Abstract
Background
Cardiovascular disease (CVD) fatality rates are higher for women as compared with men, yet obstructive coronary artery disease (CAD) is less prevalent in women. Coronary flow reserve (CFR), an integrated measure of large and small vessel CAD and myocardial ischemia, identifies patients at risk for CVD death, but is not routinely measured in clinical practice. We sought to investigate the impact of sex, CFR, and angiographic CAD severity on adverse cardiovascular events.
Methods
Consecutive patients (N=329, 43% women) referred for invasive coronary angiography after stress testing with myocardial perfusion positron emission tomography (PET) and with left ventricular ejection fraction >40% were followed (median 3.0 years) for a composite endpoint of major adverse cardiovascular events including cardiovascular death and hospitalization for nonfatal myocardial infarction (MI) or heart failure. Extent and severity of angiographic CAD were estimated using the CAD prognostic index, and CFR quantified using PET.
Results
Although women relative to men had lower pretest clinical scores, rates of prior MI and burden of angiographic CAD (p<0.001), they demonstrated greater risk of CVD events, even after adjustment for traditional risk factors, imaging findings and early revascularization (adjusted HR 2.05, 95%CI 1.05–4.02, p=0.03). Impaired CFR was similarly present among women and men, but in patients with low CFR (<1.6, n=163), women showed higher frequency of nonobstructive CAD while men showed higher frequency of severely obstructive CAD (p=0.002). After also adjusting for CFR, the effect of sex on outcomes was no longer significant. When stratified by sex and CFR, only women with severely impaired CFR demonstrated significantly increased adjusted risk of CVD events (p<0.0001, p for interaction=0.04).
Conclusions
Women referred for coronary angiography had significantly lower burden of obstructive CAD relative to men but were not protected from CVD events. Excess cardiovascular risk in women was independently associated with impaired CFR, representing a hidden biological risk, and a phenotype less amenable to revascularization. Impaired CFR, particularly absent severely obstructive CAD, may represent a novel target for CVD risk reduction.
Keywords: ischemic heart disease, women, coronary flow reserve, positron emission tomography, coronary angiography
Journal Subject Terms: Coronary Artery Disease, Ischemia, Coronary Circulation, Diagnostic Testing, Imaging, Revascularization
In the last three decades, case fatality rates for cardiovascular disease (CVD) in the United States have been higher for women as compared to men,1 yet obstructive coronary artery disease (CAD) is less prevalent in women.2–6 Luminal coronary angiography is the gold standard for diagnosis of obstructive CAD and remains a cornerstone of modern CVD care but has limited ability to identify diffuse atherosclerosis and small-vessel disease, which may contribute to adverse cardiovascular events including acute coronary syndromes, heart failure, and CVD death from plaque erosion, impaired coronary vasoreactivity, and/or microvascular dysfunction with resultant myocardial ischemia.2,7–9
Coronary flow reserve (CFR) provides a combined physiologic measure of large and small vessel CAD and myocardial ischemia, and identifies patients at risk for cardiovascular death. CFR, calculated as the ratio of hyperemic to rest myocardial blood flow, evaluates the integrated hemodynamic effects of epicardial CAD, diffuse atherosclerosis, vessel remodeling and microvascular dysfunction on myocardial perfusion. Emerging data have demonstrated that CFR measurements by noninvasive positron emission tomography (PET) can distinguish patients at highest risk for serious cardiovascular events independently of traditional measures of stress-induced ischemia or left ventricular ejection fraction (LVEF).10–14 We recently showed that impaired CFR is associated with adverse cardiovascular events independently of angiographic stenosis severity, and that baseline CFR may modify the outcomes of revascularization, especially with coronary artery bypass grafting (CABG).15
As symptomatic women are less likely than men to manifest obstructive CAD on angiography (and thus less likely to partake in any beneficial effect of revascularization), we sought to investigate the relative contributions of sex and CFR on CVD outcomes in a clinical cohort of patients referred for coronary angiography for the evaluation of CAD. We hypothesized that women relative to men would have less obstructive CAD, but not less impaired CFR as quantified by PET myocardial perfusion, and that this would be associated with a significant hidden risk of CVD events despite access to revascularization.
Methods
Study Population
Study participants were consecutive patients clinically referred for invasive coronary angiography within 90 days after stress myocardial perfusion PET at Brigham and Women’s Hospital between 2006 and 2012. Indications for testing most commonly included evaluation for chest pain, dyspnea or their combination. Patient history and medication use were ascertained at time of PET imaging. From a cohort of 841 patients, those with prior CABG, LVEF <40% or clinical diagnosis of heart failure were excluded, leaving a final cohort of 329 individuals. The median time from PET to invasive angiography was 2.6 (Q1-Q3 0.3–13.5) days. No patients had an intervening cardiovascular event or revascularization between PET and angiography. A pretest clinical score integrating age, sex, type of chest pain, prior history of myocardial infarction, presence of diabetes, hyperlipidemia, current smoking, and electrocardiographic abnormalities into a pretest probability of obstructive angiographic CAD was calculated as described previously.16 Early revascularization, considered to be triggered by imaging results, was defined as occurring within 90 days of PET.14 The study was approved by the Partners Healthcare Institutional Review Board, and conducted in accordance with institutional guidelines.
PET Imaging
Patients were imaged with a whole-body PET–computed tomography scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, WI) using 82Rubidium (1480–2200 MBq) or 13N-ammonia (700–900 MBq) as a flow tracer at rest and pharmacologic stress, as described previously.17 Computed tomography was used to correct for photon attenuation by soft tissues. For semi-quantitative assessment of myocardial scarring and ischemia, 17-segment visual interpretation of gated myocardial perfusion images was performed by experienced operators using a standard five-point scoring system.18 Summed rest and difference (stress – rest) scores were converted to percent myocardium by dividing by the maximum score of 68.19 For each of these variables, higher scores reflect larger areas of myocardial scar or ischemia, respectively. Rest LVEFs were calculated from gated myocardial perfusion images with commercially available software (Corridor4DM; Ann Arbor, Michigan).
Absolute global myocardial blood flow (MBF, in mL/min/g) was quantified at rest and at peak hyperemia using automated factor analysis and a validated two-compartment kinetic model, as described previously.17 Per-patient global CFR was calculated as the ratio of stress to rest absolute MBF for the whole left ventricle. MBF and CFR values were not clinically available to referring physicians. Radiation exposure per study was <4.6 mSV. Quantitative measures of CFR were obtained in patients undergoing PET myocardial perfusion at no additional clinical cost, imaging time or radiation exposure.
Coronary Angiography
All patients underwent selective coronary angiography using standard clinical techniques, with two or more projections obtained per vessel distribution, and angles of projection optimized for cardiac position. In each patient, the CAD prognostic index (CADPI) was quantified as described previously.15, 20 The CADPI classification is a hierarchical index (0–100) that assigns prognostic weights to increasing percent stenoses (50–100%) in one-, two-, or three-vessel classification, with higher weights for proximal left anterior descending (LAD) or left main (LM) artery involvement. Luminal diameter stenoses of the major epicardial coronary arteries were clinically graded by subjective visual consensus of experienced operators on an ordinal scale and applied to the CADPI classification in blinded fashion.
Outcomes
Subjects were followed for a median of 3.0 years (Q1-Q3 1.7–4.4) for the occurrence of major adverse cardiovascular events (MACE), including cardiovascular death and hospitalization for nonfatal myocardial infarction or heart failure. Time to first event was analyzed, Heart failure rather than repeat revascularization was selected a priori for the composite endpoint because of its emerging clinical significance and associated severe prognosis.21 Ascertainment of clinical endpoints was determined by blinded expert adjudication of the longitudinal medical record, Partners Healthcare Research Patient Data Registry, and the National Death Index. For an event to be classified as admission for nonfatal myocardial infarction or heart failure, discharge with a primary hospitalization diagnosis of myocardial infarction or heart failure, respectively, was required. The date of the last consultation was used to determine follow-up. All patients not meeting a clinical endpoint had >30 days of follow-up.
Statistical Analysis
Baseline characteristics are reported as rates with percentages (%) for categorical variables and medians with interquartile ranges (Q1-Q3) for continuous variables. We used the Fisher exact test and the Wilcoxon rank-sum test to assess differences in categorical and continuous baseline characteristics.
Cumulative event-free survival curves for the composite endpoint were compared using the log-rank test across dichotomous categories of sex, sex and CADPI clinical cutpoint of ≥37 vs. <37, and sex and CFR median (<1.6 vs. ≥1.6). The CADPI cutpoint was selected to reflect a >70% stenosis in >1 major epicardial coronary artery, a clinically actionable threshold for revascularization; this is also the cutpoint at which a survival benefit has been demonstrated previously for revascularization.20 The median CFR of 1.6 was selected as a cutpoint for simplicity in the descriptive display. This value, lower than the all-comer median cutpoint of 2,14 is consistent with the more comorbid population referred for coronary angiography.22 Where indicated (and for modeling) we report values of CADPI and CFR as continuous variables. Similar results were obtained after logarithmic transformation, and results are presented untransformed for ready clinical applicability.
Cox proportional-hazards models were used to examine the association between sex and events after controlling for effects of clinically important covariates, sequentially adding traditional clinical risk factors, noninvasive and invasive imaging factors, and finally CFR. Data were censored at the time of the last visit. Univariate associations were tested, and Cox models sequentially added sex, age, race, medical history, medications, pretest clinical score, imaging and angiography variables, with a collinearity index used to check for linear combinations among covariates, and the Akaike information criterion assessed to avoid overfitting. Final covariates were included based on clinical knowledge. To avoid overfitting, demographic and medical history variables (age, type of chest pain, prior history of myocardial infarction, presence of diabetes, hyperlipidemia, current smoking, and electrocardiographic abnormalities) were incorporated into a validated, aforementioned pretest clinical score16 in nested models, which were compared using the likelihood ratio test. The proportional hazards assumption was confirmed using martingale residuals, and time-dependent variables included as appropriate. A linear interaction term for sex and CFR was tested for significance in the final adjusted model. The final model with sex was adjusted for nonwhite race, pretest clinical score, history of PCI, hypertension and insulin use; BMI >27 kg/m2, LVEF <50%, LV ischemia >10%, CADPI, time-dependent variables of early revascularization with PCI or CABG, and CFR. Dichotomous variables were used for BMI, %LVEF and %LV ischemia to reflect clinically relevant thresholds. Adjusted event-free survival was plotted using survival probabilities from the Cox model and stratified by categories of sex, sex and CADPI, or sex and CFR. Causal mediation analysis, as recently described with survival data,23 was used to estimate the proportion of the adjusted effect of sex on outcomes that was mediated by CFR, allowing for their interaction.
In additional analysis, we stratified invasive angiographic severity by sex subgroups across medians of CFR, and used the Mantel-Haenszel Chi-Square test to assess differences across categories. We displayed data across categories of CADPI grouped for clinical significance. A p-value of <0.05 was considered to indicate statistical significance, and all tests were two-sided. No adjustment for multiple comparisons was performed. The SAS analysis system, version 9.4, was used for all analyses (SAS Institute).
Results
Baseline Characteristics
Distribution of baseline characteristics is shown in Table 1. The median (Q1-Q3) age of patients in the overall cohort was 67 (59–75) years, 42.6% were women, 24.0% were nonwhite, and median pretest clinical score was 58.2% (28.4–84.8). Nearly a third of patients had prior myocardial infarction, 31.9% had prior PCI, and 58.7% underwent revascularization by either PCI or CABG within 90 days of PET imaging. Compared to men, women (n=140) were similar in age, use of cardiovascular medications, and extent of LV ischemia and scar on semiquantitative perfusion imaging. Women, however, had lower pretest clinical scores, with lower rates of prior myocardial infarction or PCI, higher LVEF, and demonstrated significantly lower burden of obstructive angiographic disease by CADPI as compared with men (median CADPI, 32 vs. 37, respectively; p<0.001), with lower rates of early revascularization. They were also more likely than men in this angiography cohort to be nonwhite and diabetic, with higher BMI and rates of insulin use. Nearly one third of women demonstrated non-obstructive CAD (CADPI <19), as compared with 18% of men (p<0.01). Of interest, baseline CFR was similarly impaired among women and men (median CFR, 1.5 vs. 1.6, respectively; p=0.30).
Table 1.
Baseline Characteristics of Patients by Sex
| Characteristic | Overall (N=329) |
Sex | P* | |
|---|---|---|---|---|
| Women (n=140) | Men(n=189) | |||
| Demographics | ||||
| Age† y (Q1-Q3) | 67 (59–75) | 68 (59–76) | 66 (59–75) | 0.34 |
| Nonwhite race (%) | 79 (24.0) | 44 (31.4) | 35 (18.5) | 0.009 |
| Body mass index† kg/m2 | 29.9 (26.3–34.5) | 31.0 (27.2–37.6) | 28.9 (25.9–32.9) | 0.002 |
| Pretest clinical score†,‡, % | 58.2 (28.4–84.8) | 27.1 (13.1–45.7) | 78.4 (59.3–91.4) | <0.001 |
| Medical history | ||||
| Myocardial infarction (%) | 108 (32.8) | 37 (26.4) | 71 (37.6) | 0.04 |
| Percutaneous coronary intervention (%) | 105 (31.9) | 35 (25.0) | 70 (37.0) | 0.02 |
| Peripheral arterial disease (%) | 48 (14.6) | 22 (15.7) | 26 (13.8) | 0.64 |
| Diabetes mellitus (%) | 132 (40.1) | 70 (50.0) | 62 (32.8) | 0.002 |
| Hypertension (%) | 290 (88.2) | 129 (92.1) | 161 (85.2) | 0.06 |
| Dyslipidemia (%) | 241 (73.3) | 105 (75.0) | 136 (72.0) | 0.61 |
| Current smoker (%) | 29 (8.8) | 10 (7.1) | 19 (10.1) | 0.43 |
| Renal hemodialysis (%) | 11 (3.3) | 3 (2.1) | 8 (4.2) | 0.37 |
| Medications | ||||
| Antiplatelet therapy (%) | 253 (76.9) | 114 (81.4) | 139 (73.5) | 0.11 |
| Statin (%) | 231 (70.2) | 99 (70.7) | 132 (69.8) | 0.90 |
| Beta-blocker (%) | 229 (69.6) | 98 (70.0) | 131 (69.3) | 0.90 |
| Angiotensin inhibitor (%) | 149 (45.3) | 68 (48.6) | 81 (42.9) | 0.32 |
| Nitroglycerin (%) | 58(17.6) | 25 (17.9) | 33 (17.5) | 0.99 |
| Diuretic (%) | 108 (32.8) | 51 (36.4) | 57 (30.2) | 0.24 |
| Insulin (%) | 62(18.8) | 38 (27.1) | 24(12.7) | 0.002 |
| Noninvasive imaging parameters | ||||
| Left ventricular ejection fraction† % | 57 (50–65) | 62 (55–68) | 54 (49–61) | <0.001 |
| Left ventricular scar†, % | 0(0–2.9) | 0 (0–2.9) | 0 (0–4.4) | 0.82 |
| Left ventricular ischemia† % | 10.3 (5.9–16.2) | 10.3 (5.1–17.7) | 10.3 (5.9–16.2) | 0.92 |
| Stress global myocardial blood flow†, ml/g/min | 1.6(1.1–20) | 1.7(1.2–2.3) | 1.5 (1.0–1.9) | <0.001 |
| Rest myocardial blood flow†, ml/g/min | 1.0(0.8–12) | 1.1 (0.9–1.3) | 0.9 (0.7–1.1) | <0.001 |
| Coronary flow reserve† | 1.6(1.2–20) | 1.5 (1.2–1.9) | 1.6(1.2–2.0) | 0.30 |
| Impaired coronary flow reserve (<1.6) | 163 (49.5) | 76 (54.3) | 87 (46.0) | 0.15 |
| Rubidium-82 radiopharmaceutical, % | 293 (89.1) | 127 (90.7) | 166 (87.8) | 0.48 |
| Invasive angiography | ||||
| Coronary artery disease prognostic indexe†,§ | 32 (23–48) | 32 (19–37) | 37 (23–48) | <0.001 |
| Non-obstructive disease (CADPI 0–19) | 77 (23.4) | 43 (30.7) | 34 (18.0) | 0.008 |
| Any early revascularization¶ (%) | 193 (58.7) | 73 (52.1) | 120 (63.5) | 0.04 |
| Percutaneous coronary intervention (%) | 157 (47.7) | 62 (44.3) | 95 (50.3) | 0.32 |
| Coronary artery bypass surgery (%) | 39 (11.9) | 12 (8.6) | 27 (14.3) | 0.12 |
P-value is for comparison between sex groups, and is based on the Fisher’s-exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
Continuous variables are presented as medians (Q1-Q3).
Pretest clinical score is the pretest probability of >70% stenosis in >1 major coronary artery on angiography.16
Coronary artery disease prognostic index (CADPI) is a hierarchical index (0–100) assigning prognostic weights to increasing percent stenoses (50–100%) in one-, two-, or three-vessel classification, with higher weights for proximal left anterior descending or left main artery involvement. CADPI 0 (<50% stenoses), 37 (>70% stenosis in >1 major epicardial coronary artery).20
Early revascularization is defined as within 90 days of noninvasive imaging. Three patients underwent both percutaneous coronary intervention and coronary artery bypass grafting.
Sex and Clinical Events
During follow-up, 74 patients met the composite endpoint of cardiovascular death or admission for nonfatal myocardial infarction or heart failure, including 31 deaths (Table 2). Although women relative to men had lower pretest clinical scores, rates of prior MI, and burden of angiographic CAD, they demonstrated similar or greater risk of CVD events (unadjusted HR 1.95, 95%CI 1.01–2.52, p=0.047), which persisted after adjustment for traditional clinical risk factors, including pretest clinical score; nonwhite race; history of PCI, hypertension and insulin use; BMI >27 kg/m2; LVEF <50%; and LV ischemia >10% (adjusted HR 2.21, 95%CI 1.13–4.31, p=0.02) (Table 3). Accordingly, women experienced reduced event-free survival as compared to men (Figures 1A, 1B).
Table 2.
Patients Meeting Cardiovascular Endpoint*
| Outcome | No. (%) of Patients (N=329) |
|---|---|
| Total cardiovascular events | 74 (22.5) |
| Cardiovascular Death (%) | 31 (9.4) |
| Hospitalization for Nonfatal Myocardial Infarction (%) | 19 (5.8) |
| Hospitalization for Heart Failure (%) | 36 (10.9) |
Median (Q1-Q3) follow-up time was 3.0 (1.68–4.42) years. Time to first event was analyzed.
Table 3.
Multivariable-Adjusted Associations of Sex and Coronary Flow Reserve with Cardiovascular Events
| Female Sex | Coronary Flow Reserve* | Model Statistics | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Sequential Models for Total Cardiovascular Events | Hazard Ratio (95% CI) |
P | Hazard Ratio (95% CI) |
P | LR Chi-Square |
P† |
|
| ||||||
| Multivariable Model 1 | ||||||
| Traditional Clinical Factors | 2.21 (1.13–4.31) | 0.02 | 34.45 | <0.001 | ||
|
| ||||||
| Multivariable Model 2 | ||||||
| +Invasive Factors | 2.05 (1.05–4.02) | 0.03 | 47.58 | 0.02 | ||
|
| ||||||
| Multivariable Model 3 | ||||||
| +Coronary Flow Reserve | 1.81 (0.91–3.59) | 0.10 | 1.69 (1.04–2.76) | 0.03 | 52.45 | 0.03 |
|
| ||||||
| +Interaction (CFR* * Sex) | 0.04‡ | 112.07 | <0.001 | |||
| for CFR = 2.0 | 1.14 (0.49–2.72) | |||||
| for CFR = 1.6 | 1.69 (0.81–3.50) | |||||
| for CFR= 1.2 | 2.49 (1.16–5.38) | |||||
Cl indicates confidence interval.
Indicates coronary flow reserve (CFR, per -1 unit).
Indicates p value for likelihood ratio (LR) test between sequential models. First value is for comparison of multivariable Model 1 to univariate analysis of female sex.
Indicates p value for interaction. Estimated effects by level of CFR were obtained from the linear interaction of CFR and sex.
Model 1: adjusted for nonwhite race; pretest clinical score (includes age); history of percutaneous coronary intervention, hypertension, and insulin use; BMI >27 kg/nf; LVEF <50%; and LV ischemia >10%.
Model 2: adjusted for nonwhite race; pretest clinical score (includes age); history of percutaneous coronary intervention, hypertension, and insulin use; BMI >27 kg/nf; LVEF <50%; and LV ischemia >10%; coronary artery disease prognostic Index; and time-dependent revascularization with percutaneous coronary intervention or coronary artery bypass grafting within 90 days of noninvasive imaging.
Model 3: adjusted for nonwhite race; pretest clinical score (includes age); history of percutaneous coronary intervention, hypertension, and insulin use; BMI >27 kg/nF; LVEF <50%; and LV ischemia >10%; coronary artery disease prognostic Index; time-dependent revascularization with percutaneous coronary intervention or coronary artery bypass grafting within 90 days of noninvasive imaging; and CFR.
Figure 1. Freedom from Major Adverse Cardiovascular Events According to Sex (Panels A, B), Sex and Angiographic Disease (Panels C, D), or Sex and Coronary Flow Reserve (Panels E, F).
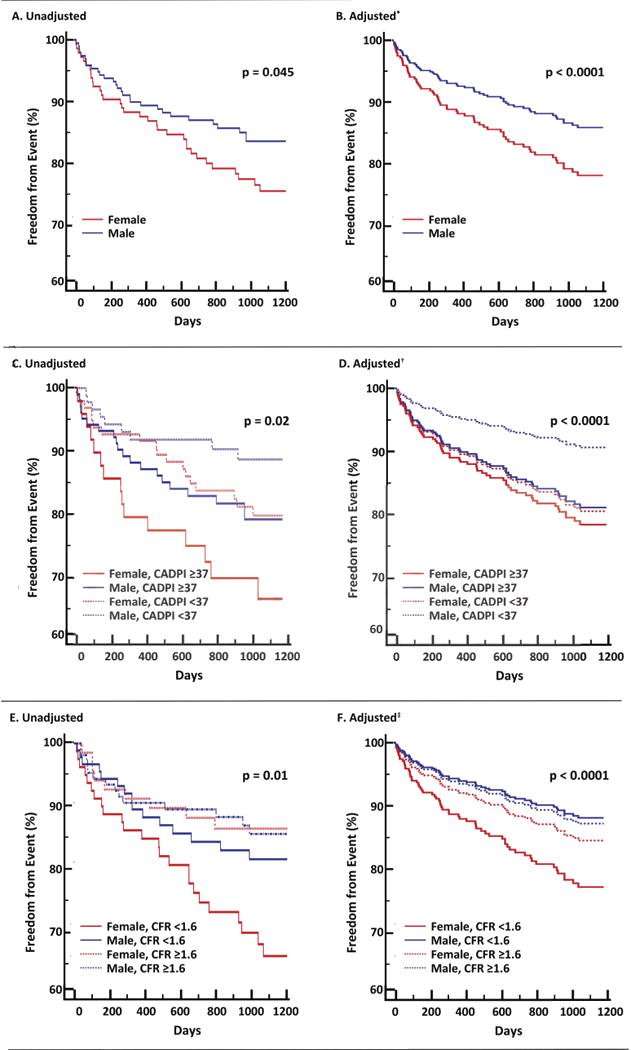
Compared to men, women experienced similar if not greater risk of cardiovascular events (Panels A, B). In men but not women, outcomes associated with presence or absence of severely obstructive CAD (Panels C, D). Not all women, but only those with impaired CFR, demonstrated the highest risk of CVD events (Panels E, F). CADPI, CAD prognostic index; CFR, coronary flow reserve.
*Adjusted for nonwhite race; pretest clinical score; history of PCI, hypertension, and insulin use; BMI >27 kg/m2; LVEF <50%; and LV ischemia >10%
†Adjusted for nonwhite race; pretest clinical score; history of PCI, hypertension, and insulin use; BMI >27 kg/m2; LVEF <50%; LV ischemia >10%; and time-dependent revascularization with PCI or CABG within 90d of noninvasive imaging
‡Adjusted for nonwhite race; pretest clinical score; history of PCI, hypertension, and insulin use; BMI >27 kg/m2; LVEF <50%; LV ischemia >10%; CAD prognostic index; time-dependent revascularization with PCI or CABG within 90d of noninvasive imaging
Sex, Angiographic Disease and Clinical Events
Addition of invasive angiographic variables into the Cox proportional-hazards model to adjust for the effect of angiographic severity and time-dependent revascularization outcomes led to improved model statistics (Table 3). However, despite manifesting a lower burden of obstructive angiographic CAD, women continued to show elevated risk of CVD events (adjusted HR 2.05, 95%CI 1.05–4.02, p=0.03) (Table 3). When survival probability was stratified by sex and CADPI, women independently of angiographic disease severity suffered higher rates of MACE, while men with less angiographic disease experienced the highest adjusted freedom from events (Figures 1C, 1D). Whereas outcomes for men were more closely associated with presence or absence of severely obstructive CAD, in women, even those with less severe CAD appeared to fare as poorly as those with severely obstructive CAD.
Sex, Angiographic Disease, CFR and Clinical Events
To investigate whether CAD severity as defined by coronary angiography may be insufficient to optimally risk-stratily symptomatic, intermediate-high risk women, we next examined the effect of CFR on cardiovascular events. Subsequent addition of CFR into the Cox proportional-hazards model to adjust for the hemodynamic burden associated with the effect of obstructive CAD as well as nonobstructive CAD and microvascular dysfunction, led to incremental improvements in model statistics (Table 3). After adjusting for CFR (adjusted HR for unit decrease in CFR, 1.69; 95%CI 1.04–2.76, p=0.03), the effect of sex on outcomes decreased and was no longer statistically significant (adjusted HR 1.81 (0.91–3.59), p=0.10). There was also a significant interaction between CFR and sex on MACE (p for interaction=0.04) such that not all women, but only those with impaired CFR demonstrated a significantly increased risk of CVD events (unadjusted p=0.01, adjusted p<0.0001) (Figures 1E, 1F). This differential effect of sex on outcomes was amplified for patients with very low CFR (Table 3, Figure 2), and not apparent in patients with CFR >2. Allowing for this interaction, CFR was estimated to mediate 40% of the observed differential effect of sex on outcomes in the adjusted model (Supplemental Table).
Figure 2. Log Adjusted Hazard for Major Adverse Cardiovascular Events in Female versus Male Sex Varies as a Function of Coronary Flow Reserve (CFR).
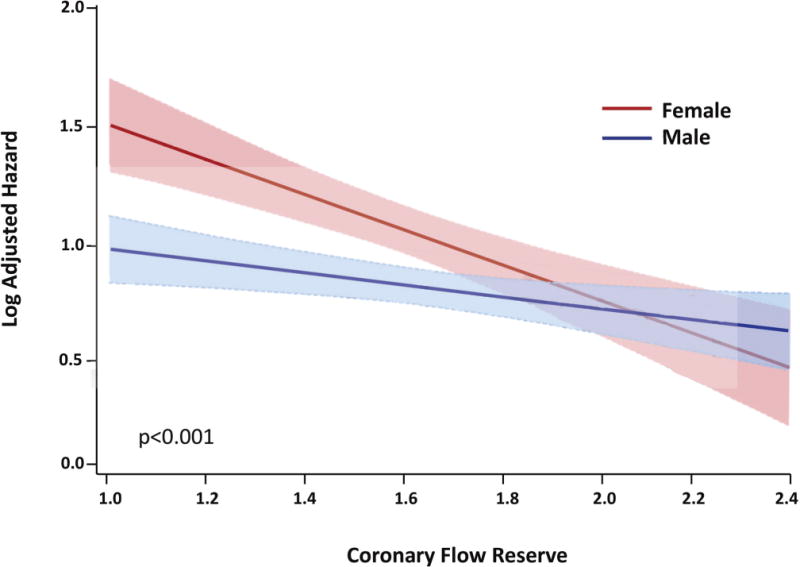
The effect of sex on cardiovascular events was modified by coronary flow reserve such that differences in outcomes between women and men were amplified for patients with very low CFR (hazard estimated from the linear interaction of CFR and sex in the final model, model p<0.001, interaction p=0.04).
To better understand the mechanism for this effect modification, we then explored the influence of patient sex on angiographic disease score across categories of CFR. CFR was similarly impaired among women and men. In patients with low CFR (<1.6, n=163), however, men had higher frequency of severely obstructive CAD (CADPI >37, 62.9% male), while women had higher frequency of non-obstructive CAD (CADPI <19, 70.4% female, p for trend=0.002) (Figure 3). Thus, fewer women with low CFR were eligible for revascularization, especially by CABG. There was a significant interaction between CFR and revascularization by CABG (p=0.01) such that patients with low CFR who underwent CABG experienced low event rates comparable to those with preserved CFR, whether or not they underwent revascularization.
Figure 3. Patients with Impaired Coronary Flow Reserve (CFR) by Coronary Artery Disease Prognostic Index (CADPI) and Sex Categories.
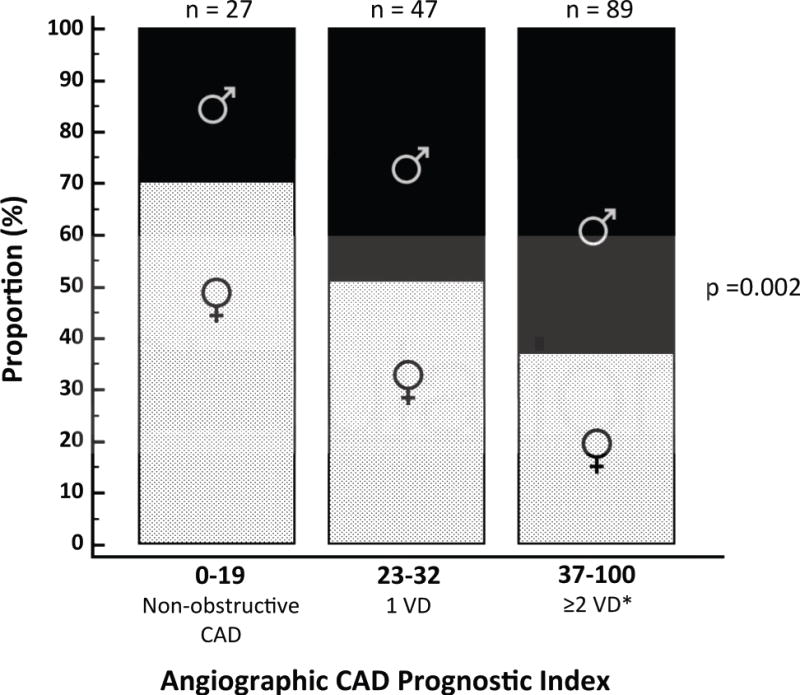
Among patients with impaired CFR (<1.6), men showed higher frequency of severely obstructive CAD (CADPI >37, 62.9% male), while women showed higher frequency of non-obstructive CAD (CADPI <19, 70.4% female, p for trend=0.002). VD, vessel disease; *CADPI 37–100 also includes 1 VD with LM or >90% proximal LAD stenosis.
Discussion
Relative to men, women referred for coronary angiography for the evaluation of CAD had lower pretest clinical risk scores, lower rates of prior MI/PCI, and lower burden of obstructive CAD by invasive angiography, but demonstrated similar if not greater adjusted risk of cardiovascular events. Here, for the first time we show that this excess risk in women was partly mediated by impaired CFR, representing a hidden biological risk. In addition to being a partial mediator, CFR also modified the effect of sex on adverse CVD events, especially for patients with very low CFR. Thus, when stratified by sex and CFR, only women with impaired CFR had significantly increased adjusted risk of CVD events.
There are several implications of these findings (Figure 4). First, although the effect of sex on CVD outcomes is multifactorial,2, 24, 25 it is mediated in a substantial manner by a hidden biological risk of ischemic heart disease. This risk may be better diagnosed through functional quantitative measures26 such as CFR, than with traditional anatomic visualization using coronary angiography. As CFR is a measure of not only obstructive CAD, but also diffuse nonobstructive CAD and microvascular dysfunction, these findings suggest that women in this angiography cohort, also disproportionately diabetic and taking insulin, exhibited a phenotype of heart disease that may still lead to adverse events6, 27, 28 despite being less amenable to focal revascularization. Instead of being interpreted as demonstrating a “false positive” (or negative) traditional ischemic evaluation, patients with impaired CFR and less obstructive CAD may be at significantly increased CVD risk despite having access to revascularization, which is fundamentally targeted at management of obstructive CAD. Thus, while providing optimal, equitable guideline-directed medical care remains a critically necessary goal for managing women with ischemic heart disease,25 doing so according to current paradigms may be insufficient to address what are likely a combination of biological as well as environmental determinants of their prognosis.
Figure 4. Conceptual Model of Prevalent Pathologic Phenotypes in Women and Men with Ischemic Heart Disease and Impact on Cardiovascular Management Strategies and Outcomes.
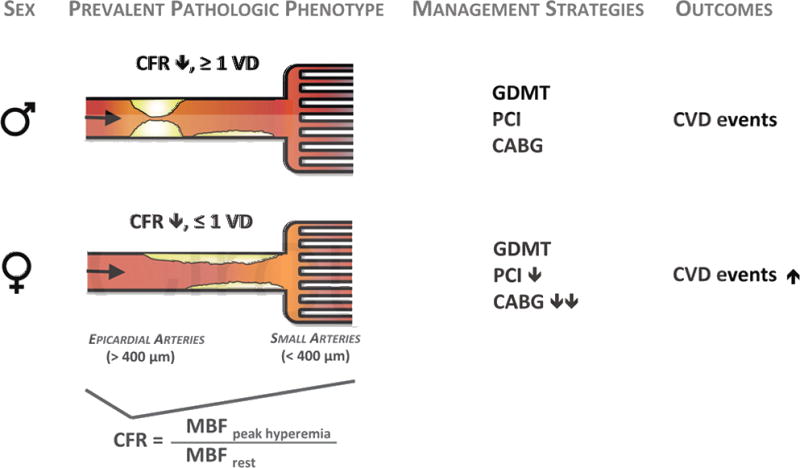
CABG, coronary artery bypass surgery; CFR, coronary flow reserve; CVD, cardiovascular disease; GDMT, guideline-directed medical therapy; MBF, myocardial blood flow; PCI, percutaneous coronary intervention; VD, vessel disease.
Second, that sex differences on outcomes of CVD vary by CFR and are amplified in those with very low CFR underscores that revascularization in certain individuals with impaired CFR may be beneficial. Our group previously showed that in a lower risk population of symptomatic patients without flow-limiting CAD (and thus, no indication for revascularization), there was no detectable difference between sexes on adverse cardiovascular events, although CFR was still associated with outcomes.29 In this case, both women and men with microvascular dysfunction (i.e. CFR <2 in the presence of normal myocardial perfusion imaging) experienced worse outcomes, although this phenotype was twice as prevalent in women as in men. That in such a lower risk group (with median CFR ≈2 and most values >1.6), no interaction was seen between sex and CFR is consistent with and now explained by our present findings (Figure 2). In that cohort, there would have been no differential impact of revascularization on outcomes, as neither women nor men were eligible for revascularization. In contrast in the current study, women fared significantly worse than men as CFR decreased. We hypothesize that this may be partly related to revascularization, and the early hazard for women demonstrated in Figure 1 would be consistent with such an effect. In patients referred for coronary angiography with impaired CFR, women demonstrated less severe angiographic disease than men, and thus, were less likely to be referred for more complete revascularization. We previously showed that baseline impaired CFR modified the effect of coronary revascularization on outcomes, especially if revascularization included CABG.15 Thus, patients with low CFR and severely obstructive CAD (a phenotype more prevalent in men) who were eligible for and underwent revascularization with CABG may have benefited preferentially.15 That the sex interaction becomes apparent only below a certain CFR threshold may reflect differences in the revascularization options for women versus men with low CFR, as a function of obstructive CAD. This may have far-reaching implications for closing the “gender gap” in cardiovascular morbidity and mortality.
Third, in cases where impaired CFR stems not from obstructive CAD (with no opportunity for revascularization to mitigate CVD risk), a novel therapeutic strategy to systemically target ischemic heart disease may be warranted. That this cohort of symptomatic intermediate-high risk women demonstrated high rates of obesity, diabetes and hypertension despite less obstructive CAD phenotypes and similar rates of cardiovascular medication use reflects larger epidemiologic trends1, 30 which may be contributing to increased prognostic risk among women, especially for subsequent heart failure events, particularly heart failure with preserved ejection fraction.21, 31–33 Impaired CFR, whether associated with diffuse nonobstructive or obstructive CAD, may provide a clue as to a common mechanism underlying ischemic cardiovascular risk in women and men. Such a mechanism may involve inflammation,34 endothelial dysfunction,35 and increased cardiomyocyte oxygen demand with ensuing microvascular ischemia, myocardial injury, and impaired cardiac mechanics.36, 37 CFR, as measured by PET, leads to meaningful risk reclassification of intermediate-risk patients, including those with diabetes12 or minimally elevated troponin36 and no flow-limiting CAD. Thus, clearer understanding of the relationship between coronary vasomotor dysfunction and CAD comorbid conditions, including insulin resistance and heart failure, may guide development of novel systemic therapies to harness the benefit of more “complete revascularization”15, 38–41 in a manner not defined by anatomy alone. The data presented here suggest that current therapies, possibly in a sex-specific manner, are insufficient to restore coronary vascular function. As such, CFR may represent an important biomarker not only for prospective studies evaluating the role of ischemia and revascularization, but also of emerging anti-inflammatory (i.e. IL-1 inhibitor,42 methotrexate43), extreme lipid-lowering (i.e. PCSK9 inhibitor44, 45) and neurohormonal-modulating (i.e. neprilysin/RAS46 and SGLT247 inhibitor) agents on cardiovascular outcomes, especially in women.
Study Limitations
Limitations of this study include its single-center observational design, in which subjects were patients clinically referred for PET myocardial perfusion imaging and subsequently referred for invasive coronary angiography. CFR results were not available to referring clinicians and thus did not affect downstream management decisions regarding catheterization or additional therapies. Our modest sample size necessitates evaluation of outcomes with a composite cardiovascular endpoint and limits extensive subgroup analysis. As with all observational cohorts, residual confounding may persist despite adjustment for baseline differences and also affect results of mediation analysis.
Recognizing these important limitations, this hypothesis-generating work may help to explain the observed gap between CVD events and CAD diagnosis in women relative to men by quantifying hidden risk of ischemic heart disease in this patient population. As such, these findings may have implications for diagnosis, risk stratification, and development of new management strategies of a clinical problem with a disproportionate impact on women’s cardiovascular health.
Conclusions
Women referred for coronary angiography had significantly lower burden of obstructive CAD relative to men, but were not protected from CVD events. Excess cardiovascular risk in women was independently associated with severely impaired CFR, representing a hidden biological risk and a phenotype less amenable to revascularization. Impaired CFR, particularly absent severely obstructive CAD, may represent a novel target for CVD risk reduction. Prospective studies are needed to evaluate the ability of CFR to reclassify risk and probe the effects of novel systemic therapies in patients across the anatomic continuum of ischemic heart disease.
Supplementary Material
Clinical Perspective.
What Is New?
Relative to men, women referred for invasive evaluation of CAD demonstrated less obstructive CAD by angiography, but greater adjusted risk of cardiovascular disease (CVD) events.
Impaired coronary flow reserve (CFR) mediated a substantial proportion (40%) of this excess risk in women, representing a hidden biological risk of ischemic heart disease (IHD).
The differential effect of sex on outcomes was amplified for patients with very low CFR (<1.6), and not apparent in patients with preserved CFR (>2). Thus, only women with severely impaired CFR demonstrated increased risk relative to men.
What Are the Clinical Implications?
The effect of sex on outcomes is mediated in part by a hidden biological risk of IHD, which may be better diagnosed using CFR.
In patients with very low CFR (<1.6) more men than women had multivessel obstructive CAD and underwent surgical revascularization, possibly mitigating their risk. Closing the “gender gap” in IHD will likely require more than equitable application of current guidelines.
CFR as a biomarker of IHD risk should be evaluated not only in prospective studies investigating the role of ischemia and revascularization, but also of emerging antiinflammatory, lipid-lowering and neurohormonal-modulating agents on CVD outcomes, especially in women.
Acknowledgments
Sources of Funding: This work was supported in part by career development awards from the Harvard Catalyst Clinical and Translational Science Center and the Harvard Medical School Office for Diversity Inclusion and Community Partnership to Dr. Taqueti.
Footnotes
Conflict of Interest Disclosures: Dr. Dorbala receives research grant support from Astellas Global Pharma Development. Dr. Murthy owns equity in General Electric. The other authors declare that they have no relationships to disclose.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C, Stroke Statistics S Heart disease and stroke statistics–2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G, Investigators W Insights from the nhlbi-sponsored women’s ischemia syndrome evaluation (wise) study: Part i: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47:S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 3.Smilowitz NR, Sampson BA, Abrecht CR, Siegfried JS, Hochman JS, Reynolds HR. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: An autopsy study. Am Heart J. 2011;161:681–688. doi: 10.1016/j.ahj.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: Evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bairey Merz CN. Sex, death, and the diagnosis gap. Circulation. 2014;130:740–742. doi: 10.1161/CIRCULATIONAHA.114.011800. [DOI] [PubMed] [Google Scholar]
- 6.Pepine CJ, Ferdinand EC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN, Committee ACiW Emergence of nonobstructive coronary artery disease: A woman’s problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66:1918–1933. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merz CN, Olson MB, McClure C, Yang YC, Symons J, Sopko G, Kelsey SF, Handberg E, Johnson BD, Cooper-DeHoff RM, Sharaf B, Rogers WJ, Pepine CJ. A randomized controlled trial of low-dose hormone therapy on myocardial ischemia in postmenopausal women with no obstructive coronary artery disease: Results from the national institutes of health/national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation (wise) Am Heart J. 2010;159:987 e981–987. doi: 10.1016/j.ahj.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA, National Heart L, Blood I Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: Results from the national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation (wise) Circulation. 2004;109:722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 9.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–2116. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima K, Javadi MS, Higuchi T, Lautamaki R, Merrill J, Nekolla SG, Bengel FM. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82rb pet perfusion imaging. J Nucl Med. 2011;52:726–732. doi: 10.2967/jnumed.110.081828. [DOI] [PubMed] [Google Scholar]
- 11.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. Long-term prognostic value of 13n-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 12.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–1868. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RS. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58:740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 15.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. doi: 10.1161/CIRCULATIONAHA.114.011939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pryor DB, Shaw L, McCants CB, Lee KL, Mark DB, Harrell FE, Jr, Muhlbaier LH, Califf RM. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Annals of internal medicine. 1993;118:81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. [DOI] [PubMed] [Google Scholar]
- 17.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)rb pet: Comparison with (13)n-ammonia pet. J Nucl Med. 2009;50:1062–1071. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, American Heart Association Writing Group on Myocardial S, Registration for Cardiac I Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 19.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 20.Mark DB, Nelson CL, Califf RM, Harrell FE, Jr, Lee KL, Jones RH, Fortin DF, Stack RS, Glower DD, Smith LR. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–2025. doi: 10.1161/01.cir.89.5.2015. [DOI] [PubMed] [Google Scholar]
- 21.Braunwald E. The war against heart failure: The lancet lecture. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 22.Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, Camici PG, Cerqueira MD, Chow BJ, Di Carli MF, Dorbala S, Gewirtz H, Gropler RJ, Kaufmann PA, Knaapen P, Knuuti J, Merhige ME, Rentrop KP, Ruddy TD, Schelbert HR, Schindler TH, Schwaiger M, Sdringola S, Vitarello J, Williams KA, Sr, Gordon D, Dilsizian V, Narula J. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639–1653. doi: 10.1016/j.jacc.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 23.Valeri L, VanderWeele TJ. Sas macro for causal mediation analysis with survival data. Epidemiology. 2015;26:e23–24. doi: 10.1097/EDE.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 24.EugenMed, Cardiovascular Clinical Study G. Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E, Franconi F, Gerdts E, Foryst-Ludwig A, Maas AH, Kautzky-Willer A, Knappe-Wegner D, Kintscher U, Ladwig KH, Schenck-Gustafsson K, Stangl V. Gender in cardiovascular diseases: Impact on clinical manifestations, management, and outcomes. Eur Heart J. 2016;37:24–34. doi: 10.1093/eurheartj/ehv598. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Fonarow GC, Mukamal KJ, Liang L, Schulte PJ, Smith EE, DeVore A, Hernandez AF, Peterson ED, Bhatt DL. Sex and race/ethnicity-related disparities in care and outcomes after hospitalization for coronary artery disease among older adults. Circ Cardiovasc Qual Outcomes. 2016;9:S36–44. doi: 10.1161/CIRCOUTCOMES.115.002621. [DOI] [PubMed] [Google Scholar]
- 26.Lee BK, Lim HS, Fearon WF, Yong AS, Yamada R, Tanaka S, Lee DP, Yeung AC, Tremmel JA. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131:1054–1060. doi: 10.1161/CIRCULATIONAHA.114.012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow BJ, Small G, Yam Y, Chen L, McPherson R, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Cury R, Delago A, Dunning A, Feuchtner G, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann PA, Kim YJ, Leipsic J, LaBounty T, Lin F, Maffei E, Raff GL, Shaw LJ, Villines TC, Min JK, Investigators C Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease: Results from the confirm (coronary ct angiography evaluation for clinical outcomes: An international multicenter registry) registry. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:981–989. doi: 10.1161/ATVBAHA.114.304351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bittencourt MS, Hulten E, Ghoshhajra B, O’Leary D, Christman MP, Montana P, Truong QA, Steigner M, Murthy VL, Rybicki FJ, Nasir K, Gowdak LH, Hainer J, Brady TJ, Di Carli MF, Hoffmann U, Abbara S, Blankstein R. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–291. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 29.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regensteiner JG, Golden S, Huebschmann AG, Barrett-Connor E, Chang AY, Chyun D, Fox CS, Kim C, Mehta N, Reckelhoff JF, Reusch JE, Rexrode KM, Sumner AE, Welty FK, Wenger NK, Anton B, American Heart Association Diabetes Committee of the Council on L, Cardiometabolic Health CoE, Prevention CoFG, Translational B, Council on H Sex differences in the cardiovascular consequences of diabetes mellitus: A scientific statement from the american heart association. Circulation. 2015;132:2424–2447. doi: 10.1161/CIR.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 31.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: A report from the women’s ischemia syndrome evaluation study and the st james women take heart project. Archives of internal medicine. 2009;169:843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Current opinion in cardiology. 2011;26:562–568. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 33.Pedrotty DM, Jessup M. “Frailty, thy name is woman”: Syndrome of women with heart failure with preserved ejection fraction. Circ Cardiovasc Qual Outcomes. 2015;8:S48–51. doi: 10.1161/CIRCOUTCOMES.115.001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC. Inflammation and microvascular dysfunction in cardiac syndrome x patients without conventional risk factors for coronary artery disease. JACC Cardiovasc Imaging. 2013;6:660–667. doi: 10.1016/j.jcmg.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Di Carli MF, Bianco-Batlles D, Landa ME, Kazmers A, Groehn H, Muzik O, Grunberger G. Effects of autonomic neuropathy on coronary blood flow in patients with diabetes mellitus. Circulation. 1999;100:813–819. doi: 10.1161/01.cir.100.8.813. [DOI] [PubMed] [Google Scholar]
- 36.Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation. 2015;131:528–535. doi: 10.1161/CIRCULATIONAHA.114.009716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 38.Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The bypass angioplasty revascularization investigation (bari) investigators. N Engl J Med. 1996;335:217–225. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 39.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, Ring S, 3rd, Bertrand M, Fuster V, Investigators FT Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 40.Frye RL, August P, Brooks MM, Hardison RM, Relsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serruys PW, Morice MC, Rappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley R, Dawkins RD, Mohr FW, Investigators S Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: Rationale and design of the canakinumab anti-inflammatory thrombosis outcomes study (cantos) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the cardiovascular inflammation reduction trial: A test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207 e115. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ, Investigators OLT Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 45.Sabatine MS, Morrow DA, de Lemos JA, Omland T, Sloan S, Jarolim P, Solomon SD, Pfeffer MA, Braunwald E. Evaluation of multiple biomarkers of cardiovascular stress for risk prediction and guiding medical therapy in patients with stable coronary disease. Circulation. 2012;125:233–240. doi: 10.1161/CIRCULATIONAHA.111.063842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P-H, Committees Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 47.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


