Supplemental Digital Content is available in the text.
Keywords: drug-eluting balloon, paclitaxel, peripheral artery disease, percutaneous treatment, randomized controlled trial
Abstract
Background:
Numerous studies have reported favorable outcomes using drug-coated balloons (DCBs) for treatment of symptomatic peripheral artery disease of the superficial femoral and popliteal arteries. However, the treatment effect compared with an uncoated balloon has differed greatly among the randomized trials, with better outcomes observed with higher-dose DCBs. This European trial was designed to assess the safety and effectiveness of a next-generation low-dose (2-µg/mm2 surface dose of paclitaxel) DCB.
Methods:
This was a prospective, randomized, multicenter, single-blinded trial. Patients were randomized (3:1) to treatment with a low-dose DCB or an uncoated percutaneous transluminal angioplasty (PTA) balloon. The primary safety end point was a composite of freedom from device- and procedure-related death through 30 days after the procedure and freedom from target limb major amputation and clinically driven target lesion revascularization through 12 months after the procedure. The primary effectiveness end point was primary patency at 12 months.
Results:
Patients were randomized to treatment with a DCB (222 patients, 254 lesions) or uncoated PTA balloon (72 patients, 79 lesions) after successful predilatation. Mean lesion length was 7.2 and 7.1 cm, and 19.2% and 19.0% of lesions represented total occlusions, respectively. The primary safety end point was met, and superiority was demonstrated; freedom from a primary safety event was 94.1% (193 of 205) with DCB and 83.3% (50 of 60) with PTA, for a difference of 10.8% (95% confidence interval, 0.9%–23.0%). The primary effectiveness end point was met, and superiority of DCB over PTA was achieved (83.9% [188 of 224] versus 60.6% [40 of 66]; P<0.001). Outcomes with DCB were also superior to PTA per the Kaplan-Meier estimate for primary patency (89.0% versus 65.0% at 365 days; log-rank P<0.001) and for rates of clinically driven target lesion revascularization (5.9% versus 16.7%; P=0.014).
Conclusions:
Superiority with a low-dose DCB for femoropopliteal interventions was demonstrated over PTA for both the safety and effectiveness end points.
Clinical Trial Registration:
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01858363.
Editorial, see p 2237
Peripheral artery disease contributes to significant morbidity and mortality, affecting ≈27 million adults in Europe and North America.1 The overall prevalence of peripheral artery disease is estimated to be 3% to 10% and increases to 15% to 20% in adults >80 years of age.2 The superficial femoral artery (SFA), the longest artery in the human body, is exposed to the highest levels of dynamic mechanical stress and is involved in the majority of patients with peripheral artery disease. It is challenging to maintain patency after revascularization of the SFA. Primary patency rates of ≈55% can be reached with optimal percutaneous transluminal angioplasty (PTA) at 1 year.3,4 Elective stenting has raised this closer to 80%.5 However, hesitance exists concerning the elective use of stents given the burden of treating in-stent restenosis and the desire to avoid an unnecessary permanent implantation. Therefore, PTA with provisional stenting still represents the most widely adopted endovascular treatment in this patient population.
Stents coated with paclitaxel, an antiproliferative agent, have proven safety and have shown superior 12-month effectiveness compared with bare metal stents.6,7 Recent research suggests that paclitaxel drug-coated balloons (DCBs) are viable alternatives to drug-eluting stents, with the added advantage of avoidance of a permanent implantation. Several studies demonstrate that DCBs are safe with durable clinical outcomes.4,8–11 Current commercially available DCBs all contain the same drug (paclitaxel) but have different doses or surface concentrations (2–3.5 µg/mm2), excipients, coating methods, and drug formulations (crystalline, amorphous, or hybrid). Bench and preclinical tests confirm that drug tissue uptake, residency, drug loss, and drug effect are different across DCB platforms.12–14
The first-in-human trial with this low-dose DCB was promising.8 The primary patency rates were 89.5% and 80.3% at 12 and 24 months, respectively. The objective of the present study was to assess the safety and effectiveness of this low-dose DCB compared with a standard PTA balloon in symptomatic patients with SFA and/or proximal popliteal artery disease.
Methods
Study Design
This was a multicenter, single-blinded, randomized controlled trial conducted at 18 centers in Germany and Austria to assess the safety and effectiveness of a low-dose DCB (Stellarex, Spectranetics Corp, Colorado Springs, CO) versus a standard PTA balloon in symptomatic patients with SFA and/or proximal popliteal artery disease. The study was approved by the ethics committee at each participating center, and patients provided signed written informed consent before enrollment. The study was prospectively registered (http://www.clinicaltrials.gov; unique identifier: NCT01858363).
Patient Selection
Eligible patients reported moderate to severe claudication or ischemic rest pain (Rutherford class 2–4) with angiographic evidence of >70% stenosis within the SFA and/or popliteal artery, 1 or 2 de novo or restenotic lesions with a cumulative length of 30 to 200 mm, and reference vessel diameter of 4 to 6 mm. Important patient eligibility criteria are detailed in Table I in the online-only Data Supplement.
Randomization, Blinding, and Data Quality
After successful lesion crossing and predilatation with ≤70% residual stenosis and no flow-limiting dissection, patients were randomized to treatment with DCB or PTA with blocked allocation with a 3:1 ratio stratified by site. Patients requiring provisional stent placement after predilatation underwent postdilatation with DCB and were not randomized but instead were assigned to the stent cohort and analyzed separately. Investigators and research staff at the study centers were not blinded to treatment assignment given visual differences in the study devices. Patients remained blinded to treatment assignment throughout the study.
Independent core laboratories analyzed all images, including duplex ultrasound (VasCore, Massachusetts General Hospital, Boston, MA) and angiography (SynvaCor, Springfield, IL). Core laboratory readers remained blinded to treatment assignment. A blinded Clinical Events Committee who did not participate in the study adjudicated all adverse events. An independent Data Safety and Monitoring Board monitored the study for safety. Data were monitored for accuracy with 100% source document verification.
Study Device and Procedure
The DCB coating includes a low dose (2 μg/mm2) of paclitaxel with a polyethylene glycol excipient. The DCB is coated while unfolded and partially inflated; then it is deflated and folded into the final configuration. This coating method allows most of the drug to be protected by the balloon folds during delivery to the target lesion and provides a uniform circumferential delivery to the artery. The DCB is available in 4-, 5-, and 6-mm diameters and 40-, 80-, and 120-mm lengths.
Patients received dual antiplatelet therapy before the procedure per hospital standard of care. After the procedure, the study protocol required patients to take acetylsalicylic acid for the duration of the study and recommended the additional use of clopidogrel for 30 days after the procedure or for 90 days if a stent was placed. Balloon length was required to be ≥10 mm longer than the predilatation balloon length, and balloon inflation time was ≥1 minute in each group. Patients with residual stenosis >30% or flow-limiting dissection after treatment with the study device underwent postdilatation. In the DCB group, postdilatation was performed with DCB or PTA. In the PTA group, postdilatation was performed with an uncoated balloon catheter only. If postdilatation was unsuccessful, provisional stent placement was performed.
Patient Follow-Up
Patients returned for clinical visits at 1, 6, and 12 months that included clinical assessment, functional status (excluding 1 month), adverse events, medication compliance, and duplex ultrasound examination. Patient follow-up is ongoing for up to 5 years, with the primary effectiveness and safety results at 12 months of follow-up presented here.
Outcomes
The primary safety end point was a composite of freedom from device- and procedure-related death through 30 days after the procedure and freedom from target limb major amputation and clinically driven target lesion revascularization (CD-TLR) through 12 months. The primary effectiveness end point was primary patency (per lesion) through 12 months, defined as the absence of target lesion restenosis (duplex ultrasound–determined peak systolic velocity ratio ≤2.5) and freedom from CD-TLR. CD-TLRs were adjudicated by a blinded Clinical Events Committee and defined as a revascularization of the target lesion with a peak systolic velocity ratio ≥2.5 by duplex ultrasound (or percent diameter stenosis >50% by angiography) and worsening of Rutherford classification or abnormal ankle-brachial index (ABI) that was clearly referable to the target lesion. Worsening was defined as Rutherford increase of at least 1 class from the earliest postprocedural measurement or an ABI decrease >0.15 from the maximum early postprocedural level. The degree of stenosis was determined by independent, blinded core laboratories. Technical success (per lesion) was defined as final in-lesion residual diameter stenosis ≤50% determined by the angiographic core laboratory without a device malfunction. Clinical success (per patient) required technical success without a procedural major adverse event (MAE). Lesion success (per lesion) required final in-lesion residual diameter stenosis of ≤50% determined by the angiographic core laboratory. Procedural success (per patient) was defined as lesion success without a procedural MAE. Additional outcomes included MAEs defined as cardiovascular death, target limb amputation, or CD-TLR, as adjudicated by the blinded Clinical Events Committee. Other secondary end points included change in ABI, walking impairment questionnaire score, Rutherford classification, and walking distance compared with baseline. ABI was defined as the ratio of the highest ankle systolic pressure to the highest brachial systolic pressure. The change in walking distance was assessed by a treadmill test or 6-minute walk test and calculated per subject, with the baseline result compared with the result at 12 months using the same assessment method.
Statistical Analysis
The primary safety hypothesis was that freedom from a primary safety end point at 1 year with DCB would be noninferior to PTA. A noninferiority margin of 5% absolute difference was deemed a clinically nonsignificant difference. A priori, if noninferiority was met and the lower 95% confidence limit was >0%, superiority would be claimed. The primary effectiveness hypothesis was that primary patency at 1 year would be superior with DCB versus PTA. Given 3:1 randomization, 90% power, 1-sided α of 0.025, absolute difference of 5% noninferiority margin for safety, and estimated safety and effectiveness end-point rates of 80% with DCB and 60% with PTA, 176 patients were required for the primary safety end point and 280 for the primary effectiveness end point.
Continuous data are reported as mean and SD; categorical data are reported as frequency and percentage. Comparisons of baseline characteristics were performed with independent-samples t test, Fisher exact test, or χ2 test as appropriate. Noninferiority was assessed with a Farrington-Manning exact test, and superiority was evaluated with a χ2 test. The Kaplan-Meier method and log-rank tests were used to evaluate time-to-event outcomes, including primary patency and freedom from CD-TLR through 12 months of follow-up. Time-to-event effectiveness outcomes are displayed through 395 days (12-month follow-up plus 30-day visit window). All analyses were prespecified in a statistical analysis plan. Data were analyzed with SAS version 9.3 or higher (SAS Institute, Cary, NC).
Role of the Funding Source
The clinical trial was designed by the principal investigator (H.S.), Philippe Marco, MD, and the study sponsor. Study data were collected, monitored, and analyzed by the study sponsor (currently The Spectranetics Corp). Investigators (H.S. and M.B.) prepared the first draft of the manuscript, which was then critically reviewed and edited by the other authors. The study sponsor had the right to review, but not to approve, the final manuscript. The authors had full access to all data and take full responsibility for the accuracy, completeness, and integrity of the reported analyses and data interpretation.
Results
Patient Enrollment and Follow-Up
Between December 2012 and April 2015, 294 patients were randomized to DCB (222 patients, 254 lesions) or PTA (72 patients, 79 lesions) at 18 centers in Germany and Austria (listed in the online-only Data Supplement). Data are reported separately for 33 patients who underwent provisional stent placement after failed predilatation. One patient who was not randomized was treated with DCB and excluded from all analyses (Figure 1).
Figure 1.
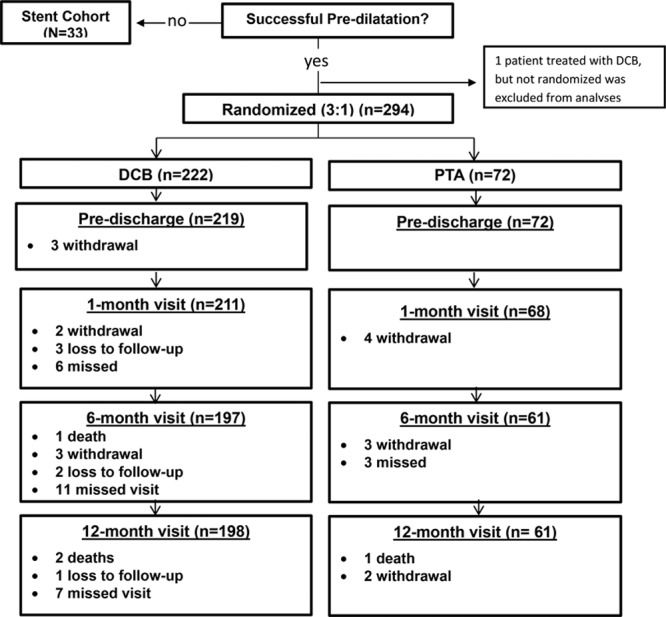
Patient flow diagram. Twelve-month follow-up visit completed in 89% treated with a drug-coated balloon (DCB) and 85% treated with percutaneous transluminal angioplasty (PTA).
Patient and Procedural Data
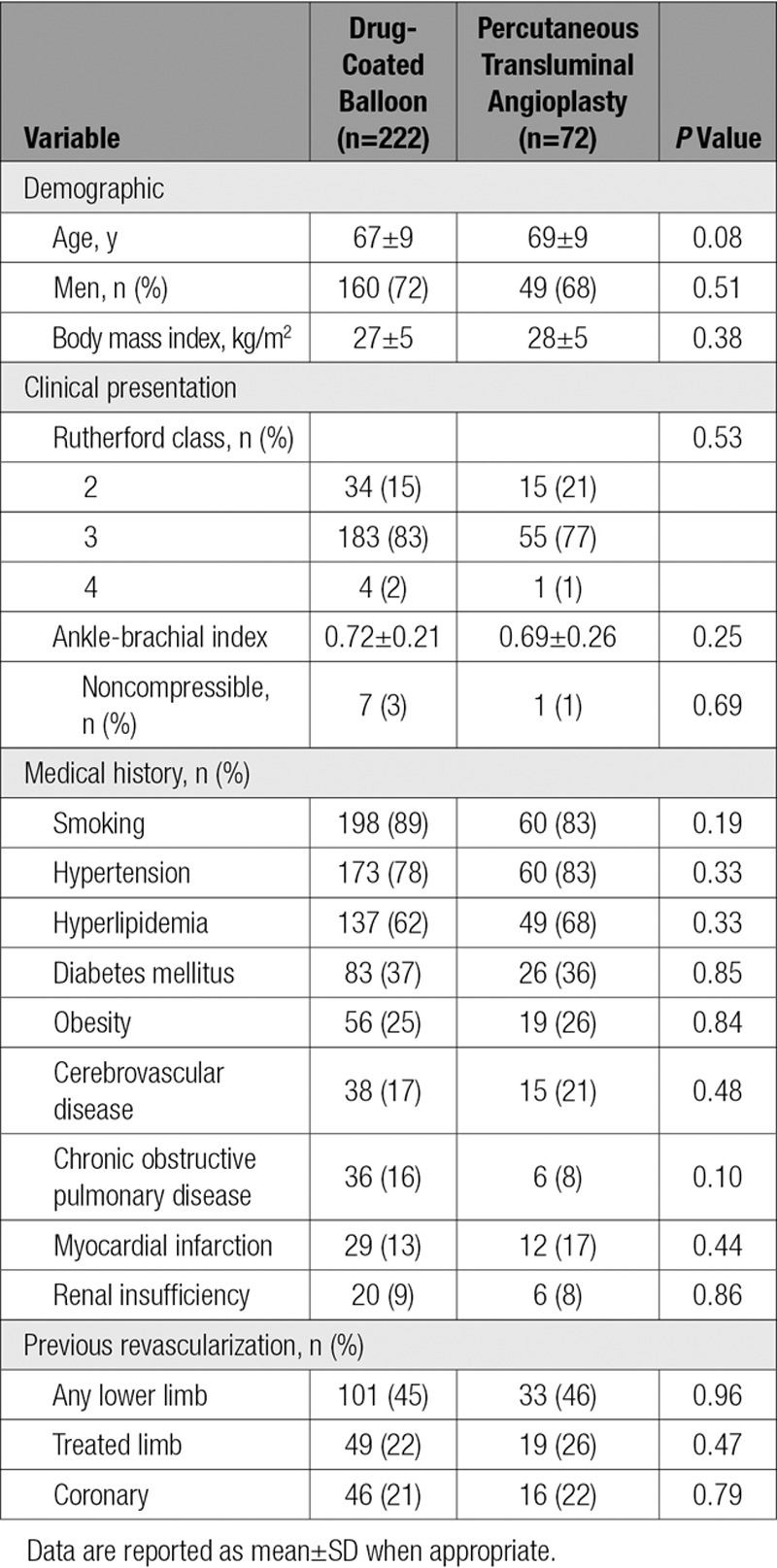
No statistically significant differences were noted in baseline patient characteristics between groups (Table 1). Lesion characteristics were comparable across the randomized cohorts, including mean lesion length (7.2 versus 7.1 cm), diameter stenosis (79% versus 81%), and total occlusions (both 19%). The only significant difference between groups was noted in reference vessel diameter (5.0±0.8 versus 4.8±0.7 mm; P=0.01; Table 2). Procedural outcomes were not statistically different between DCB and PTA, including flow-limiting dissection (0.4% versus 0%), provisional stent placement (15% versus 11%), and diameter stenosis after the procedure (24% versus 23%; Table 3). Comparing DCB with PTA acute success rates showed the following: technical success, 99.2% versus 100%; clinical success, 99.1% versus 100%; lesion success, 99.6% versus 100%; and procedural success, 99.5% versus 100%.
Table 2.
Baseline Lesion Characteristics
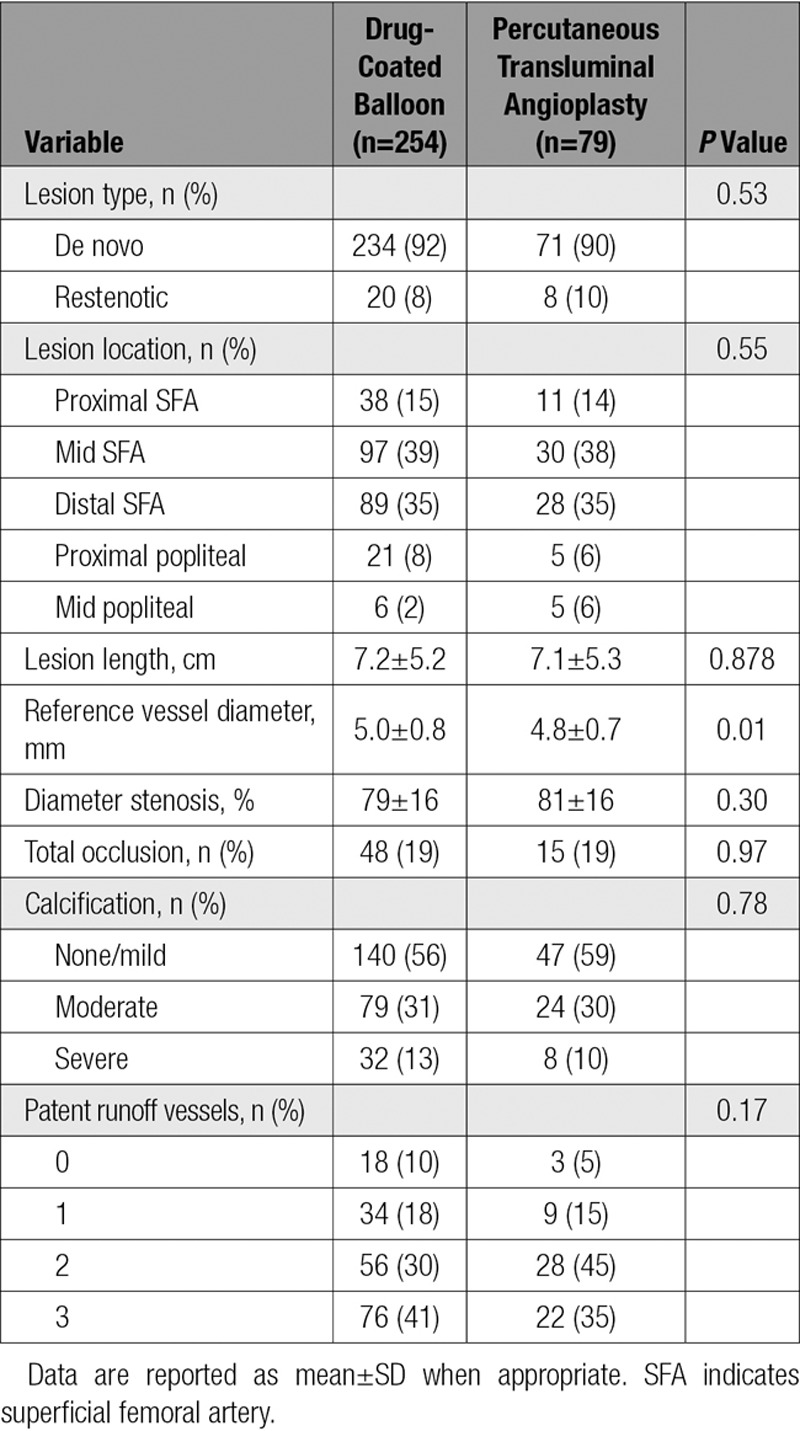
Table 3.
Procedural Data
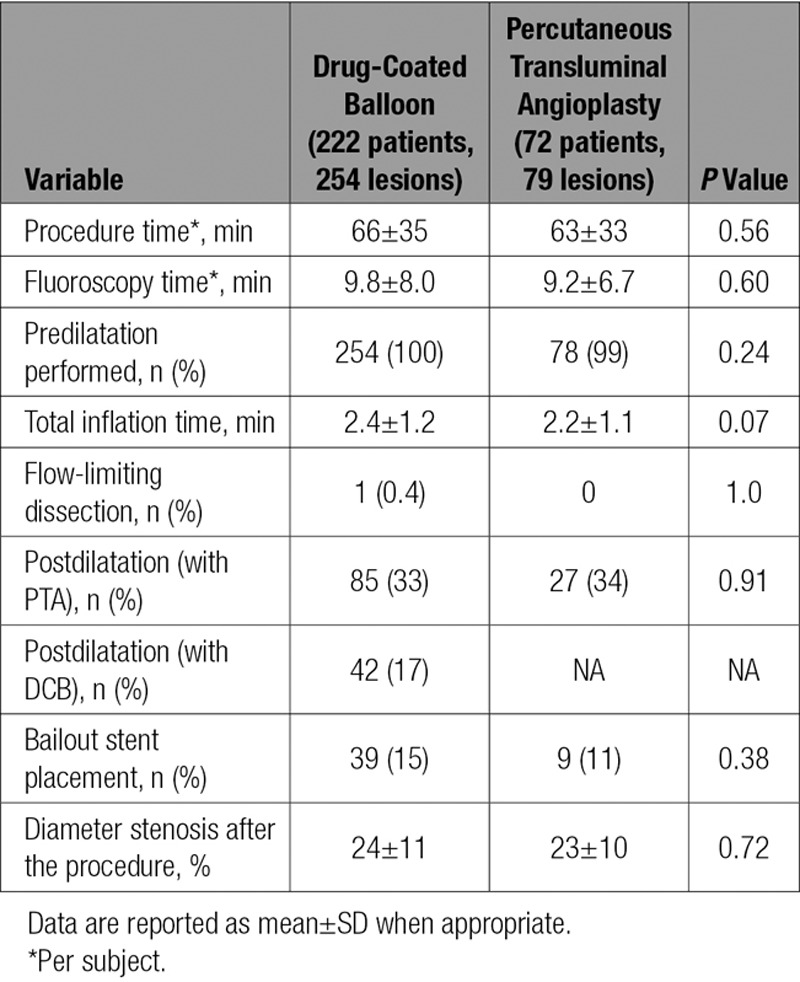
Table 1.
Baseline Patient Characteristics
Safety Outcomes
Freedom from a primary safety event was 94.1% (193 of 205) with DCB and 83.3% (50 of 60) with PTA, for a difference of 10.8% (95% confidence interval, 0.9%–23.0%). The lower limit of the 95% confidence interval of the difference was greater than −5%; thus, noninferiority was established. In addition, superiority of DCB was established because the lower confidence interval limit exceeded 0. The Kaplan-Meier freedom from CD-TLR estimates were 94.8% for DCB and 85.3% for PTA at 365 days (log-rank P=0.010; Figure 2). A total of 20 MAEs were reported in 14 DCB patients (6.8%) and 12 MAEs were reported in 11 PTA patients (18.0%; P=0.008). For DCB versus PTA, CD-TLR was 5.9% versus 16.7% (P=0.014), cardiovascular death was 1.0% versus 1.6% (P=0.542), and target limb amputation was 0.5% versus 0% (P>0.99). The target limb amputation was a minor amputation (toe) 354 days after the procedure. No major amputations were reported in either cohort.
Figure 2.
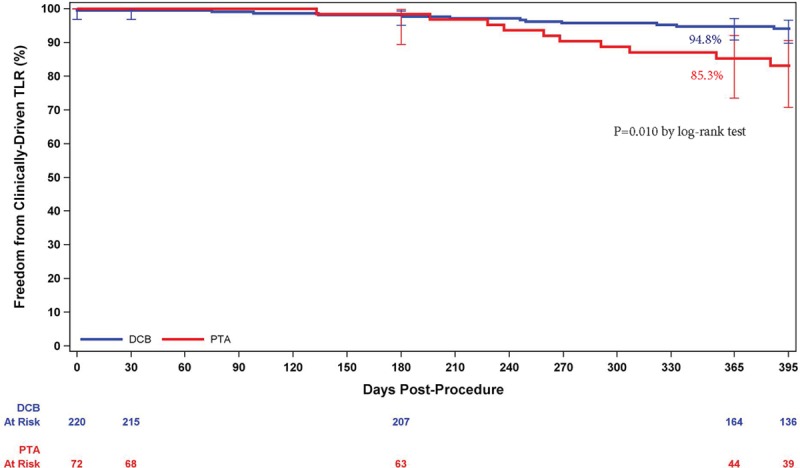
Freedom from clinically driven target lesion revascularization (CD-TLR) by Kaplan-Meier was significantly higher in the drug-coated balloon (DCB) group than the percutaneous transluminal angioplasty (PTA) group (P=0.010 by log-rank test; 94.8% vs 85.3% at day 365). Bars represent 95% confidence intervals.
Effectiveness Outcomes
The primary effectiveness outcome, primary patency proportional rates per assessable lesion through the 12-month follow-up (day 395), was 83.9% (188 of 224) for DCB and 60.6% (40 of 66) for PTA, for a difference of 23.3% (95% confidence interval, 10.6%–36.1%; P<0.001). Therefore, the primary effectiveness end point was also met, and superiority over PTA was demonstrated.
The Kaplan-Meier primary patency rate was 89.0% for DCB and 65.0% for PTA at day 365 (log-rank P<0.001; Figure 3). In addition, significantly favorable outcomes were observed with the DCB when primary patency was evaluated by sex (Figure 4) and diabetes status (Figure 5). The primary patency rate in the DCB cohort was 89.2% in patients with diabetes mellitus and 88.8% in patients without diabetes mellitus (P=0.4724). Likewise, no statistical difference in the primary patency rates was observed in the DCB cohort when stratified by sex (90.4% in men and 85.3% in women; P=0.3064).
Figure 3.

Primary patency by Kaplan-Meier was significantly higher in the drug-coated balloon (DCB) group than the percutaneous transluminal angioplasty (PTA) group (P<0.001 by log-rank test; 89.0% vs 65.0% at day 365). Bars represent 95% confidence intervals.
Figure 4.
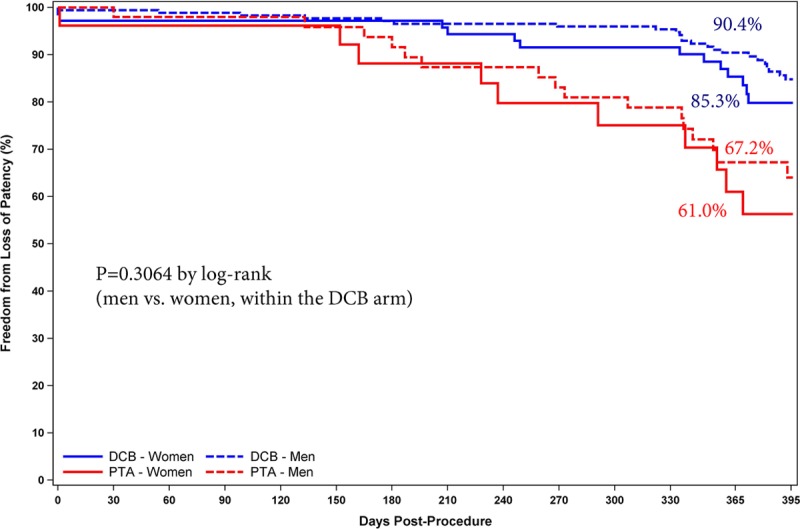
Primary patency by Kaplan-Meier was similar between men and women (P=0.31 by log-rank test; 90.4% vs 85.3% at day 365) in the drug-coated balloon (DCB) cohort. PTA indicates percutaneous transluminal angioplasty.
Figure 5.
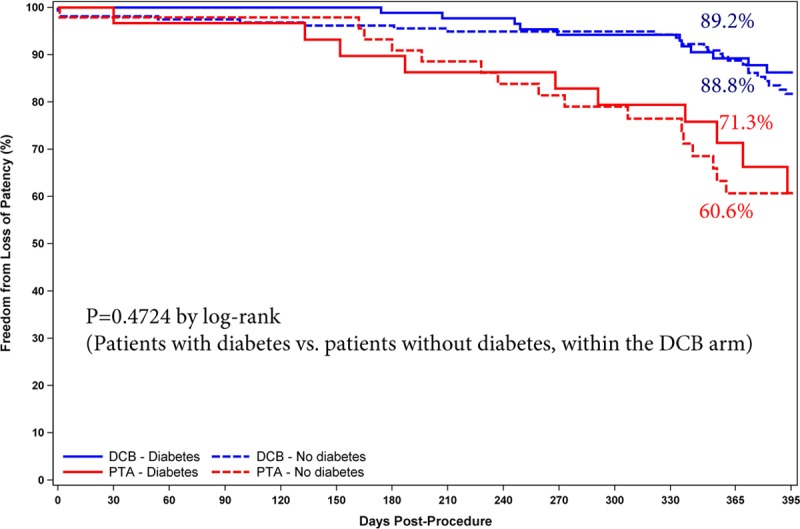
Primary patency by Kaplan-Meier was similar between patients with and without diabetes mellitus (P=0.47 by log-rank test; 89.2% vs 88.8% at day 365) in the drug-coated balloon (DCB) cohort. PTA indicates percutaneous transluminal angioplasty.
This study allowed postdilatation with a DCB in the DCB arm. This occurred in 24 lesions (17%). When these lesions are excluded from the analysis, the primary patency rate at day 365 is 88.4%. To assess the impact of the statistically significant difference between groups in baseline reference vessel diameter (P=0.012) on the primary end points, logistic regression models were used to provide both adjusted and unadjusted comparisons. For both primary effectiveness and safety, baseline reference vessel diameter is significantly associated with outcomes. However, there is no statistical evidence of interaction effects, and the impact on the treatment group comparisons is minimal, with unadjusted odds ratios for DCB versus PTA versus adjusted odds ratios of 3.394 versus 3.105 for patency and 3.217 versus 2.827 for safety. Therefore, this difference does not affect the overall study conclusions.
At 12 months, a similar percentage of patients in both the DCB and PTA cohorts had improvements in ABI (83.9% and 76.8%), Rutherford classification (89.2% and 86.2%), and walking distance (77.1% and 72.1%). ABI improvement was similar with DCB (0.71±0.20 to 0.93±0.14) and PTA (0.66±0.27 to 0.90±0.16) through 12 months. These similar outcomes were achieved with a significant, almost 3-fold lower rate of CD-TLR in the DCB cohort (5.9% [12 of 205] versus 16.7% [10 of 60]; P=0.014).
Outcomes With Provisional Stent Placement
A total of 33 patients were enrolled in a nonrandomized cohort after provisional stent placement for suboptimal predilatation. After suboptimal predilatation, these patients were not randomized; all were stented and then treated with a DCB. The majority of these patients (75.8%) were men with a mean age of 66±8 years. Comorbidities included hypertension (78.8%), hyperlipidemia (69.7%), diabetes mellitus (36.4%), and previous coronary revascularization (30.3%). The mean lesion length was 8.8 cm, 54.5% were chronic total occlusions, and 15.6% were severely calcified. At 12 months, the primary patency was 78.8% (26 of 33) and CD-TLR rate was 12.1% (4 of 33).
Within the randomized DCB cohort, a bailout stent was placed in 38 patients (39 of the 42 target lesions in this subgroup). The mean lesion length was 8.4 cm; 40.5% were chronic total occlusions, and 11.9% were severely calcified. The 12-month primary patency rate was 75.0% (30 of 40) in this cohort. A total of 181 patients with 209 lesions were treated with a DCB and not stented. The mean lesion length was 6.9 cm; 14.9% were chronic total occlusions, and 12.9% were severely calcified. The primary patency rate in this cohort was 85.9% (158 of 184). Primary patency was not statistically different in patients treated with versus without bailout stent placement in the DCB group (P=0.09).
Discussion
This was the first randomized controlled trial to assess the effectiveness and safety of the Stellarex DCB versus standard uncoated PTA to treat SFA and/or popliteal artery disease. The trial demonstrated superior safety and effectiveness outcomes in the DCB arm and validated the results of the previously reported first-in-human study.8 The core laboratory–adjudicated primary patency rate at day 365 was 89.0% in the DCB arm of this trial, a rate comparable to that observed in the first-in-human study (89.5%), validating those early promising outcomes. Uniquely, this study allowed postdilatation with a DCB in the DCB arm. When these lesions are excluded from the patency analysis, there is a negligible change in the patency rate at day 365 (88.4%).
There are 2 other published randomized trials comparing DCB and PTA in similar patient populations and characterized by the same rigorous trial design and conduct related to 2 DCBs of different drug doses, 2.0 µg/mm2 and 3.5 µg/mm2.3,4 Outcomes in the present study are comparable to those of the IN.PACT SFA Trial (Drug-Coated Balloon Versus Standard Percutaneous Transluminal Angioplasty for the Treatment of Superficial Femoral and/or Popliteal Peripheral Artery Disease),4,10 which assessed the IN.PACT Admiral DCB versus PTA with very similar trial design, end point definitions, and rigorous conduct. The IN.PACT SFA Trial randomized 331 patients with a mean lesion length of ≈9 cm and demonstrated significantly higher patency rates for the DCB arm at 360 days, per the Kaplan-Meier estimate (87.5% versus 66.8%). Two-year data for this trial have been reported, and no late catchup was observed; 2-year patency rates were 78.9% versus 50.1% in the PTA arm (log-rank P<0.001), demonstrating a sustained clinical benefit after short-term exposure to an effective DCB. The most important differentiator between the IN.PACT Admiral DCB and the Stellarex DCB is the amount of paclitaxel on the balloon surface. The IN.PACT Admiral DCB has a drug dose surface concentration of 3.5 compared with 2 µg/mm2 on the Stellarex DCB. Drug pharmacokinetics is a key variable affecting DCB performance. Optimizing drug tissue uptake and retention while minimizing drug loss during transit and inflation remains the key goals of modern DCB technologies.12,15 The ILLUMENATE EU RCT (Prospective, Randomized, Multi-Center, Single-Blind Study for the Treatment of Subjects Presenting with De Novo Occluded/Stenotic or Re-occluded/Restenotic Lesions of the Superficial Femoral or Popliteal Arteries using a Paclitaxel-Coated or Bare Percutaneous Transluminal Angioplasty Balloon Catheter) is the first trial to demonstrate that angioplasty with a low-dose DCB is able to achieve clinical outcomes comparable to that of a DCB with 75% more drug. The clinical implications of the higher drug dose coating formulation are unclear; however, low-dose DCBs carry the potential to reduce distal drug embolization,16 which may translate into a safety advantage in specific patient populations and anatomic settings.
LEVANT 2 (Lutonix Paclitaxel-Coated Balloon for the Prevention of Femoropopliteal Restenosis Trial 2) assessed the safety and effectiveness of the Lutonix DCB, which also has a 2 µg/mm2 surface concentration of paclitaxel.3 This trial also had a blinded duplex ultrasound core laboratory assessing patency and the same peak systolic velocity ratio threshold to determine patency in a binary fashion. The trial randomized 476 patients with a mean lesion length of 6.3 cm in both groups. At 12 months, the patency rate was significantly higher in the DCB arm (73.5% versus 56.8%; P<0.001), although considerably lower than the rate observed in the present trial and in the IN.PACT SFA trial. There was no statistical difference between groups for rates of target lesion revascularization (12.3% versus 16.8%; P=0.21). To date, 2-year outcomes have not been published but have been reported.17 The primary patency rate was 58.6% versus 53.0% (log-rank P=0.05) and freedom from CD-TLR was 82% versus 79% (P=NS) at 2 years, questioning the durability of outcomes with this low-dose DCB.
The effectiveness of a DCB may be affected by several components, including the antirestenotic drug, excipient, drug morphology, balloon material, manufacturing process, and drug pharmacokinetic properties and bioavailability.15 More studies are needed to better understand the contribution of each of these factors and their role in DCB effectiveness.
Post hoc analyses show that the Stellarex DCB maintained a significant treatment effect compared with PTA in both critical subsets of women and patients with diabetes mellitus. These findings are important because diabetes mellitus has been identified as an independent predictor of decreased long-term primary patency after PTA/stenting,18 and there was no treatment effect observed for the Lutonix DCB in women in the LEVANT 2 Trial.19
The PTA data indicate that the control arm treatment was optimal with high short-term success rates and a relatively high patency rate. At 12 months, the patency rate was 65.0%, which is consistent with previous control arm data in prior randomized controlled DCB trials (IN.Pact SFA PTA arm patency rate, 66.8%) but nearly twice that for PTA used in previously published bare metal stent trials (33%).20 Despite these optimal results, superiority was demonstrated with the DCB.
Limitations
Several limitations of this study deserve further discussion. Although the Clinical Events Committee, Data Safety and Monitoring Board, and core laboratory personnel were blinded to treatment, physicians were not blinded because of the visible coating on the DCB catheter. These data cannot be generalized to other DCBs because head-to-head comparative trials have not been completed. Finally, patients were selected with the use of strict inclusion and exclusion criteria; therefore, generalizability of these data to real-world cases may be limited.
Conclusions
This randomized trial of a low-dose DCB demonstrated superior safety and effectiveness outcomes over standard PTA in the treatment of symptomatic SFA and/or popliteal peripheral artery disease.
Acknowledgments
The authors thank Larry Miller, PhD, and Meghan Schadow, MS, for editorial assistance, and Teresa Yurik, MS, for statistical support.
Sources of Funding
The study was sponsored by the Spectranetics Corp, Maple Grove, MN.
Disclosures
Drs Schroeder and Werner have received research grants and speaking and consulting honoraria from Spectranetics Corp. Dr Brodmann has received speaking honoraria from BARD, Medtronic, Bayer Health Care, Daiichi, and Böhringer Ingelheim. She has also participated on Scientific Advisory boards for Medtronic, Spectranetics Corp, and Intact Vascular. Dr Jaff has served as a noncompensated advisor to Abbott Vascular, Boston Scientific, Cordis, and Medtronic Vascular and has served as a board member of VIVA Physicians, a 501(c)(3) not-for-profit education and research organization. All other authors report no conflicts.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.116.026493/-/DC1.
Circulation is available at http://circ.ahajournals.org.
Clinical Perspective
What Is New?
In symptomatic patients with superficial femoral and/or proximal popliteal artery disease, this low-dose drug–coated balloon is safer and more effective than an uncoated percutaneous transluminal angioplasty balloon through 12 months of follow-up.
What Are the Clinical Implications?
A low-dose drug–coated balloon is a promising treatment option in symptomatic patients with superficial femoral and/or popliteal artery disease.
References
- 1.Belch JJ, Topol EJ, Agnelli G, Bertrand M, Califf RM, Clement DL, Creager MA, Easton JD, Gavin JR, 3rd, Greenland P, Hankey G, Hanrath P, Hirsch AT, Meyer J, Smith SC, Sullivan F, Weber MA Prevention of Atherothrombotic Disease Network. Critical issues in peripheral arterial disease detection and management: a call to action. Arch Intern Med. 2003;163:884–892. doi: 10.1001/archinte.163.8.884. doi: 10.1001/archinte.163.8.884. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfield K, Jaff MR, White CJ, Rocha-Singh K, Mena-Hurtado C, Metzger DC, Brodmann M, Pilger E, Zeller T, Krishnan P, Gammon R, Müller-Hülsbeck S, Nehler MR, Benenati JF, Scheinert D LEVANT 2 Investigators. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–153. doi: 10.1056/NEJMoa1406235. doi: 10.1056/NEJMoa1406235. [DOI] [PubMed] [Google Scholar]
- 4.Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR IN.PACT SFA Trial Investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. doi: 10.1161/CIRCULATIONAHA.114.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vardi M, Novack V, Pencina MJ, Doros G, Burke DA, Elmariah S, Cutlip DE, Mauri L, Yeh RW. Safety and efficacy metrics for primary nitinol stenting in femoropopliteal occlusive disease: a meta-analysis and critical examination of current methodologies. Catheter Cardiovasc Interv. 2014;83:975–983. doi: 10.1002/ccd.25179. doi: 10.1002/ccd.25179. [DOI] [PubMed] [Google Scholar]
- 6.Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Zeller T, Roubin GS, Burket MW, Khatib Y, Snyder SA, Ragheb AO, White JK, Machan LS Zilver PTX Investigators. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011;4:495–504. doi: 10.1161/CIRCINTERVENTIONS.111.962324. doi: 10.1161/CIRCINTERVENTIONS.111.962324. [DOI] [PubMed] [Google Scholar]
- 7.Dake MD, Scheinert D, Tepe G, Tessarek J, Fanelli F, Bosiers M, Ruhlmann C, Kavteladze Z, Lottes AE, Ragheb AO, Zeller T Zilver PTX Single-Arm Study Investigators. Nitinol stents with polymer-free paclitaxel coating for lesions in the superficial femoral and popliteal arteries above the knee: twelve-month safety and effectiveness results from the Zilver PTX single-arm clinical study. J Endovasc Ther. 2011;18:613–623. doi: 10.1583/11-3560.1. doi: 10.1583/11-3560.1. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder H, Meyer DR, Lux B, Ruecker F, Martorana M, Duda S. Two-year results of a low-dose drug-coated balloon for revascularization of the femoropopliteal artery: outcomes from the ILLUMENATE first-in-human study. Catheter Cardiovasc Interv. 2015;86:278–286. doi: 10.1002/ccd.25900. doi: 10.1002/ccd.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinstner CM, Lammer J, Willfort-Ehringer A, Matzek W, Gschwandtner M, Javor D, Funovics M, Schoder M, Koppensteiner R, Loewe C, Ristl R, Wolf F. Paclitaxel-eluting balloon versus standard balloon angioplasty in in-stent restenosis of the superficial femoral and proximal popliteal artery: 1-Year Results of the PACUBA Trial. JACC Cardiovasc Interv. 2016;9:1386–1392. doi: 10.1016/j.jcin.2016.04.012. doi: 10.1016/j.jcin.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Laird JR, Schneider PA, Tepe G, Brodmann M, Zeller T, Metzger C, Krishnan P, Scheinert D, Micari A, Cohen DJ, Wang H, Hasenbank MS, Jaff MR IN.PACT SFA Trial Investigators. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-month results of IN.PACT SFA. J Am Coll Cardiol. 2015;66:2329–2338. doi: 10.1016/j.jacc.2015.09.063. doi: 10.1016/j.jacc.2015.09.063. [DOI] [PubMed] [Google Scholar]
- 11.Giacoppo D, Cassese S, Harada Y, Colleran R, Michel J, Fusaro M, Kastrati A, Byrne RA. Drug-coated balloon versus plain balloon angioplasty for the treatment of femoropopliteal artery disease: an updated systematic review and meta-analysis of randomized clinical trials. JACC Cardiovasc Interv. 2016;9:1731–1742. doi: 10.1016/j.jcin.2016.06.008. doi: 10.1016/j.jcin.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Gongora CA, Shibuya M, Wessler JD, McGregor J, Tellez A, Cheng Y, Conditt GB, Kaluza GL, Granada JF. Impact of paclitaxel dose on tissue pharmacokinetics and vascular healing: a comparative drug-coated balloon study in the familial hypercholesterolemic swine model of superficial femoral in-stent restenosis. JACC Cardiovasc Interv. 2015;8:1115–1123. doi: 10.1016/j.jcin.2015.03.020. doi: 10.1016/j.jcin.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Milewski K, Afari ME, Tellez A, Aboodi MS, Kim JS, Cheng Y, Conditt GB, McGregor JC, Yi GH, Stenoien M, Langanki D, Krueger CG, Kaluza GL, Granada JF. Evaluation of efficacy and dose response of different paclitaxel-coated balloon formulations in a novel swine model of iliofemoral in-stent restenosis. JACC Cardiovasc Interv. 2012;5:1081–1088. doi: 10.1016/j.jcin.2012.06.012. doi: 10.1016/j.jcin.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Cremers B, Biedermann M, Mahnkopf D, Böhm M, Scheller B. Comparison of two different paclitaxel-coated balloon catheters in the porcine coronary restenosis model. Clin Res Cardiol. 2009;98:325–330. doi: 10.1007/s00392-009-0008-2. doi: 10.1007/s00392-009-0008-2. [DOI] [PubMed] [Google Scholar]
- 15.Katsanos K. Paclitaxel-coated balloons in the femoropopliteal artery: it is all about the pharmacokinetic profile and vessel tissue bioavailability. JACC Cardiovasc Interv. 2016;9:1743–1745. doi: 10.1016/j.jcin.2016.06.047. doi: 10.1016/j.jcin.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 16.Kolodgie FD, Pacheco E, Yahagi K, Mori H, Ladich E, Virmani R. Comparison of particulate embolization after femoral artery treatment with IN.PACT Admiral versus Lutonix 035 paclitaxel-coated balloons in healthy swine. J Vasc Interv Radiol. 2016;27:1676–1685.e2. doi: 10.1016/j.jvir.2016.06.036. doi: 10.1016/j.jvir.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 17.Laurich C. Lutonix Global Real World SFA Registry: interim results & first look at LEVANT 2 24 month results. http://investorrelations.crbard.com/phoenix.zhtml?c=91501&p=irol-sec&secCat01.1_rs=71&secCat01.1_rc=10&control_searchbox=&control_year=2015&control_selectgroup=0. Accessed February 25, 2017.
- 18.Abularrage CJ, Conrad MF, Hackney LA, Paruchuri V, Crawford RS, Kwolek CJ, LaMuraglia GM, Cambria RP. Long-term outcomes of diabetic patients undergoing endovascular infrainguinal interventions. J Vasc Surg. 2010;52:314–22.e1. doi: 10.1016/j.jvs.2010.03.015. doi: 10.1016/j.jvs.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration. Summary of safety and effectiveness data (SSED): LUTONIX® 035 Drug Coated Balloon PTA Catheter. http://www.accessdata.fda.gov/cdrh_docs/pdf13/P130024S009B.pdf. Accessed February 25, 2017.
- 20.Rocha-Singh KJ, Jaff MR, Crabtree TR, Bloch DA, Ansel G VIVA Physicians, Inc. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv. 2007;69:910–919. doi: 10.1002/ccd.21104. doi: 10.1002/ccd.21104. [DOI] [PubMed] [Google Scholar]


