Abstract
Purpose of review
Current WHO-recommended first-line therapy in low-income and middle-income countries has been very successful in saving millions of lives but still has toxicity concerns and a low barrier to resistance.
Recent findings
Two candidate antiretrovirals may substantially transform first-line therapy in low-income and middle-income countries, yielding a safer, more robust and cheaper regimen. Tenofovir alafenamide carries toxicity and cost benefits over tenofovir disoproxil fumarate. Dolutegravir could replace efavirenz, with substantial toxicity, resistance and cost benefits. However, these drugs are currently not manufactured together in developed countries, for commercial reasons.
Summary
We describe a large randomized controlled study testing a combination of these candidate antiretrovirals against the current first-line recommendation that commenced recruitment in early 2017. We justify the study design and discuss how we will deal with complex issues such as tuberculosis and pregnancy.
Keywords: antiretrovirals, dolutegravir, first-line, tenofovir alafenamide, WHO
INTRODUCTION
Since 2010, the WHO has recommended a single regimen as preferred first-line antiretroviral treatment (ART) for HIV that is well tolerated in pregnancy and can be taken with first-line tuberculosis (TB) treatment (ref). Millions are now receiving the fixed-dose combination (FDC) of tenofovir disoproxil fumarate (TDF), lamivudine or interchangeable emtricitabine (FTC), often collectively referred to as XTC and efavirenz (EFV). The regimen has been very successful in treating tens of millions but has a low resistance barrier in the presence of even slightly compromised adherence, and significant side effects in a minority of people with HIV. The availability of new antiretrovirals at potentially similar or even lower prices than those in the current first-line regimen has led to consideration of the use of these agents in first-line treatment [1▪,2,3▪▪]. Tenofovir alafenamide (TAF; Gilead Health Sciences, California, USA) and particularly dolutegravir (DTG; ViiV Healthcare, London) are considered two potentially transformative antiretrovirals that address tolerability and toxicity as well as resistance for low-income and middle-income countries (LMICs). Recent WHO guidelines have recommended DTG as an alternative to EFV [4▪,5▪▪]. Both agents are covered in detail in other articles in this issue of Current Opinion.
Box 1.
no caption available
HISTORY OF THE ADVANCE STUDY
ADVANCE was conceived after a WHO Think Tank on ART Optimization at the 2015 Conference on Retroviruses and Opportunistic Infections (CROI) by an informal group of clinicians, activists, academics, public health specialists and generic pharmaceutical company representatives from St. Stephen's AIDS Trust, HIV i-Base, the Clinton Health Access Initiative (CHAI), Wits Reproductive Health and HIV Institute (Wits RHI) and Mylan, a generic pharmaceutical manufacturer. Previously, several expert ART optimization meetings – including the Second Conference on Antiretroviral Drug Optimization – had identified DTG/TAF/XTC as a potentially more durable, tolerable, safer and affordable regimen for LMICs than the current first-line. In the absence of sufficient evidence, such a recommendation had not moved beyond the discussion stage.
The group considered the ADVANCE trial – a randomized controlled trial (RCT) of potential new first-line regimens – to be necessary for two reasons. First, it was felt that WHO was unlikely to firmly recommend new regimens in the absence of robust evidence from an RCT, which would compromise confidence by governments to adopt the new regimens and generic manufacturers to scale up production. Second, and linked to the first, the originator companies of TAF (Gilead Sciences) and DTG (ViiV Healthcare) are commercial competitors, and although both have granted extensive licences to generic manufacturers in LMICs, they are unlikely to pursue an expensive regulatory study combining their drugs for markets that do not yield profits. Registration studies for new antiretrovirals for high-income countries typically do not include populations important in LMICs, such as pregnant women, children and adolescents, and those with TB and viral hepatitis.
The study was designed over the next 3 months, with details ironed out during the subsequent negotiations with donors and other stakeholders, and agreement that it could occur in a single site at Wits RHI in Johannesburg, South Africa. Existing large studies in Johannesburg run by the Wits RHI investigators, funded by the Bill and Melinda Gates Foundation and South African Medical Research Council, suggested that this site would suffice, especially as the study will recruit adolescents, and the site has access to this cohort.
In the second half of 2015, an opportunity for cofunding of the study arose separately with United States Agency for International Development (USAID) and Unitaid, with community mobilization and clinician education, a key aspect supporting the study. Both applications were successful, and funding began to flow during 2015 and 2016 for study design, regulatory submissions and engagement with other stakeholders, including the originator companies, both of whom agreed to donate study drug. Formal engagement with the South African government gained high-level support for the study, especially when potential cost savings were noted [6]. This engagement, along with CHAI, has catalysed a series of discussions with senior government officials as well as generic manufacturers, preparing the country for the next antiretroviral tender, which, by virtue of the size of South Africa's epidemic, accounts for almost a third of all global generic antiretroviral consumption. USAID asked that the National Institutes of Health International Data Safety Monitoring Board (DSMB) for HIV studies be used as the formal DSMB, which suggested several modifications to the protocol.
During this time, the original informal group was formalized as the OPTIMIZE Consortium, and included WHO, CHAI and various academic groups, as well as the pan-African community group AfroCAB, with a broader mandate around improving antiretroviral regimens. Collaborations were extended to other researchers working on similar projects, including within the National Institutes for Health and AIDS Clinical Trials Group led projects, and the protocol circulated freely.
The ADVANCE protocol was finalized during 2016, largely by email but using one formal scientific advisory group meeting at the 2016 CROI, and successfully submitted to the various regulators within South Africa. The last study drug was received at the site in December 2016, and the first 45 patients enrolled in the study in February 2017, with active support for recruitment from South Africa's Treatment Action Campaign. Enrolment is projected to be completed by the end of 2017, with 48-week results available during the first quarter of 2019.
CRITICAL DEBATES SURROUNDING THE STUDY DESIGN
The critical inclusion and exclusion criteria are detailed in Table 1. TAF was selected for study as the likely nucleotide reverse transcriptase inhibitor to succeed TDF, and its low dose suggested significant cost savings over TDF in a generic market; preliminary data also suggested less renal and bone-density impact. DTG, with proven tolerability benefits over EFV and a far greater resistance barrier, was selected as a cost neutral or even cheaper option. No other integrase inhibitor was available at the time that combined cost benefit and potential for daily FDC development, with studies planned to look at what happens to those who develop TB or pregnancy while on study. It was felt that it was important to keep the study as close to ‘real world’ as possible, so exclusion criteria were kept to a minimum. TB and pregnancy are being specifically studied in separate research projects, so excluding these patients at baseline was felt to be acceptable, but patients who fall pregnant or get TB will be retained.
Table 1.
Summary of the ADVANCE study
| Investigator driven, study drug donated by originator companies |
| Conducted in Johannesburg, start date: beginning 2017 |
| Noninferiority design, ∼1100 participants, over 96 weeks |
| Eligibility criteria |
| Needing first-line ART |
| No TB treatment, not pregnant at baseline (addressed in other studies; those contracting TB or falling pregnant may continue in the study) |
| No CD4 threshold |
| >12 years of age, >40 kg (weight band driven by weight restrictions on EFV) |
| Compares three combinations: DTG/TAF vs. DTG/TDF vs. EFV/TDF, all with FTC |
| Primary endpoint: viral suppression at 48 weeks |
| Pharmacokinetic sampling of both DTG/TAF in those who contract TB or become pregnant |
ART, antiretroviral treatment; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; TAF, tenofovir alafenamide; TB, tuberculosis; TDF, tenofovir disoproxil fumarate. Adopted with permission [6].
A noninferiority design was selected, and initially compared TAF with TDF in the presence of XTC (ADVANCE will use FTC) and DTG, as DTG had clear benefits over EFV, as a two-arm study. However, several commentators and donors felt that the level of evidence for starting millions of people on a new regimen needed to be robust, and that this justified including a third arm, the standard of care arm being TDF/FTC/EFV (Fig. 1). The TDF/FTC/DTG arm was retained, in case issues with TAF arose, and allowing development of DTG-containing regimens to continue. This added to cost and complexity, but after consulting with stakeholders, community advisors and the DSMB, was felt to be sufficiently compelling to accommodate.
FIGURE 1.
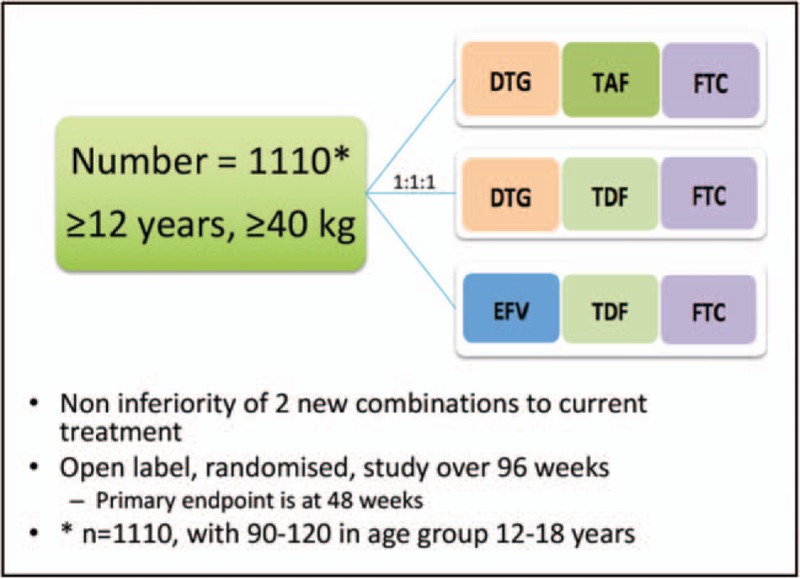
ADVANCE study design.
The study uses traditional 48-week and 96-week virological suppression endpoints and toxicity monitoring seen in similar registration trials, including renal and bone endpoints. Pharmacokinetic substudies have been nested within the main study, along with quality of life, sleep and psychiatric symptom questionnaires, to carefully map the side effects seen within the various regimens.
At the end of 2015, Gilead brought the ADVANCE group's attention to new data surrounding a major potential drug interaction with TAF and rifampicin. The study was adapted to accommodate this, with patients who develop TB being moved from TAF to TDF, and for DTG, doubling the dose and giving the drug twice daily.
In 2016, the DSMB requested that adolescents, a group thought to be important to include in the protocol, be analysed separately, and the adult portion of the protocol powered separately. This also added to cost and complexity, and increased the size of the study, but again was felt to be compelling enough to accommodate.
CONCLUSION
Traditionally, drug and regimen development have been largely led by the pharmaceutical industry or independent researchers. ADVANCE represents a unique consortium of stakeholders, including policy makers, nongovernmental agencies, activists, generic manufacturers and originators, that has allowed a major randomized controlled study to be designed, funded and started in exactly 2 years. This new collaborative approach to the rapid design and implementation of critical strategy studies deserves more attention. Similar approaches that prioritize public-health interests and engage communities may allow for more responsive, rapid and relevant research to be pursued.
Acknowledgements
We would like to thank all the members of the OPTIMIZE consortium, and the supporting committees, for their support to ADVANCE. The views of this study are those of the authors and do not necessarily reflect the views of USAID, Unitaid, SA MRC or the SA or US governments.
Financial support and sponsorship
The study is supported by the United States President's Emergency Plan for AIDS Relief (PEPFAR)/United States Agency for International Development (USAID) and Unitaid, as well as the South African Medical Research Council (MRC) in a broader collaboration addressing drug formulation and introduction, called OPTIMIZE. The study drug is donated by the originator companies, ViiV Healthcare (UK) and Gilead Sciences (USA).
Conflicts of interest
W.D.F.V. has received speaking honoraria for pharmaceutical and managed care organizations, as well as for participation on advisory boards (all less than $1000/year).
The United States Agency for International Development (USAID) invests in OPTIMIZE through its support of a global consortium, led by Wits RHI, that includes ICAP at Columbia University, Mylan Laboratories, the University of Liverpool and the Medicines Patent Pool. USAID is a key implementing agency of the US President's Emergency Plan for AIDS Relief (PEPFAR) and is responsible for over half of all PEPFAR programmes with activities focused in 35 priority countries and regions, mainly in sub-Saharan Africa and Asia. For more information, please visit:www.usaid.gov.
Unitaid finds new and better ways to prevent, test and treat HIV, tuberculosis and malaria quickly and more affordably. It takes game-changing ideas and turns those into practical solutions that can help accelerate the end of the three diseases. Established in 2006 by Brazil, Chile, France, Norway and the United Kingdom, Unitaid plays an important part in the global effort to defeat HIV, tuberculosis and malaria. For more information, please visit:www.unitaid.org.
ADVANCE is supported by Unitaid and the United States Agency for International Development (USAID), through the President's Emergency Plan for AIDS Relief (PEPFAR), as well as the South African Medical Research Council. Study drug has been donated by Gilead Sciences and ViiV Healthcare.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Barnhart M, Shelton JD. ARVs: the next generation. Going boldly together to new frontiers of HIV treatment. Glob Health Sci Pract 2015; 3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]; A good review of newer candidate antiretrovirals.
- 2.Raffi F, Pozniak AL, Wainberg MA. Has the time come to abandon efavirenz for first-line antiretroviral therapy? J Antimicrob Chemother 2014; 69:1742–1747. [DOI] [PubMed] [Google Scholar]
- 3▪▪.Clinton Health Access Initiative. The state of the antiretroviral drug market in low-and middle-income countries, 2015–2020. Published October 21st, 2016. Available from: http://www.clintonhealthaccess.org/arv-mket-report-2016/. [Accessed 20 March 2017] [Google Scholar]; This invaluable resource documents the most recent data on costs and antiretroviral usage.
- 4▪.WHO. Geneva. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach – Second edition. Published June 2016. Available from: http://www.who.int/hiv/pub/arv/arv-2016/en/. [Accessed 1 October 2016] [Google Scholar]; Low-income and middle-income countries use WHO antiretroviral guidelines to guide drug choices for national guidelines.
- 5▪▪.I-Base. Fit for purpose: antiretroviral treatment optimisation (Feb 2017). London. Published Feb 2017. Available from: http://i-base.info/fit-for-purpose-feb-2017/. [Accessed 20 March 2017] [Google Scholar]; This guide categorizes all current critical antiretroviral treatment registration studies, as well as discussing new approached to diagnosing and treating HIV, hepatitis and tuberculosis.
- 6.Venter WF, Kaiser B, Pillay Y, et al. Cutting the cost of South African antiretroviral therapy using newer, safer drugs. S Afr Med J 2016; 107:28–30. [DOI] [PubMed] [Google Scholar]


