohistochemical techniques, however, have made this designation obsolete. The true incidence remains unknown, but CCS is believed to represent less than one percent of all sarcomas.
Presentation of case
A 22-year-old patient presented with a mass sized 2.6 × 2.7 × 2.7 cm of the left gluteal region, pain, and malaise. Initially, the symptoms were interpreted as an infection. Subsequent, pathological diagnosis after surgical removal was tentatively MM albeit definitive pathological diagnosis was CCS.
Discussion
The patient of this case underwent definitive surgical treatment with 2 cm margin. In spite of time delay, because of prolonged time for definitive diagnosis, PET-CT and sentinel lymph node biopsy did not show any metastasis. One-year postoperatively, multidisciplinary follow-up is without suspicion of relapse.
Conclusion
Accurate and timely diagnosis of CCS are imperative, as initial misdiagnosis, may cause delay and further tumour growth, which is correlated to the prognosis.
1. Introduction
Clear cell sarcoma of tendons and aponeuroses or soft tissue or simply just clear cell sarcoma (CCS) are tumours of very low incidence derived from neural crest cells originating from the soft tissue, representing an estimated one percent of all sarcomas, yet, the true incidence remains unknown [1], [2]. In 2013 the annual incidence of all sarcomas of the soft tissue were 220 in Denmark [3]. Hitherto, no descriptions of CCS from Denmark are available in the English literature, and only three occurrences of CCS are reported in Denmark in the period from 2009 to 2013 [3]. This case is disseminated according to the SCARE criteria [4]. The treatment of this patient was managed at a university hospital in Denmark.
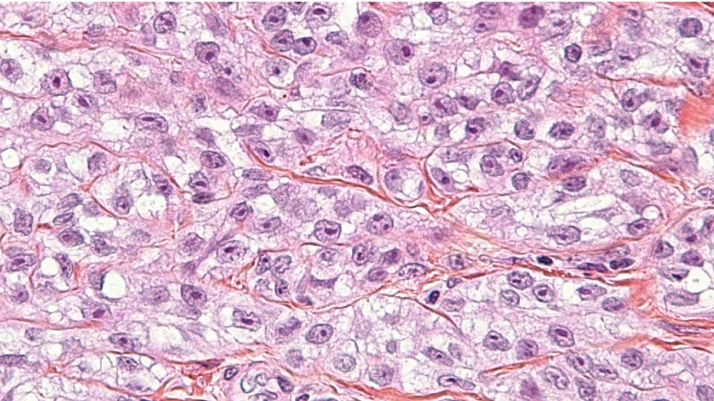
CCS shares morphological similarities with malignant melanoma (MM), hence, the key process in the diagnosis of CCS is to distinguish it from other tumours, i.e. MM in particular, to facilitate fast, correct, and appropriate treatment [5], [6]. The neoplastic cells of CCS are clear or pale, appear polygonal to fusiform with eosinophilic to amphophilic cytoplasm, and have centrally located uniform and round nuclei with prominent basophilic nucleoli, similar to that of MM [5]. The clear cell appearance is due to the accumulation of glycogen (Fig. 1). CCS was previously characterised as MM of the soft tissue, but contemporary techniques have made this designation obsolete [5]. Debut is on average during the third decade, the male-female ratio is approximately 1:1, although CCS seems to be slightly more common in females. The lower limbs are most frequently affected [5]. The most common presentation is a slowly growing and tender or painful lump of the extremities. As the tumour grows, it invades nearby tissues and over time, symptoms of more advanced cancer might develop, including i.a. weight loss, malaise and loss of appetite. The 5-year survival is estimated to 63 percent and tumour size is implicated in the prognosis [7], [8].
Fig. 1.
Periodic acid-Schiff stain. Clear cell sarcoma. Clear cell appearance because of glycogen accumulation in cytoplasm.
2. Presentation of case
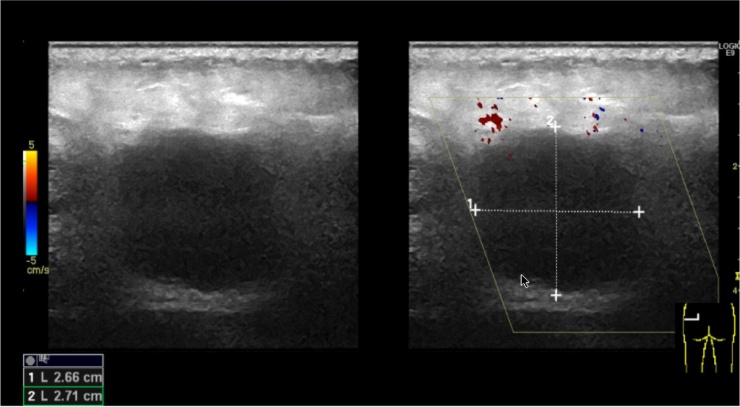
A 22-year-old university student with no previous medical history presented to the Accident and Emergency Department after referral from her General Practitioner with a growing mass of the left gluteal region. The patient had experienced local tenderness and malaise for approximately one week prior to contact. No previous trauma was present and the patient was not on any medication. There was no family history of any cancers and the patient did not smoke. An ultrasound showed a well-delimited mass of 2.6 × 2.7 × 2.7 cm below skin, but above the gluteal muscles (Fig. 2). Initially, the process was suspected to represent an infection. Hence, an operation was made to evacuate the potential abscess. During the operation a centrally located solid mass was noted, which the surgeon removed in toto. Subsequent pathological diagnosis was MM and the patient was referred to the Department of Plastic Surgery for further treatment. An interim PET-CT revealed no metastases. At the Department of Plastic Surgery, in general anaesthesia the patient had further surgery to remove any remaining tumour and to make sentinel lymph node biopsy. The surgeon was a Consultant Plastic Surgeon. The lymph node biopsy revealed no tumour cells. A pathological revision of the tumour concluded that the tumour represented CCS with intact surgical margins of two cm. For follow-up, the patient has been referred to a multidisciplinary sarcoma centre. Included in the follow-up are chest x-rays and PET-CTs. One-year postoperatively, the patient adheres to the follow-up programme without any signs of relapse.
Fig. 2.
Preoperative ultrasonography of the patient’s gluteal region.
3. Discussion
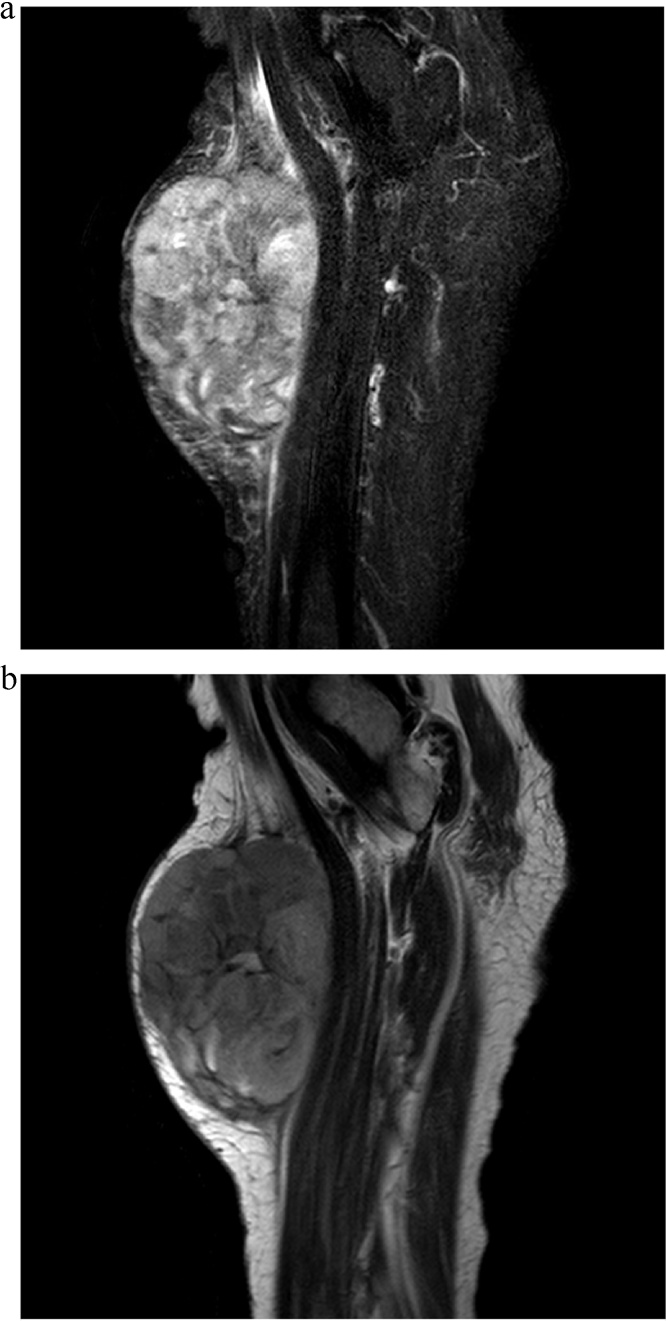
The present case of CCS represents an unfortunately typical course of CCS, as the tumour was misdiagnosed initially (Fig. 3a and b). In this case a 22-year-old lady presented with localised pain, an expanding mass, and lethargy. In previous cases of CCS manifestations resembling the current case were evident [1], [2], [7]. Mean age and localisation of tumour are parallel to the present case, as the lower limbs seem to be most frequently affected [9]. In a retrospective study of patients with CCS, the tumours occurred predominantly in young patients between the age of 15 and 35 years [9], Furthermore, females were slightly more affected than male patients [9]. The current case is therefore a common presentation of CSS and in spite of this, the diagnosis was not made primarily, which further underlines the importance to consider both CCS and MM in cases of doubt to ensure timely and correct diagnosis for optimal treatment. Fortunately in the present case, the tumour size remained within the reach of surgery despite delay and the patient remains cancer free.
Fig. 3.
(a) Typical appearance of clear cell sarcoma on MRI enhanced by contrast administration. b Typical appearance of clear cell sarcoma on MRI without contrast administration.
The treatment protocols of both CCS and MM was followed in the current case, as MM of this size should be surgically removed with a margin of two cm [10]. No current national guideline for CCS exists, however, a surgical margin of at least one cm is accepted domestically [3]. Follow-up differs, as chemotherapy may be beneficial in selected cases of MM, whereas CCS does not seem to respond to neither chemotherapy nor radiation [11], [12]. In all cases a multidisciplinary approach for follow-up is recommended.
Regarding pathological examination, no common signs or symptoms, atypically localisation, and/or presentation of MM should warrant further examinations, if the histological features of the tumour presents as typically to MM, considering CCS as main differential diagnosis [7]. Keep in mind that approximately two thirds of all CCS contain melanin, and therefore are S-100 positive, whereas the immunological profile for CCS is typically positive for melan-A, HMB-45, and microphthalmia transcription factor [7]. In 90% of all cases of CCS polymerase chain reaction and fluorescence in situ hybridisation can detach the translocation (t[12; 22] [q13;q12]) or a resultant EWSR1-ATF1 fusion gene unique for CCS [7], [13].
4. Conclusion
Although, CCS is a rare tumour, it should be kept in mind in cases of patients presenting with tumours of the soft tissue. Furthermore, an initial diagnosis of MM in tumours of the soft tissue should warrant further examinations to exclude CCS, for the optimal specific treatment, albeit all delays should be diminished if possible, as more time spend on diagnosis potentially equals a larger tumour and a more sinister prognosis.
Conlicts of interest
None of the authors have any conflict of interests to declare.
Funding
None.
Ethical approval
This case report did not require ethical approval.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author contributions
Jacob Juel: drafted the first version of the manuscript and critical revision of the manuscript for important intellectual content.
Rami Mossad Ibrahim: critical revision of the manuscript for important intellectual content.
Guarantor
Dr Jacob Juel.
Acknowledgment
None.
References
- 1.Dim D.C., Cooley L.D., Miranda R.N. Clear cell sarcoma of tendons and aponeuroses: a review. Arch. Pathol. Lab. Med. 2007;131:152–156. doi: 10.5858/2007-131-152-CCSOTA. [DOI] [PubMed] [Google Scholar]
- 2.Pavlidis N.A., Fisher C., Wiltshaw E. Clear-cell sarcoma of tendons and aponeuroses: a clinicopathologic study. Presentation of six additional cases with review of the literature. Cancer. 1984;54:1412–1417. doi: 10.1002/1097-0142(19841001)54:7<1412::aid-cncr2820540730>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Dansk Sarkom Database (DSD) (2013).
- 4.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P. A protocol for the development of reporting criteria for surgical case reports: the SCARE statement. Int. J. Surg. 2016;27:187–189. doi: 10.1016/j.ijsu.2016.01.094. [DOI] [PubMed] [Google Scholar]
- 5.Ozuguz P., Kocak M., Atasoy P., Vargel I., Cavusoglu T. Clear cell sarcoma. Indian Dermatol. Online J. 2014;5:488–490. doi: 10.4103/2229-5178.142515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nugent S.L., Dim D.C., Bridge J.A., Ioffe O.B. Clear cell sarcoma of soft tissue metastatic to the ovaries: a heretofore unreported occurrence. Int. J. Gynecol. Pathol. 2009;28:234–238. doi: 10.1097/PGP.0b013e31818d10a8. [DOI] [PubMed] [Google Scholar]
- 7.Hisaoka M., Ishida T., Kuo T.-T., Matsuyama A., Imamura T., Nishida K. Clear cell sarcoma of soft tissue: a clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am. J. Surg. Pathol. 2008;32:452–460. doi: 10.1097/PAS.0b013e31814b18fb. [DOI] [PubMed] [Google Scholar]
- 8.Hocar O., Le Cesne A., Berissi S., Terrier P., Bonvalot S., Vanel D. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 52 cases. Dermatol. Res. Pract. 2012;2012:984096. doi: 10.1155/2012/984096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung E.B., Enzinger F.M. Malignant melanoma of soft parts. A reassessment of clear cell sarcoma. Am. J. Surg. Pathol. 1983;7:405–413. doi: 10.1097/00000478-198307000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Lucas D.R., Nascimento A.G., Sim F.H. Clear cell sarcoma of soft tissues. Mayo clinic experience with 35 cases. Am. J. Surg. Pathol. 1992;16:1197–1204. doi: 10.1097/00000478-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Jones R.L., Constantinidou A., Thway K., Ashley S., Scurr M., Al-Muderis O. Chemotherapy in clear cell sarcoma. Med. Oncol. 2011;28:859–863. doi: 10.1007/s12032-010-9502-7. [DOI] [PubMed] [Google Scholar]
- 12.Al-Absi E., Farrokhyar F., Sharma R., Whelan K., Corbett T., Patel M. A systematic review and meta-analysis of oncologic outcomes of pre- versus postoperative radiation in localized resectable soft-tissue sarcoma. Ann. Surg. Oncol. 2010;17:1367–1374. doi: 10.1245/s10434-009-0885-7. [DOI] [PubMed] [Google Scholar]
- 13.Wang W.-L., Mayordomo E., Zhang W., Hernandez V.S., Tuvin D., Garcia L. Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts) Mod. Pathol. 2009;22:1201–1209. doi: 10.1038/modpathol.2009.85. [DOI] [PubMed] [Google Scholar]