Abstract
Purpose of review
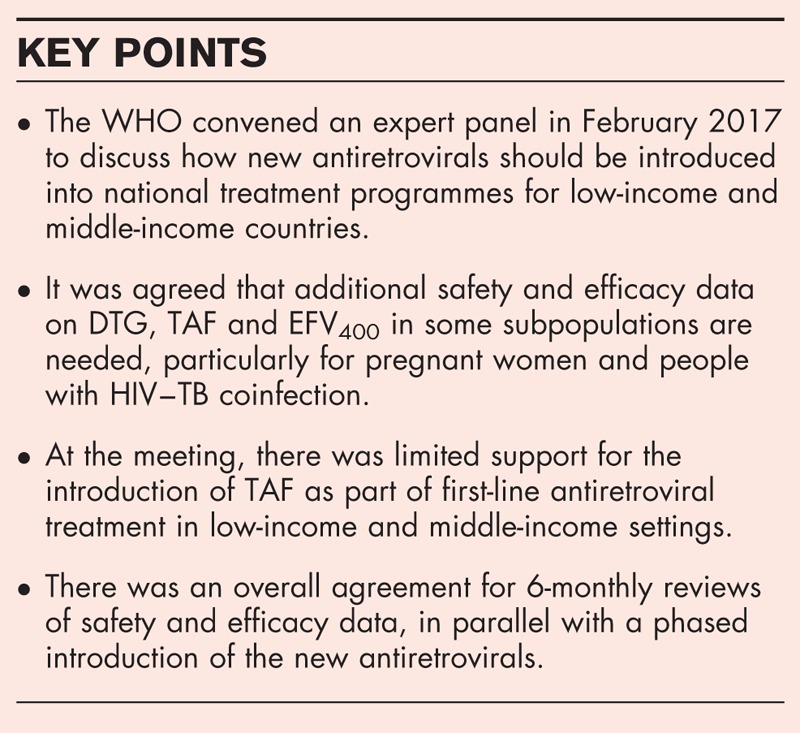
To discuss barriers and opportunities for the introduction of new antiretrovirals into national treatment programmes in low-income and middle-income countries to support further treatment scale-up. Invitees to a WHO Think Tank in February 2017 evaluated recently published results.
Recent findings
There is not sufficient clinical experience of dolutegravir (DTG), tenofovir alafenamide (TAF) or efavirenz 400 mg (EFV400) to recommend their use in pregnancy. Outcomes from births and assessment of congenital anomalies need to be evaluated from several hundred pregnant women. Clinical experience of these treatments during rifampicin-based treatment for tuberculosis is also required. This could be difficult for TAF, which is currently contraindicated with TAF. Changes in second-line treatment from two nucleoside analogues + protease inhibitor plus ritonavir will require new randomized trials of alternative combinations.
Conclusion
Additional safety and efficacy data on DTG, TAF and EFV400 in some subpopulations are needed before a large introduction in national treatment programmes. There is currently limited support for the introduction of TAF as part of first-line antiretroviral treatment in low-income and middle-income settings. There was an overall agreement for 6-monthly reviews of safety and efficacy data, in parallel with a phased introduction of the new antiretrovirals.
Keywords: antiretroviral treatment, drug–drug interactions, HIV–TB coinfection, pregnant women
INTRODUCTION
WHO guidelines currently recommend first-line treatment for HIV with tenofovir disoproxil fumarate (TDF) and lamivudine (3TC) or emtricitabine (FTC) and either the nonnucleoside efavirenz (EFV) or the integrase inhibitor dolutegravir (DTG) [1]. Treatment guidelines in high-income settings have recently been revised to recommend first-line use of integrase inhibitors in preference to EFV [2–4]. The recommended second-line treatment is with two nucleos(t)ide analogues and a boosted protease inhibitor: this is consistent across treatment guidelines.
DTG, the new nucleotide analogue tenofovir alafenamide (TAF), and the NNRTI EFV at the reduced 400 mg once daily dose (EFV400) are becoming available as low-cost generics. Widespread use of these new options could lower the unit costs of antiretroviral treatment and enhance the treatment coverage capacity in countries [5,6,7▪]. However, evidence for the efficacy and safety of these drugs in pregnant women, children and tuberculosis (TB) coinfection is limited [8▪▪]. In addition, the role of DTG in second-line treatment, after first-line virological failure, is unclear. Several clinical trials are underway to assess the pharmacokinetics, efficacy and safety of these new antiretrovirals in pregnant women and TB-coinfected patients and to compare DTG with EFV, and TDF with TAF in low-income and middle-income countries (LMICs). Most of these trials will not generate results until 2019–2020. By that time, it is possible that several million people will already have been started on DTG, TAF and other new antiretrovirals.
The risks of adverse outcomes in pregnancy, or congenital anomalies in the infants, need to be considered when introducing new antiretroviral drugs into national programmes. Several recent studies have reported associations between use of certain antiretrovirals during pregnancy and adverse birth outcomes. An analysis of 6500 women in Botswana showed that those treated with TDF/3TC/EFV before conception were significantly less likely to have preterm deliveries or infants low birth weight, compared with mothers treated with the protease inhibitor lopinavir/ritonavir (LPV/r) [9]. In the randomized PROMISE study, women treated with TDF/3TC + LPV/r were significantly more likely to have adverse birth outcomes than those treated with zidovudine/3TC + LPV/r [10].
Another consideration is the compatibility of new antiretroviral drugs with rifampicin-based TB treatment. People with TB coinfection are typically under-represented in Phase 3 clinical development programmes for new antiretrovirals. However, HIV–TB coinfection is common in LMICs, and this drug interaction with rifampicin-based treatment is an important issue to be considered by HIV treatment programmes in these settings [8▪▪].
In February 2017, the WHO held a ‘Think-Tank’ meeting in Seattle, the United States of America. There were 60 experts invited, including members of the WHO HIV Guidelines committee, specialists in paediatrics and HIV drug resistance, UNITAID, the Clinton Health Access Initiative, USAID, Centres for Disease Control and PEPFAR.
The two main questions discussed at this WHO Think-Tank meeting were
-
(1)
Is there already enough evidence to support the efficacy and safety of DTG, TAF and EFV400 to justify their use in millions of people in LMICs?
-
(2)
What clinical trials and pharmacovigilance studies are needed to assess drug safety when these new treatments are used more widely?
Box 1.
no caption available
DOLUTEGRAVIR: OVERALL EFFICACY AND SAFETY
DTG is recommended as an alternative first-line treatment to EFV in the current WHO-consolidated ARV guidelines [1]. The efficacy of DTG has been established in studies of naïve and pretreated patients [11–15]. This alternative status from WHO reflects the limited information about the use of DTG in pregnant women and TB coinfection available in end of 2015, when these guidelines were formulated. In addition, there might be additional safety concerns with the use of this integrase inhibitor in real-life settings, beyond the controlled environment and selected patient population of clinical trials.
DOLUTEGRAVIR IN PREGNANT AND BREASTFEEDING WOMEN
In the registrational trials programme for DTG, there is very little clinical experience of treatment during pregnancy, reflecting the general precautionary approach to enroling pregnant women, and women in general in clinical trials of antiretrovirals [16]. DTG has high penetration across the placenta, unlike some other antiretrovirals [17]. High DTG concentrations in the developing embryo might protect against vertical HIV transmission, but might also increase the risk of adverse birth outcomes. In animal toxicology studies, there was no evidence for adverse effects from DTG treatment during pregnancy [18].
During the original clinical trials programme for DTG, women were advised to use contraception, and any women who became pregnant were discontinued from treatment with DTG [11,12]. These measures, although typical for early clinical development studies, mean that there are few women with treatment outcome data available.
Safety data for DTG during pregnancy is currently from the originator company database of Phase 2 and 3 clinical trials, postmarketing surveillance and from one prospective study – IMPAACT P1026s [19]. There have been 112 live births from these sources of data. Table 1 shows the congenital anomalies recorded in these infants. In addition to the outcomes from live births, there was one case of spontaneous abortion with foetal dystrophy after use of DTG in the first trimester. In the IMPAACT P1026s study, there was one additional case of polydactyly that was not included in the summary because it was not judged to be related to treatment.
Table 1.
Congenital anomalies for infants after in-utero exposure to dolutegravir
| Study | Congenital anomalies |
| IMPAACT P1026s (2/15) | Multicystic dysplastic right kidney |
| Cyst, left kidney | |
| DTG Phase 3 studies (1/30) | Right ventricular septal defect |
| DTG postmarketing (5/67) | Polydactyly |
| Polydactyly and syndactyly | |
| Polydactyly | |
| Intracranial calcifications, intrauterine growth retardation | |
| Bilateral hydroureter, right hydronephrosis, pyelocaliectasis |
DTG, dolutegravir.
It is not possible to evaluate the safety of DTG in pregnancy from the current database because the sample size is too small and potential confounders have not been assessed. The reports from postmarketing surveillance could be subject to reporting bias, if clinicians are more likely to report results from infants with congenital anomalies. Overall birth outcomes, such as spontaneous abortion or premature birth, also need to be evaluated.
At the 2017 WHO Think-Tank meeting, only a minority of participants considered that the safety database in pregnancy was sufficient to recommend giving pregnant women DTG in national treatment programmes in LMICs. The Brazilian Ministry of Health is starting to introduce DTG nationally but not for pregnant women or those with TB coinfection. Botswana is currently the only LMIC where DTG is being widely used for pregnant women. There are three randomized clinical trials of DTG versus EFV in pregnancy in progress: DOLPHIN-1, DOLPHIN-2 and VESTED [20,21]. A pharmacokinetic study is also in progress [22]. The details of these studies are shown in Table 2. These randomized studies will not report results until 2019–2020. Until then, results will only be available from nonrandomized studies, which could be more difficult to interpret.
Table 2.
Key randomized clinical trials evaluating new antiretrovirals: pregnant women
| Clinical trial | Treatment arms | Inclusion | Objective | Results |
| Dolphin-1 | 2NRTI + EFV | Pregnant women | Efficacy | 2018 |
| N = 60 | 2NRTI + DTG | (Uganda) | Birth outcomes | |
| Dolphin-2 | 2NRTI + EFV | Pregnant women | Efficacy | 2020 |
| N = 250 | 2NRTI + DTG | (Uganda) | Birth outcomes | |
| VESTED | TDF/FTC/EFV | Pregnant women | Efficacy | 2020 |
| N = 549 | TDF/FTC/DTG | (International) | Birth outcomes | |
| TAF/FTV/DTG | ||||
| SSAT 063 | 2NRTI + EFV400 | Pregnant women | PK, outcomes | 4Q17 |
| N = 25 |
2NRTI, two nucleoside analogues; DTG, dolutegravir; EFV, efavirenz; EFV400, efavirenz 400 mg; FTC, emtricitabine; PK, Pharmacokinetics; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
By July 2017, the current database on pregnant women should be supplemented by analysis of the Antiretroviral Pregnancy Registry, a European study of pregnancy outcomes (EPPIIC) and data from over 400 pregnant women treated in Botswana. A review of this larger dataset in late 2017 could inform considerations on the use of pregnant women with DTG in LMICs.
DOLUTEGRAVIR AND TB COINFECTION
When used with rifampicin, the dose of DTG needs to be increased to 50 mg twice daily [23] because of drug–drug interactions. DTG is being evaluated with rifampicin in several new studies [24–26], as shown in Table 3. This issue is discussed in other parts of the supplement.
Table 3.
Key randomized clinical trials evaluating new antiretrovirals: TB coinfection
| Clinical trial | Treatment arms | Inclusion | Objective | Results |
| SSAT 062 | EFV400 + rifampicin | Healthy volunteers | PK | 4Q17 |
| N = 20 | ||||
| RADIO | DTG + rifampicin | Healthy volunteers | PK | 4Q17 |
| N = 20 | ||||
| NIH | DTG + rifapentine | Healthy volunteers | PK | Suspended |
| N = 20 | ||||
| INSPIRING | DTG + 2NRTIs | HIV–TB coinfection | 48-week efficacy | 4Q17 |
| N = 125 | EFV + 2NRTIs | With rifampicin | ||
| RIFT | TAF + rifampicin | Healthy volunteers | PK | 4Q17 |
| N = 20 |
2NRTI, two nucleoside analogues; DTG, dolutegravir; EFV, efavirenz; EFV400, efavirenz 400 mg; PK, Pharmacokinetics; TAF, tenofovir alafenamide; TB, tuberculosis.
USE OF INTEGRASE INHIBITORS AND THE RISK OF IMMUNE RECONSTITUTION INFLAMMATORY SYNDROME
Immune reconstitution inflammatory syndrome (IRIS) occurs most often among people with low CD4 cell count initiating first-line antiretroviral treatment. Integrase inhibitors suppress HIV RNA levels more quickly than other antiretroviral drug classes [27]. A rapid recovery of immune function during first-line antiretroviral treatment can cause immune reactions to existing infections, often with severe and paradoxical effects. Inflammatory symptoms such as severely swollen lymph nodes (lymphadenopathy), tachycardia, fever and the worsening of symptoms of opportunistic infections can emerge and may require hospitalization and/or corticosteroid treatment.
Data from two recent studies from nonrandomized cohort studies in France and The Netherlands reported an association between the use of integrase inhibitors and a higher risk of IRIS.
In the Dutch study [28▪], IRIS was either diagnosed by a clinician or classified by the French 2004 definition (atypical tumour or opportunistic infection presentation accompanied by viral load decline or CD4 increase). According to these definitions, 38% of those who started integrase inhibitor treatment and 16% of those starting any other treatment regimen developed IRIS. Patients taking integrase inhibitor treatment were significantly more likely to develop IRIS according to either definition [odds ratio 3.25, 95% confidence interval (CI) 1.83–5.80].
In the French study [29▪], severe IRIS leading to hospitalization developed in 3% of patients in the integrase inhibitor group versus 1.5% in the nonintegrase inhibitor group, a relative risk of 1.99 (95% CI 1.09–3.47). IRIS was most frequently related to tuberculosis, to Mycobacterium avium and to progressive multifocal leukoencephalopathy.
These two cohort studies are not randomized trials, so there is the potential for bias and confounding in the reported association with IRIS. However, randomized clinical trials comparing first-line treatment with integrase inhibitors and other treatment classes have typically excluded people with the highest risk of IRIS (patients with low CD4 cell counts, active TB or other opportunistic infections) [11,12]. It will therefore be important to monitor the risk of IRIS in national treatment programmes using first-line DTG in case a rise in its occurrence is observed.
The results from randomized trials in an appropriate patient population are not yet available and so cannot be used to evaluate the risk of IRIS from use of integrase inhibitors in LMICs. The Spanish ADVANZ-4 trial is evaluating first-line treatment with DTG versus darunavir plus ritonavir (DRV/r) in 108 patients with baseline CD4 counts below 100 cells/μl [30]. This trial is limited in sample size to a statistically significant risk of clinical IRIS, but includes detailed evaluations of immune function and is expected to produce results in late 2017. As shown in Table 4, the other large randomized trials of first-line DTG versus EFV that could include patients with low CD4 cell counts and/or Centres for Disease Control (CDC) stage C disease – ADVANCE and NAMSAL – will not report 48-week results until 2019 [31,32].
Table 4.
Key randomized clinical trials evaluating new antiretrovirals: first-line and second-line treatments
| Clinical trial | Treatment arms | Inclusion | Objective | Results |
| First-line treatment | ||||
| ADVANCE | TDF/FTC/EFV | Naïve | 48-week efficacy | 2019 |
| N = 1100 | TDF/FTC/DTG | (South Africa) | ||
| TAF/FTC/DTG | ||||
| NAMSAL | TDF/3TC/EFV400 | Naïve | 48-week efficacy | 2019 |
| N = 606 | TDF/3TC/DTG | (Cameroun) | ||
| ADVANZ-4 | ABC/3TC/DTG | Naïve | 48-week efficacy | 4Q17 |
| N = 108 | ABC/3TC/DRV/r | (Spain) | IRIS | |
| Second-line treatment | ||||
| WHRI 052 | 2NRTI + LPV/r | Switch | 48-week efficacy | 2018 |
| N = 300 | 2NRTI + DRV/r 400 mg | (South Africa) | ||
| DAWNING | 2NRTI + DTG | First-line failure | 48-week efficacy | 3Q17 |
| N = 612 | 2NRTI + PI/r | (International) | ||
| D2EFT | DTG + DRV/r | First-line failure | 48-week efficacy | |
| N = 610 | 2NRTI + DRV/r | (International) | ||
2NRTI, two nucleoside analogues; 3TC, lamivudine; DTG, dolutegravir; DRV/r, darunavir plus ritonavir; EFV, efavirenz; EFV400, efavirenz 400 mg; FTC, emtricitabine; LPV/r, lopinavir/ritonavir; PI/r, protease inhibitor plus ritonavir; TAF, tenofovir alafenamide TDF, tenofovir disoproxil fumarate.
Until more evidence becomes available on this issue, strict clinical monitoring for IRIS may be required for patients starting first-line, integrase-based treatment with known risk factors for IRIS, to check for evidence of emerging IRIS.
USE OF DOLUTEGRAVIR AND OTHER SIDE EFFECTS
Results from nonrandomized cohort studies suggest a higher risk of CNS adverse events for DTG compared with other integrase inhibitors [33–37]. In addition, there has been a report of two cases of myocarditis on DTG [38], with one additional case of myocarditis in the FLAMINGO trial, part of the Phase 3 development programme [12]. These results need to be evaluated in the context of the overall safety profile of DTG from randomized clinical trials: there have been fewer discontinuations for all adverse events on DTG compared with either EFV in the SINGLE study [11] or with DRV/r in the FLAMINGO study [12].
TENOFOVIR ALAFENAMIDE: OVERALL SAFETY AND EFFICACY
In a recent meta-analysis of randomized clinical trials comparing TAF versus the original prodrug of TDF, there was no difference in the risk of adverse events, serious adverse events or discontinuations for adverse events between the two forms of the drug [39]. However, there were significant differences in mean change in bone and renal laboratory markers, favouring TAF, whereas mean changes in blood lipids showed a benefit for TDF [8▪▪]. The clinical significance of these mean changes in laboratory markers for patients in mass treatment programmes in LMICs is unclear. There is very limited clinical experience of TAF in pregnancy.
TENOFOVIR ALAFENAMIDE IN PREGNANT AND BREASTFEEDING WOMEN
The intracellular concentration of tenofovir diphosphate is four to five times higher for TAF compared with TDF [40]. It is unclear whether this higher intracellular concentration could lower the risk of vertical HIV transmission and/or increase the risk of adverse birth outcomes. There is currently very little clinical experience of TAF in pregnancy. No data are available on placental or breast milk passage of TAF in humans [41▪].
The safety database of TAF from the originator company included birth outcomes in only 12 live infants, of whom two had a congenital anomaly. One infant was born with tricuspid atresia, a large ventricular septal defect and died within 10 min of birth. The other infant was born with patent foramen ovale. The trial investigators did not consider either of these anomalies as related to their antiretroviral treatment. In addition, there were 17 induced terminations, with two congenital anomalies. One embryo had Trisomy 18 (Edwards’ syndrome) that was considered possibly related to TAF because the mother was taking TAF at the time of conception. The other embryo had Trisomy 21 (Down's syndrome) that was not considered to be treatment-related because the mother was not taking TAF at the time of conception.
It is not clear whether there is a clinical trial programme in place to properly evaluate the safety of TAF in pregnant women. Results from the Antiretroviral Pregnancy Registry are accumulating slowly, and the clinical trial database is limited in size. The VESTED study will compare TAF with TDF in over 500 pregnant women and their infants [21]. There should be approximately 180 pregnant women treated with TAF in this study. However, results will not be available until 2020. Other studies such as IMPAACT P1026s may only provide a small number of mother–infant pairs with outcome data [19].
At the 2017 WHO Think-Tank meeting, very few participants supported a recommendation to allow treatment of pregnant women with TAF in LMICs. The lack of pharmacokinetic and safety data from pregnant women was noted as a key concern.
TENOFOVIR ALAFENAMIDE IN HIV–TB COINFECTION
TAF is currently contraindicated for treatment with rifampicin, because the results of an interaction study with carbamezapine suggested that there would be significant reductions in tenofovir concentrations [41▪]. A new pharmacokinetic interaction study (RIFT, Table 3) is in progress, in 20 healthy volunteers, to investigate the effects of rifampicin on TAF. This study will include analysis of intracellular tenofovir diphosphate concentrations. At the 2017 Think-Tank meeting, there was no support for using TAF with rifampicin, given the current contraindication and lack of clinical evidence.
EFAVIRENZ 400 MG ONCE DAILY
The recommendation to use the 400-mg dose of EFV is supported by results from the ENCORE-1 study and the substudy of pharmacokinetics (PK)/PD [42,43]. There were significantly fewer EFV-related clinical adverse events at the 400-mg dose (38%) compared with the 600-mg dose (48%). A detailed PK/PD analysis of this study showed that the lower EFV concentrations at the 400-mg dose were not associated with a loss of virological efficacy [43].
EFAVIRENZ 400 MG IN PREGNANT WOMEN
There is extensive clinical experience of TDF/XTC/EFV in pregnant women, using the standard 600-mg once daily dose of EFV, which is recommended by WHO for treatment of pregnant women. The SSAT 063 study is in progress to evaluate the pharmacokinetics of EFV 400 mg in pregnant women (Table 2). At the meeting, some participants questioned the priority of adopting the low dose of EFV versus switching to DTG.
EFAVIRENZ 400 MG IN HIV–TB COINFECTION
Pharmacokinetic studies showed that rifampicin-based treatment leads to short-term reductions in EFV drug levels during the first 1–2 weeks of treatment, but after longer term treatment in combination with rifampicin-based combinations increases in EFV drug levels have been observed consistently across several studies [44]. However, these overall trends could differ by ethnicity, as suggested in the STRIDES study [45]. The efficacy of EFV-based treatment is similar for people either taking or not taking rifampicin-based treatment (in contrast to nevirapine, which shows lower efficacy when coadministered with rifampicin) [26].
There is a new pharmacokinetic study in progress – SSAT 062 – evaluating the interaction between EFV400 and rifampicin. The first phase of this study is in people with HIV infection in the United Kingdom and Uganda. The first results are expected by the end of 2017 (Table 3).
At the meeting, a minority of participants supported the use of EFV400 in combination with rifampicin. Most people wanted to see the results from the SSAT 062 study before making a firm recommendation.
The consensus was to continue using rifampicin-based treatment for HIV–TB coinfected people, despite the drug-interaction issues. The pharmacokinetic studies of DTG, TAF and EFV400 with rifampicin should generate results by the end of 2017. These results could allow planning of new clinical studies. For example, the pharmacokinetic interaction studies with TAF are likely to show lower concentrations of tenofovir diphosphate with rifampicin. However, if this concentration is still above the levels seen for TDF without rifampicin, this could still be therapeutic.
FIRST-LINE TREATMENT: CONTINUE WITH EFAVIRENZ OR SWITCH TO DOLUTEGRAVIR?
At the 2017 WHO Think-Tank meeting, there were equal numbers of participants who favoured a switch to first-line TDF/XTC/DTG in LMICs versus keeping country programmes using TDF/XTC/EFV.
The arguments in favour of switching to DTG included potential cost-savings, improved tolerability, encouraging evidence from treatment in North America and Europe and a higher barrier to drug resistance for DTG. The arguments supporting maintaining the status quo of EFV included TB coinfection, which was considered central to HIV infection in sub-Saharan Africa. The complexity of doubling the dose of DTG when using rifampicin was seen as a problem by some participants. Also, there was some concern over the emerging adverse event profile of DTG and the need for more intensive pharmacovigilance. Some participants favoured a phased introduction of DTG, excluding pregnant women and TB coinfected people from using DTG in national programmes until a more reliable safety database was available.
SECOND-LINE TREATMENT: FUTURE ALTERNATIVES TO 2NRTI + PI/R
At the 2017 WHO Think-Tank meeting, there was strong consensus that second-line treatment should be with two nucleoside analogues (NRTIs) with a boosted protease inhibitor. This is because of the strong evidence base from randomized clinical trials, which has shown no advantage of other treatment strategies. For example, in the EARNEST and SECOND-LINE studies, there was no improvement in efficacy for using combinations of a protease inhibitor and an integrase inhibitor second-line, versus 2NRTI + protease inhibitor plus ritonavir (PI/r). This high efficacy for 2NRTI + PI/r combinations was seen despite the presence of high-level NRTI resistance at baseline in the EARNEST study [46,47].
There are three studies in progress that might change this paradigm. The DAWNING study is comparing 2NRTI + DTG with 2NRTI + LPV/r for patients who have failed virologically on first-line treatment but have at least one active NRTI, according to genotypic resistance analysis. Results from the DAWNING study are expected by September 2017 [15]. Even if this study does show similar efficacy for DTG and LPV/r as second-line treatment, it may be difficult to apply this strategy in LMICs where there is restricted availability of genotypic resistance testing.
The D2EFT trial [48] is comparing a new combination of DRV/r + DTG versus the standard of care 2NRTI + DRV/r treatment in patients who have failed virologically on first-line treatment. Resistance testing is also permitted in this study to guide the choice of NRTIs, if locally available – again this could limit the application of the results to LMICs where resistance testing is not available. Another issue with this treatment strategy is the prevalence of Hepatitis B in sub-Saharan Africa, which ranges from 6.5% in Zambia to 25% in Zimbabwe [49]. Combinations of protease inhibitors with integrase inhibitors would not suppress HBV DNA replication. Therefore, before starting PI-integrase combinations, there would need to be systematic screening for Hepatitis B to avoid flares among people previously taking TDF/FTC or TDF/3TC.
Finally, the WHRI 052 study is evaluating a switch from LPV/r to a lower dose of DRV/r 400/100 mg once daily for 300 patients on second-line treatment with HIV RNA suppression (Table 4). If successful, the results could justify widespread switching to the lower dose of DRV/r for people with HIV RNA suppression. However, it will be important to start other studies to evaluate the lower dose of DRV/r in people with virological failure on first-line treatment. Currently, DRV/r is not available as a heat stable coformulation and is significantly more expensive than either LPV/r or atazanavir/ritonavir, which limits its widespread use in LMICs [6]. A reduction of the DRV/r dose to 400/100 mg once daily could lower the price to the same range as the other protease inhibitors, while avoiding the gastrointestinal adverse events and twice daily dosing of LPV/r.
CONCLUSION
After review of the current clinical trial data, it was agreed that the evidence base for evaluating the safety and efficacy of DTG, TAF and EFV400 needs to be improved to justify expanding treatment with these new drugs in millions of people in LMICs. Results from several key randomized clinical trials, such as NAMSAL, ADVANCE, D2EFT and VESTED, are not expected for at least another 2 years. Therefore, it will be important to analyse other datasets, even if nonrandomized, in the interim.
The current evidence for the safety and efficacy of DTG, TAF and EFV400 was not considered strong enough to justify widespread introduction of these antiretrovirals in LMICs. This situation could change within the next 3 years, as results emerge from ongoing clinical trials.
By July 2017, there should be a large enough database of pregnant women treated with DTG for a first review of birth outcomes and congenital anomalies. This review could be repeated at the end of 2017, once the database has grown further. The outcomes from the pregnant mothers treated in Botswana will be of key interest in these reviews.
The reports of IRIS and CNS adverse events on DTG need to be followed up with a systematic review of clinical trials and cohort studies. The cohort studies could provide valuable information on the safety of DTG in patients typically excluded from Phase 2 and 3 studies, because of CDC C disease, low CD4 cell counts or HIV–TB coinfection. These are the patients most likely to develop IRIS.
There was agreement that 6-monthly reviews of safety and efficacy should be started to be continued until the evidence is sufficient to change WHO recommendations on the use of these drugs in pregnant women, HIV–TB coinfection and people with low CD4 cell counts.
Acknowledgements
We would like to thank all of the experts who attended the WHO Think-Tank meeting in Seattle for their helpful advice and comments.
List of participants for Think Tank 2017: HIV treatment transition and drug sequencing in the context of new ARVs.
Panel of invited experts (Adults): Adele Benzaken, MoH, Brazil; Aleny Couto, MoH Mozambique; Amandine Cournil, ANRS/IRD, France; Amy Lyn, USAID, USA; Anton Pozniak, Chelsea and Westminster Hospital, UK; Beatriz Grinsztejn, Oswaldo Cruz Foundation, MoH, Brazil; Benjamin Young, IAPAC, USA; Carmen Perez Casas, UNITAID, Switzerland; Carolyn Amole, CHAI, USA; Celicia Serenata, Witwatersrand University, Johannesburg, South Africa; Charles Flexner, Johns Hopkins University, USA; Danielle Ferris, UNITAID, Switzerland; David Cooper, Kirby Institute, Australia; Deenan Pillay, Africa Center for Health, South Africa; Diane Havlir, UCSF, USA; Emily Harris, USAID, USA; Francois Venter, Witwatersrand University, Johannesburg, South Africa; Kevin de Cock, CDC, Kenya; James Hakim, University of Zimbabwe, Zimbabwe; Juliana Silva, CDC, USA; Laura Broyles, CDC, USA; Lana Lee, USAID, USA; Lisa Nelson, OGAC, USA; Lynne Mofenson, EGPAF, USA; Marta Boffito, Chelsea & Westminster Hospital, UK; Melinda Watkins, CHAI, USA; N Kumarasamy, YRG Care, India; Nikos Dedes, EATG, Greece; Paul Domanico, CHAI, USA; Pedro Cahn, Fundacion Huespede, Argentina; Refeletswe Lebelonyane, MoH, Botswana; Richard Chaisson, Johns Hopkins University, USA; Roy Gulick, Cornell University, USA; Sandeep Juneja, MPP, Switzerland; Shannon Hader, CDC, USA; Stefano Vella, Istituto Superiore di Sanita, Italy; Tendani Gaolathe, MoH Botswana; Zhang Fujie, Beijing Ditan Hospital, China.
Panel of invited experts (Paediatrics): Diana Gibb, MRC, UK; David Burger, UMC, Netherlands; Elaine Abrams, Columbia University, USA; George Siberry, OGAC, USA; Polly Clayden, HIV i-Base, UK; Fernando Pascual, MPP, Switzerland; Tim Cressey, PHPT, Thailand.
WHO staff & consultants: Gottfried Hirnschall, WHO/HIV/ODH, Geneva; Meg Doherty, WHO/HIV/TAC, Geneva; Marco Vitoria, WHO/HIV/TAC, Geneva; Martina Penazzato, WHO/HIV/TAC, Geneva; Lara Vojnov, WHO/HIV/TAC, Geneva; Ying Ru Lo, WHO/WPRO, Philippines; Michael Jordan (consultant), Tufts University, USA; Andrew Hill (consultant), University of Liverpool, UK.
Financial support and sponsorship
The Think-Tank meeting and publication were supported by a research grant from the WHO.
Conflicts of interest
A.H. has received consultancy payments from Janssen and Teva, not connected with this project. A.P. has received consultancy payments from Gilead, Janssen, BMS, Merck and ViiV and Cipla, not connected with this project. M.V., N.F. and P.C. report no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. 2nd ed. 2015. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. [Accessed 22 October 2016]. [Google Scholar]
- 2.Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults [2016] recommendations of the International Antiviral Society – USA Panel. JAMA 2016; 316:191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department for Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2016. Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Accessed 20 March 2017]. [Google Scholar]
- 4.European AIDS Clinical Society Guidelines for HIV treatment. 2016. Available at: http://www.eacsociety.org/files/guidelines_8.2-english.pdf. [Accessed 20 March 2017]. [Google Scholar]
- 5.Venter F, Kaiser B, Pillay Y, et al. Cutting the cost of South African antiretroviral therapy using newer, safer drugs. South Afr Med J 2017; 107:28–30. [DOI] [PubMed] [Google Scholar]
- 6.Clinton Health Access Initiative. ARV market report. The state of the antiretroviral drug market in low- and middle-income countries. 2015. Available at: http://www.clintonhealthaccess.org/content/uploads/2015/11/CHAI-ARV-Market-Report-2015_FINAL.pdf. [Accessed 25 August 2016]. [Google Scholar]
- 7▪.Barnhart M, Shelton J. ARVs: the next generation. Going boldly together to new frontiers of HIV treatment. Glob Health Sci Prac 2015; 1:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]; Summary of potential prices of antiretroviral treatment.
- 8▪▪.Vitoria M, Hill AM, Ford N, et al. Choice of antiretroviral drugs for continued treatment scale-up in a public health approach: what more do we need to know? J Int AIDS Soc 2016; 19:20504. [DOI] [PMC free article] [PubMed] [Google Scholar]; Summary of efficacy and safety data for dolutegravir, tenofovir alafenamide and efavirenz 400 mg once daily.
- 9.Zash R, Jacobson D, Diseko M, et al. Adverse birth outcomes differ by art regimen from conception in Botswana. CROI conference, 13–16 February 2017, Seattle, Washington. [Abstr 25]. [Google Scholar]
- 10.Chi BH, Farhad M, Owor M, et al. Antenatal Antiretroviral Therapy and Adverse Birth Outcomes: The PROMISE trial. Conference on Retroviruses and Opportunistic Infections February 2017. Seattle, WA, USA. Abstract 776. [Google Scholar]
- 11.Walmsley S, Baumgarten A, Berenguer J, et al. Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naïve patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 2015; 70:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2015; 383:2222–2231. [DOI] [PubMed] [Google Scholar]
- 13.STRIVING Study – switch from current treatment to dolutegravir. 2017. Available at: https://clinicaltrials.gov/ct2/show/results/NCT02105987?term=dolutegravir&rank=95. [Accessed 3 March 2017]. [Google Scholar]
- 14.Llibre JM, et al. Phase III SWORD 1 & 2: Switch to DTG+RPV maintains virologic suppression through 48 weeks. Presented at: Conference on Retroviruses and Opportunistic Infections; 13–16 February 2017; Seattle. [Google Scholar]
- 15.DAWNING Study: dolutegravir versus lopinavir/ritonavir as second-line treatment. Available at: https://clinicaltrials.gov/ct2/show/NCT02227238?term=dolutegravir&rank=110. [Accessed 3 March 2017]. N = 612, Completion in August 2017. [Google Scholar]
- 16.Exclusion of pregnant women from HIV clinical trials. https://www.ncbi.nlm.nih.gov/pubmed/26361171. [Accessed 1 March 2017]. [Google Scholar]
- 17.Lewis JM, Railton E, Riordan A, et al. Early experience of dolutegravir pharmacokinetics in pregnancy: high maternal levels and significant foetal exposure with twice-daily dosing. AIDS 2016; 30:1313–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolutegravir prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204790lbl.pdf. [Accessed 20 March 2017]. [Google Scholar]
- 19.IMPAACT 1026s: pharmacokinetic properties of antiretroviral and related drugs during pregnancy and postpartum. https://clinicaltrials.gov/ct2/show/NCT00042289?term=dolutegravir&rank=131. [Accessed 3 March 2017]. N = 1700. End date October 2018. [Google Scholar]
- 20.DOLPHIN 1: dolutegravir in pregnant women. Available at: https://clinicaltrials.gov/ct2/show/NCT02245022?term=dolutegravir&rank=17. [Accessed 3 March 2017]. N = 60. [Google Scholar]
- 21.NIH/NIAID VESTED Study: dolutegravir versus efavirenz in pregnant women. Available at: https://clinicaltrials.gov/ct2/show/NCT03048422?term=dolutegravir&rank=109. [Accessed 3 March 2017]. N = 549. End date May 2020. [Google Scholar]
- 22.ING200336, pharmacokinetic and safety study in pregnant women with human immuno virus infection. Available at: https://clinicaltrials.gov/ct2/show/NCT02075593?term=dolutegravir&rank=129. [Accessed 3 March 2017]. N = 25. End date October 2021. [Google Scholar]
- 23.Dooley KE, Sayre P, Borland J, et al. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin or once daily with rifabutin: results of a phase 1 study among healthy subjects. J Acquir Immune Defic Syndr 2013; 62:21–27. [DOI] [PubMed] [Google Scholar]
- 24.Brooks K, Pau A, George J, et al. Early termination of a PK study between dolutegravir and weekly isoniazid/rifapentine. CROI Conference, 13–16 February 2017, Seattle, Washington [Abstr 409a]. [Google Scholar]
- 25.INSPIRING Study: dolutegravir versus efavirenz in TB co-infection. Available at: https://clinicaltrials.gov/ct2/show/NCT02178592?term=dolutegravir&rank=89. [Accessed 3 March 2017]. N = 125, Results end 2017. [Google Scholar]
- 26.Swaminathan S, Padmapriyadarsini C, Venkatesan P, et al. Efficacy and safety of once-daily nevirapine- or efavirenz-based antiretroviral therapy in HIV-associated tuberculosis: a randomized clinical trial. Clin Infect Dis 2011; 53:716–724. [DOI] [PubMed] [Google Scholar]
- 27.Rutherford GW, Horvath H. Dolutegravir plus two nucleoside reverse transcriptase inhibitors versus efavirenz plus two nucleoside reverse transcriptase inhibitors as initial antiretroviral therapy for people with HIV: a systematic review. PLoS One 2016; 11:e0162775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪.Wijting I, et al. Integrase inhibitors are an independent risk factor for IRIS; an ATHENA-Cohort study. Conference on Retroviruses and Opportunistic Infections (CROI 2017), Seattle, abstract 731, 2017. [Google Scholar]; Cohort study showing an association between integrase inhibitors and an increased risk of immune reconstitution inflammatory syndrome (IRIS).
- 29▪.Duterte M, et al. Initiation of ART based on integrase inhibitors increases the risk of IRIS. Conference on Retroviruses and Opportunistic Infections (CROI 2017), Seattle, abstract 732, 2017. [Google Scholar]; Cohort study showing an association between integrase inhibitors and an increased risk of IRIS.
- 30.Spanish Immune Recovery study: dolutegravir versus darunavir/ritonavir in patients with CD4 counts <100/μl. Available at: https://clinicaltrials.gov/ct2/show/NCT02337322?term=dolutegravir&rank=56. [Accessed 3 March 2017]. [Google Scholar]
- 31.NAMSAL Study. Dolutegravir versus efavirenz in first-line treatment. Available at: https://clinicaltrials.gov/ct2/show/NCT02777229?term=dolutegravir&rank=58. [Accessed 3 March 2017]. [Google Scholar]
- 32.ADVANCE Study: Dolutegravir versus efavirenz in first-line treatment. Available at: https://www.usaid.gov/sites/default/files/documents/1864/ADVANCE-Trial-Landscape-508.pdf [Date last accessed: 3 March 2017]. [Google Scholar]
- 33.LLibre J, Esteve A, Miro J, et al. Discontinuation of DTG, EVG/c and RAL due to toxicity in a prospective cohort. CROI conference, February 13–16, Seattle, Washington. [Abstr 651]. [Google Scholar]
- 34.de Boer M1, van den Berk G, van Holten N, et al. Intolerance of dolutegravir containing cART regimens in real life clinical practice. AIDS 2016; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann C, Welz T, Sabranski M, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med 2017; 18:56–63. [DOI] [PubMed] [Google Scholar]
- 36.Yagura H, Watanabe D, Nakauchi T, et al. Effect of dolutegravir plasma concentration on central nervous system side effects. CROI conference, February 13–16, Seattle, Washington. [Abstr 426]. [Google Scholar]
- 37.Hsu A, Henegar C, Carpio F, et al. Psychiatric disorders observed in HIV+ patients using 6 common third agents in opera. CROI conference, February 13–16, Seattle, Washington. [Abstr 664]. [Google Scholar]
- 38.Mahlab-Guri K, Asher I, Rosenberg-Bezalel S, et al. Two case reports of severe myocarditis associated with the initiation of dolutegravir treatment in HIV patients. Medicine 2016; 95:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Lu X, Yang X, Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy. Medicine 2016; 95:e5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray A, Fordyce M, Hitchcock M. Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res 2016; 125:63–70. [DOI] [PubMed] [Google Scholar]
- 41▪.Tenofovir alafenamide prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207561s000lbl.pdf [Date last accessed: 20 March 2017]. [Google Scholar]; Contraindication for use of TAF with rifampicin.
- 42.Carey D, Puls R, Amin J, et al. ENCORE-1 Study Group. Efficacy and safety of efavirenz 400 mg daily versus 600 mg daily: 96-week data from the randomised, double-blind, placebo-controlled, non-inferiority ENCORE1 study. Lancet Infect Dis 2015; 15:793–802. [DOI] [PubMed] [Google Scholar]
- 43.Dickinson L, Amin J, Else L, et al. Pharmacokinetic and pharmacodynamic comparison of once-daily efavirenz (400 mg vs 600 mg) in treatment-naïve HIV-infected patients: results of the ENCORE-1 study. Clin Pharm Therap 2015; 98:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill A, Khoo S, Back D, et al. The drug interaction between rifampicin and efiavirenz is time-dependent: systematic review of 12 pharmacokinetic studies. World AIDS Conference, Melbourne, Australia, July 2014 [Abstr MOPE040]. [Google Scholar]
- 45.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365:1482–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paton N, Kityo C, Hoppe A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med 2014; 371:234–247. [DOI] [PubMed] [Google Scholar]
- 47.Amin J, Boyd MA, Kumarasamy N, et al. Raltegravir non-inferior to nucleoside based regimens in second-line therapy with lopinavir/ritonavir over 96 weeks: a randomised open label study for the treatment of HIV-1 infection. PLoS One 2015; 10:e0118228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D2EFT Study. Dolutegravir plus darunavir/ritonavir as a second-line treatment. Available at: https://clinicaltrials.gov/ct2/show/NCT03017872?term=dolutegravir&rank=45. [Accessed 3 March 2017]. N = 610. [Google Scholar]
- 49.World Health Organisation. Hepatitis B factsheet. Available at: http://www.who.int/mediacentre/factsheets/fs204/en/. [Accessed 20 March 2017]. [Google Scholar]


