Abstract
Ghrelin and peptide YY (PYY) are peptides generally produced by the gastrointestinal organs which are involved in appetite regulation via highly specialized centers in the brain. Abnormal plasma ghrelin and PYY levels compared with controls have been reported for subjects with Prader-Willi syndrome (PWS) which is characterized by infantile hypotonia, poor suck reflex and failure to thrive followed by hyperphagia and marked obesity in early childhood. We studied gene expression of ghrelin, peptide YY, and their receptors (i.e., GHS-R1a, GHS-R1b, and NPY2R) in six different brain regions (frontal cortex, temporal cortex, visual cortex, pons, medulla, and hypothalamus) obtained from three subjects with PWS, two individuals with Angelman syndrome, and six controls to determine if expression of these genes is detectable in different regions of the brain in subjects with and without PWS. In general, expression of these genes using RT-PCR was detected in all subjects and no obvious differences were seen in their pattern of expression between subjects with or without PWS. Additional studies including quantitative gene expression measurements will be required to further evaluate the role of these genes in the eating disorder seen in PWS.
Keywords: ghrelin, peptide YY, receptors, human brain, Prader-Willi syndrome
Introduction
Ghrelin is a growth hormone-releasing peptide which acts as an endogenous ligand of the growth hormone secretagogue receptors (GHS-Rs). Two transcriptional subtypes, generated by alternative splicing of a single gene have been described for GHS-R and known as 1a and 1b. Specific functional differences of these two receptor subtypes are not known; however, it has been suggested that only the 1a subtype is biologically active (1–3). Gene expression studies using RT-PCR of rat testis suggested strong expression of mRNA for GHS-R1a from puberty onward, while in earlier stages of testicular development, GHS-R1b is predominantly expressed (4).
Ghrelin has been reported to be expressed in the human and rat adrenal cortex (5–7). In addition, Carraro et al (3) studied expression of the GHS-R receptors in human adrenal specimens using cDNA as a template from two females and six males (age range, 39–80 years old) previously diagnosed with kidney cancer. Expression of GHS-R1a was detected in the adrenal cortex from all eight subjects, while mRNA for GHS-R1b was observed only in six of the studied samples. No expression was found for GHS-R1a in the adrenal cortex obtained from two subjects (76- and 79-year-old males).
Fasting plasma ghrelin levels are reportedly high in subjects with Prader-Willi syndrome (8–10) while peptide YY levels have been understudied (10). Both peptides are involved in eating behavior (i.e., ghrelin produced by the stomach stimulates eating while peptide YY produced by the intestine inhibits eating). The genes for ghrelin, GHS-Rs, peptide YY (PYY) and NPY2R (peptide YY receptor) are localized on chromosomes 3p26, 3q26, 17q21 and 4q31, respectively.
Prader-Willi syndrome (PWS) is an imprinting disorder due to chromosome 15 abnormalities, usually a paternal deletion of the 15q11-q13 region. It is considered the most common genetic cause of marked obesity in humans. Hyperphagia is a clinical feature of PWS in early childhood and gastric rupture is a relatively common cause of death (11). However, infantile hypotonia, poor suck reflex and failure to thrive are present before the onset of hyperphagia and subsequent obesity. Angelman syndrome (AS), a sister imprinting syndrome to PWS, has few overlapping features including obesity but due to lack of maternally expressed genes from the 15q11-q13 region.
Both ghrelin and peptide YY act at the brain level, particularly in the hypothalamus, although there is a paucity of gene expression data from the brain in the general population and no studies reported in subjects with eating disorders. We examined these genes and their receptors at the expression level in different brain regions including the hypothalamus and cortex from subjects with and without PWS.
Materials and methods
Materials
Three subjects with Prader-Willi syndrome (PWS) (1-year-old female with 15q11-q13 deletion, 32-year-old female with 15q11-q13 deletion, and 32-year-old female with 15q11-q13 deletion), two individuals with Angelman syndrome (AS) (4-year-old male with 15q11-q13 deletion and 43-year-old female with 15q11-q13 deletion), and six controls (1-year-old female, 37-year-old male, 39-year-old female, 49-year-old male, 71-year-old female, and 72-year-old male). Tissue from six brain regions (frontal cortex, temporal cortex, visual cortex, pons, medulla, and hypothalamus) were obtained from the subjects at the time of autopsy, frozen immediately and stored at −80°C. The tissue was obtained following informed consent from funded brain and tissue banks for developmental disorders [(University of Maryland, Baltimore, MD; University of Miami, Miami, FL; and University of California, Los Angeles (UCLA)]. cDNA from four control subjects were purchased from Invitrogen (Carlsbad, CA). All subjects were Caucasian.
Methods
Total RNA was extracted using standard Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using 1 μg of total RNA, 2.5 μM random hexamers and 1st strand cDNA synthesis kits following manufacturer’s protocols (Roche, Indianapolis, IN). Gene specific primers were used to PCR amplify a fragment from the coding regions of ghrelin, GHS-Rs, PYY, and NPY2R. GAPDH, as a positive control, was also amplified from the studied tissues to verify the presence of RNA. SNRPN, a paternally expressed gene absent from the 15q11-q13 region in subjects with PWS was used as a negative control. PCR products were analyzed by electrophoresis on a 2% agarose gel stained with ethidium bromide and visualized under UV light. The primer sequence and their annealing temperature are shown in Table I. The primers for ghrelin and GHS-R1b were synthesized according to the sequence reported by Carraro et al (3). For GHS-R1a, the primers used were reported by Korbonits et al (12). MacVector version 2.0 computer program was utilized to design primers for the PYY, NPY2R, SNRPN, and GAPDH genes and sequence data available from Genbank. The fragments were amplified at 40 PCR cycles and gene expression; if any, was recovered.
Table I.
The primers used for the RT-PCR in our study.
| Gene | Primer’s sequence | Product size (bp) | Annealing temprature (°C) |
|---|---|---|---|
| Ghrelina | 5′-AGCCTCCTGCTCCTCGGCAT-3′ | 339 | 62 |
| 5′-TGTGGGCGATCACTTGTCGGCT-3′ | |||
| GHS-R1ab | 5′-TCGTGGGTGCCTCGCT-3′ | 65 | 59 |
| 5′-CACCACTACAGCCAGCATTTTC-3′ | |||
| GHS-R1ba | 5′-TGGAGCACGAGAACGGCA-3′ | 304 | 62 |
| 5′-AGGCACAGGGAGAGGATAGGA-3′ | |||
| PYY | 5′-GGACAGGCTTCTTTCCAAAACG-3′ | 158 | 62 |
| 5′-TTCTGGGGTCGGGAGTGCGTATGC-3′ | |||
| NPY2R | 5′-GCTGGCTAATCATCGGACAGAC-3′ | 140 | 60 |
| 5′-GCACCTATTGGACCCATTTTCAG-3′ | |||
| SNRPN | 5′-CGCTTACACCTGAGACGAACTACAG-3′ | 818 | 58 |
| 5′-TGGAGCCTGGTTTTTGCTTG-3′ | |||
| GAPDH | 5′-TGACAACTTTGGTATCGTGGAAGG-3′ | 130 | 58 |
| 5′-AGGGATGATGTTCTGGAGAGCC-3′ |
The primers for ghrelin and GHS-R1b were synthesized according to the sequence reported by Carraro et al. (3).
The primers for GHS-R1awere synthesized according to the sequence reported by Korbonits et al (12). The primers for the other genes were designed using sequence data available from Genbank.
Results
The results from RT-PCR experiments are shown in Figs. 1–3. Fig. 1 represents RT-PCR gel images for expression of ghrelin, GHS-R1a, and GHS-R1b genes from six different brain regions obtained from subjects with PWS and AS, and for controls. Expression was detected for ghrelin and its two receptors from all samples (Fig. 1). PYY was amplified from brain regions for all subjects except for pons in two subjects (39-year-old control female and 32-year-old PWS female) (Fig. 2). A detectable amplified product was observed for the peptide YY receptor (NPY2R) for all samples studied (Fig. 2). For validation purposes, a representative PCR amplified product from each gene was sequenced and the result matched the corresponding gene sequence. As anticipated, no RT-PCR signal was observed for the SNRPN gene in subjects with PWS while the correct size product was amplified from the subjects with AS or for controls (Fig. 3). Expression for the control gene (GAPDH) was detected in brain tissues for all subjects (data not shown).
Figure 1.
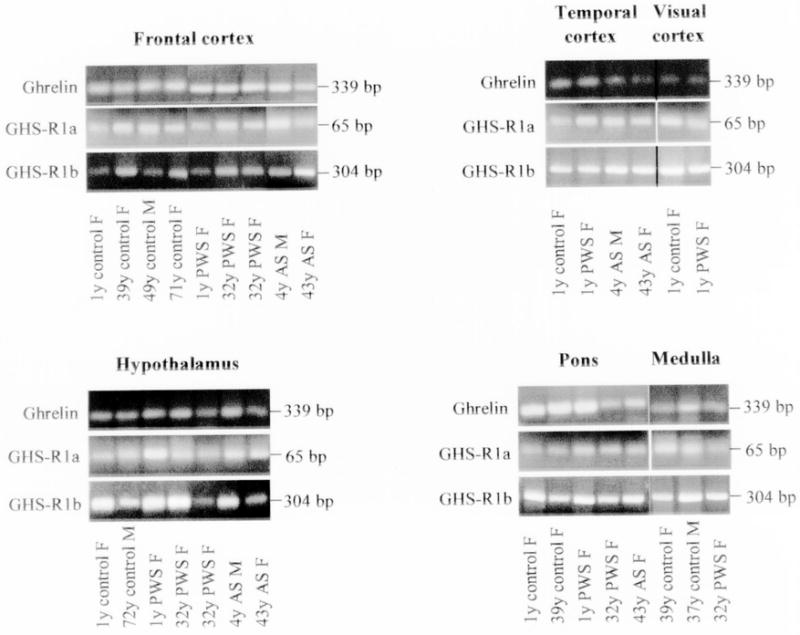
Ethidium bromide stained 2% agarose gel showing RT-PCR amplified fragments of human ghrelin, GHS-R1a and GHS-R1b genes using specific primers with RNA isolated from different brain regions of patients with Prader-Willi and Angelman syndromes and control subjects. The expected size for each amplified fragment is shown to the right of each gel image.
Figure 3.

Ethidium bromide stained 2% agarose gel showing an RT-PCR amplified fragment of human SNRPN gene using specific primers with RNA isolated from frontal cortex of patients with Prader-Willi and Angelman syndromes and control subjects. The amplified gene fragment is 818 bp. As expected, the amplified fragment of this imprinted gene was not present in the patients with Prader-Willi syndrome.
Figure 2.
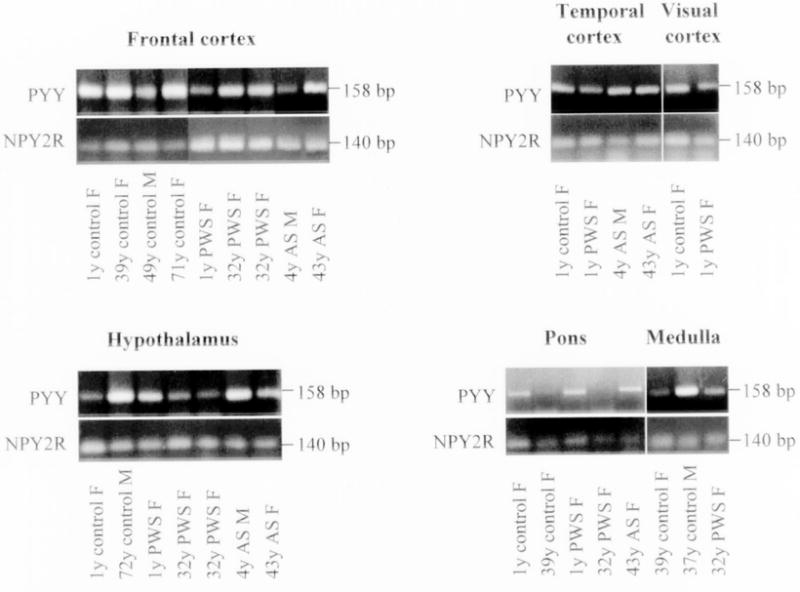
Ethidium bromide stained 2% agarose gel showing RT-PCR amplified fragments of human PYY and NPY2R genes using specific primers with RNA isolated from different brain regions of patients with Prader-Willi and Angelman syndromes and control subjects. The expected size for each amplifier fragment is shown to the right of each gel image.
Discussion
Appetite is controlled by several interconnected pathways in highly specialized centers in the brain influenced by neuropeptides produced by the gastrointestinal system. Food intake is regulated by the hypothalamus including the melanocortin and neuropeptide Y (NPY) systems in the arcuate nucleus (13). Furthermore, the involvement of ghrelin and peptide YY in stimulation or inhibiting of food intake has been very well documented. However, limited gene expression data are available for these neuropeptides and their receptors in any organ system.
Gene expression of ghrelin and the two growth hormone secretagogue receptors have been recently studied in tissues other than the brain such as human testis and testicular tumors (2), human adrenal cortex (3), and fetal thyroid and follicular tumors (14).
Due to the lack of gene expression data for ghrelin, peptide YY, and their receptors in the brain, we attempted to identify the distribution pattern of these genes in different regions of the brain (frontal cortex, temporal cortex, visual cortex, pons, medulla, and hypothalamus) for three subjects with PWS (a genetic imprinting syndrome and eating disorder), two subjects with AS (a second imprinting syndrome but without an obesity disorder) and six controls. The results from our study confirm that expression was present for ghrelin, GHS-R1a, GHS-R1b, PYY, and NPY2R in all six brain regions studied including the hypothalamus from subjects with PWS and AS, and for controls. However, PYY expression was not seen on reported assays in pons tissue from two subjects (one PWS and one control). For the rest of these genes, expression was present in all examined tissues.
The objective of our study was to investigate the presence or absence of the specific gene products involved in eating behavior in subjects with hyperphagia and obesity (i.e., PWS) and controls. The study was not carried out as a quantitative experiment for gene expression analysis; however, an equal amount of template (cDNA) was used from different sources under the same PCR condition (including cycle number). If the intensity of bands would be considered as a measurement for a relative quantitation of gene products, possible differences can be seen at the expression level between different sources. For example, a higher expression of the GHS-Ra1 mRNA can be seen in the hypothalamus from the one-year-old subject with PWS compared with the gene product from the age/sex matched control in repeated assays (Fig. 1). A preliminary quantitative RT-PCR was performed to evaluate this observation (data not shown) following the protocols reported previously (15). In agreement with the RT-PCR results, this quantitative RT-PCR assay suggested a higher expression of the GHS-R1a mRNA in the hypothalamus of the one-year-old PWS subject compared with the same age and sex control.
Changes in the balance of expression between the two alternative splicing growth hormone secretagogue receptors (i.e., GHS-R1a and GHS-R1b) have been reported for the rat testis at different developmental stages (4). In our study, mRNA from both GHS-R receptors were detected in all the studied tissues from the brain and no specific pattern was noticed in comparison between their amplified products considering age.
In summary, we report the gene expression pattern of ghrelin, peptide YY and their receptors in six brain regions in subjects with PWS using RT-PCR and comparison with AS and controls. No obvious or consistent differences in expression were detected in comparison with subjects without PWS. However, a detailed quantitative comparison of these genes involved in appetite-regulating pathways will require further experimentation to gain a better understanding of the role of these genes in PWS and its eating disorder.
Acknowledgments
Funding support for this study was partially provided by a grant from the Prader-Willi Syndrome Foundation (GL 01.3945).
References
- 1.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, van der Ploeg LH, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 2.Gaytan F, Barreiro ML, Caminos JE, Chopin LK, Herington AC, Morales C, Pinilla L, Paniagua R, Nistal M, Casanueva FF, Aguilar E, Dieguez C, Tena-Sempere M. Expression of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in normal human testis and testicular tumors. J Clin Endocrinol Metab. 2004;89:400–409. doi: 10.1210/jc.2003-031375. [DOI] [PubMed] [Google Scholar]
- 3.Carraro G, Albertin G, Abudukerimu A, Aragona F, Nussdorfer GG. Growth hormone secretagogue receptor subtypes 1a and 1b are expressed in the human adrenal cortex. Int J Mol Med. 2004;2:295–298. [PubMed] [Google Scholar]
- 4.Barreiro ML, Suominen JS, Gaytan F, Pinilla L, Chopin LK, Casanueva FF, Dieguez C, Aguilar E, Tena-Sempere M, Toppari J. Developmental, stage-specific, and hormonally regulated expression of growth hormone secretagogue receptor messenger RNA in rat testis. Biol Reprod. 2003;68:1631–1640. doi: 10.1095/biolreprod.102.008862. [DOI] [PubMed] [Google Scholar]
- 5.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 6.Andreis PG, Malendowicz LK, Trejter M, Neri G, Spinazzi R, Rossi GP, Nussdorfer GG. Ghrelin and growth hormone secretagogue receptor are expressed in the rat adrenal cortex: evidence that ghrelin stimulates the growth, but not the secretory activity of adrenal cells. FEBS Lett. 2003;536:173–179. doi: 10.1016/s0014-5793(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 7.Tortorella C, Macchi C, Spinazzi R, Malendowicz LK, Trejter M, Nussdorfer GG. Ghrelin, an endogenous ligand for the growth hormone-secretagogue receptor, is expressed in the human adrenal cortex. Int J Mol Med. 2003;12:213–217. [PubMed] [Google Scholar]
- 8.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 9.Haqq AM, Farooqi IS, O’Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL, LaFranchi SH, Purnell JQ. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader-Willi syndrome. J Clin Endocrinol Metab. 2003;88:174–178. doi: 10.1210/jc.2002-021052. [DOI] [PubMed] [Google Scholar]
- 10.Butler M, Bittel D, Talebizadeh Z. Plasma peptide YY and ghrelin levels in infants and children with Prader-Willi syndrome subjects. J Pediatr Endocrinol Metab. 2004;17:1177–1184. doi: 10.1515/jpem.2004.17.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler MG, Thompson T. Prader-Willi syndrome: clinical and genetic findings. Endocrinology. 2000;10:3S–16S. doi: 10.1097/00019616-200010041-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korbonits M, Bustin SA, Kojima M, Jordan S, Adams EF, Lowe DG, Kangawa K, Grossman AB. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J Clin Endocrinol Metab. 2001;86:881–887. doi: 10.1210/jcem.86.2.7190. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 14.Volante M, Allia E, Fulcheri E, Cassoni P, Ghigo E, Muccioli G, Papotti M. Ghrelin in fetal thyroid and follicular tumors and cell lines: expression and effects on tumor growth. Am J Pathol. 2003;162:645–654. doi: 10.1016/S0002-9440(10)63858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bittel DC, Kibiryeva N, Talebizadeh Z, Butler MG. Microarray analysis of gene/transcript expression in Prader-Willi syndrome: deletion versus UPD. J Med Genet. 2003;40:568–574. doi: 10.1136/jmg.40.8.568. [DOI] [PMC free article] [PubMed] [Google Scholar]


