Abstract
OBJECTIVE
Assess postoperative morbidity and patient reported outcomes following unilateral and bilateral breast reconstruction in patients with unilateral breast cancer.
BACKGROUND
Relatively little is known about the morbidity associated with and changes in quality of life experienced by patients who undergo contralateral prophylactic mastectomy (CPM) and breast reconstruction. This information would be valuable for decision making in patients with unilateral breast cancer.
METHODS
Women undergoing mastectomy and breast reconstruction for unilateral breast cancer were recruited for this prospective observational study. Postoperative complications following implant and autologous breast reconstruction in patients undergoing unilateral or bilateral mastectomy were recorded. Preoperative and one year patient reported outcomes were measured. Univariate tests and logistic regression analyses were performed, studying the effects of reconstructive method, laterality, and risk factors on surgical complication rates, patient satisfaction and anxiety.
RESULTS
We identified 1144 women who underwent either unilateral (47.2%) or bilateral (52.8%) mastectomies with reconstruction. Bilateral autologous (OR 1.73, 95% CI 1.07–2.81) and implant reconstructions (OR 1.73, 95% CI 1.22–2.47) were associated with a higher risk of complications compared to unilateral reconstructions. Baseline anxiety was greater in women who chose bilateral compared to unilateral implant reconstructions (p=0.001). There was no difference in anxiety levels between groups postoperatively. Postoperatively, women who chose CPM with implant reconstructions were more satisfied with their breasts than women with unilateral reconstructions (p=0.034).
CONCLUSIONS
Though higher postoperative complications were observed following CPM and reconstruction, these procedures were associated with decreased anxiety levels and improved satisfaction with breasts for women who underwent implant reconstructions.
Keywords: Contralateral Prophylactic Mastectomy, Breast Reconstruction, Complications, Patient Reported Outcomes, Satisfaction, Anxiety, MROC, BREAST-Q
INTRODUCTION
The benefit of a contralateral prophylactic mastectomy (CPM), in patients without a genetic predisposition or oncologic risk factors remains unclear. Yet trends demonstrate that the number of women with stage I-III breast cancer opting for CPM in the Surveillance, Epidemiology, and End Results (SEER) registry more than doubled (4.2% to 11%) over a six-year period.1 Moreover, the proportion of breast conserving procedures performed for treatment of early stage breast cancer has declined, with a compensatory rise in the number of CPMs.2 The role of breast reconstruction in the choice to undergo CPM is also unclear, but it appears to influence patient decision-making. For example, women who choose a bilateral mastectomy undergo reconstruction at rates nearly double those in patients with unilateral mastectomy.2
The choice to undergo a CPM involves a number of tradeoffs to be weighed by the patient. On one hand is the possibility of greater morbidity following bilateral mastectomy with reconstruction. On the other is the potential for improvements in health-related quality-of-life (HR-QOL) aspects such as breast cancer related anxiety and body image (including satisfaction with breast appearance and symmetry).3 Effectively balancing these tradeoffs is a significant challenge for patients who are often actively wrestling with a recent diagnosis of breast cancer. Prospectively obtained objective data on the expected morbidity and HR-QOL that results after reconstruction in patients who opt for CPM instead of a unilateral mastectomy would be essential to help facilitate well informed patient decisions.
The aim of this study is to prospectively measure surgical complications and patient reported outcomes (PROs) in a multicenter cohort undergoing either unilateral or bilateral breast reconstruction for unilateral breast cancer. The first hypothesis is that women who choose CPM have greater surgical complication rates than women with unilateral mastectomies. The second hypothesis is that bilateral reconstructions result in greater HR-QOL relative to similar unilateral reconstructions.
METHODS
Study Population
Patients were recruited as part of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study, a five-year prospective, multicenter cohort study of mastectomy reconstruction patients funded by the National Cancer Institute. Women 18 years or older undergoing first-time, immediate post-mastectomy breast reconstruction for unilateral carcinoma in situ or cancer treatment were eligible for participation. Women who underwent bilateral mastectomy had the contralateral breast removed prophylactically (CPM) with no genetic, pathologic or radiographic abnormalities present at the time of surgery. Both unilateral and bilateral implant or abdominally based autologous reconstructions were included. Choices of reconstructive options were based on patient and surgeon preferences. Patients were excluded from the study if they answered less than 50% of the preoperative baseline panel of questionnaires; these questionnaires were a combination of PRO instruments and questions on demographic information. Patients were also excluded if they had metastatic breast cancer (Stage IV disease), had a prior mastectomy or underwent bilateral reconstruction with two different methods (Figure 1, Supplemental Digital Content 1, study flow diagram). Over 60 plastic surgeons from 11 centers in the USA (Michigan, New York, Illinois, Ohio, Massachusetts, Washington, D.C., Georgia and Texas) and Canada (British Columbia and Manitoba) contributed patients to the study, which began in February 2012. Appropriate Institutional Review Board (IRB) approval was obtained from all participating sites.
Figure 1.
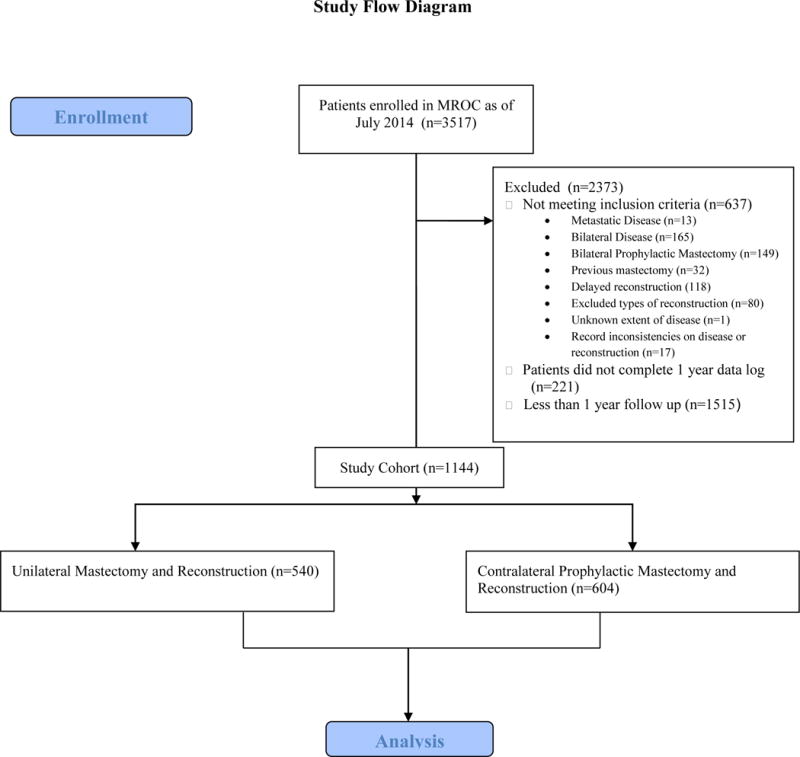
Study flow diagram. (Supplemental Digital Content 1)
Data Collection
Patients enrolled in the MROC Study completed a series of questionnaires at five specific time-points while enrolled in the study: preoperatively; and one week; three months; one year; and two years post reconstruction. Site staff also performed medical chart reviews and collected clinical data preoperatively, and at one-week, one-year, and two-years post reconstruction. Patients were recruited in person preoperatively and completed the questionnaires either electronically or on paper. These questionnaires consisted of a series of validated PRO instruments that solicited information on health-related quality of life outcomes, treatment satisfaction and socio-demographics. The medical record for each patient was also accessed to obtain demographic and clinical data related to treatment, reconstruction method, and postoperative complications retrospectively. For this study we analyzed PRO data collected preoperatively and at 1 year as well as complications at one year postoperatively.
Questionnaires
The BREAST-Q, is a validated, procedure specific PRO instrument for women undergoing different types of breast surgery.4 This questionnaire is composed of independent scales, in a variety of domains that assess both satisfaction and health-related quality of life outcomes. Response options are four-point scales ranging from one (very dissatisfied) to four (very satisfied). Item responses for each scale are summed and transformed utilizing the Q-Score program5 into a range from 0 to 100 for each scale. Higher scores are indicative of greater patient satisfaction.
The Generalized Anxiety Disorder 7 (GAD 7) Scale is a self-reported questionnaire for screening and measuring the severity of generalized anxiety disorder.6 The questionnaire consists of seven items that are summed to form a total score. A higher score correlates to more anxiety over the past two weeks. The Patient Reported Outcome Measurement Information System (PROMIS-29) is a self-administered survey for patient reported symptoms. The PROMIS-29 is a short profile form of a longer instrument developed for use in a wide range of conditions.7 The anxiety instrument measures patient reported anxiety with the questionnaire consisting of four items. A higher score correlates to more anxiety over the past seven days.
Dependent Variables
The dependent variables of the analysis were complications and PRO scores (BREAST-Q, GAD 7, and PROMIS Anxiety). Major complications were defined as complications that required an operative intervention or readmission while minor complications could be managed conservatively on an outpatient basis. Major complications included hematoma, infection requiring intravenous antibiotics, total flap loss, partial flap loss, implant or tissue expander removal, implant malposition, implant leakage or rupture, and capsular contracture (Baker grade III and IV). Minor complications included wound dehiscence, infection requiring oral antibiotics only, mastectomy skin flap necrosis, fat necrosis, scarring, and seroma.
Independent Variables
Demographic variables included age, race, ethnicity (Hispanic/Non-Hispanic), body mass index (BMI), marital status, highest level of education, and household income. The racial group “other” included Asians, Pacific Islanders, Hawaiians, and American Indians. Highest level of education obtained was defined as college degree or not. Household income was categorized into low income (<$50,000 per year), mid income ($50,000 to $99,999 per year), and high income (>$100,000 per year). Clinical variables included comorbidities (Charlson Score), extent of disease, histology, chemotherapy, and radiation therapy. The extent of disease was defined as the following: local disease - disease confined to the breast; or regional disease- axillary or internal mammary lymph nodes positive for cancer. Chemotherapy and radiation therapy were defined as receipt before or after reconstruction.
Statistical Analysis
Sociodemographic and clinical characteristics were compared between those who underwent unilateral versus bilateral reconstruction using chi-square or Fisher’s exact test. All analyses presenting differences in complications between unilateral and bilateral groups were separated by reconstruction type – autologous or implant. Rates of specific types of minor and major complications are given as the observed proportion for those with unilateral versus bilateral reconstructions. To compare the proportion of women who had any complication to those with none by laterality, chi-square tests were performed. A stepwise model building technique with logistic regression was used to build a multivariable model for the odds of any complication. Sociodemographic and clinical characteristics were potential independent variables in the model with the criteria to entry of 0.30 and the criteria to stay of 0.35.
PRO analysis preoperatively and at one year was measured by the BREAST-Q (five scales: satisfaction of breasts, psychological well-being, physical well-being chest, physical well-being abdomen, sexual well-being), PROMIS 29 (anxiety section), and GAD 7. Changes in PRO scores from baseline to 1 year were analyzed for individual patients. Some of the PRO measures were missing for a proportion of individuals at baseline and at the one year follow-up. To account for this missingness, multiple imputations with chained equations were used to create 10 complete imputed data sets. PROs at one year were approximately normally distributed. Separate linear regression models were run for women who had implants and autologous reconstruction, respectively. Initially, changes in PRO scores were modelled univariably to find the difference in those who had bilateral versus unilateral reconstruction. Then, multivariable models were run controlling for age, BMI, race (white, black, or other), and prior receipt of chemotherapy. Analyses were performed for each imputed data set and the results were combined using Rubin’s rules.8 Analyses were performed in R using packages MI and Zelig.9,10
RESULTS
Between February 2012 and July 2014, 3517 patients were recruited from 11 centers in Canada and the USA. Of these, 1144 patients (32.53 %) met the inclusion criteria and had complete data available on post-reconstruction complications and PROs at one year. Details of the study cohort selection are presented in a flow diagram (Figure 1). Among eligible patients, 540 underwent unilateral reconstruction: 343 with implants and 197 with autologous tissue. In comparison 604 patients had CPM with bilateral reconstruction: 482 with implants and 122 with autologous tissue. Table 1 outlines the demographic and oncologic characteristics of the study cohort. Patients who underwent CPM were more likely to be white, younger, college graduates, and earn a higher income compared with those who chose unilateral mastectomy (P< .05).
Table 1.
Patient Socio-Demographic and Clinical Characteristics (N=1144)
| Variable | Unilateral n= 540 |
Bilateral n= 604 |
p-value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age | <0.0001 | ||||
| ≤30 | 7 | 1.3 | 22 | 3.6 | |
| 30–39 | 36 | 6.7 | 122 | 20.2 | |
| 40–49 | 177 | 32.8 | 261 | 43.2 | |
| 50–59 | 184 | 34.1 | 147 | 24.3 | |
| ≥60 | 136 | 25.2 | 52 | 8.6 | |
| BMI | 0.08 | ||||
| ≤30 | 400 | 74.1 | 473 | 78.3 | |
| >30 | 140 | 25.9 | 130 | 21.5 | |
| Missing | 0 | 0 | 1 | 0.2 | |
| Race | 0.043 | ||||
| White | 460 | 85.2 | 539 | 89.2 | |
| Black | 43 | 8.0 | 28 | 4.6 | |
| Other | 32 | 5.9 | 29 | 4.8 | |
| Missing | 5 | 0.9 | 8 | 1.3 | |
| Ethnicity | 0.88 | ||||
| Hispanic | 31 | 5.7 | 36 | 6.0 | |
| Non-Hispanic | 498 | 92.2 | 556 | 92.1 | |
| Missing | 11 | 2.0 | 12 | 2.0 | |
| Education Level | 0.009 | ||||
| College degree | 381 | 70.6 | 467 | 77.3 | |
| No college degree | 157 | 29.1 | 135 | 22.4 | |
| Missing | 2 | 0.4 | 2 | 0.3 | |
| Employed | 0.004 | ||||
| Yes | 356 | 65.9 | 446 | 73.8 | |
| No | 177 | 32.8 | 152 | 25.2 | |
| Missing | 7 | 1.3 | 6 | 1.0 | |
| Income | 0.016 | ||||
| <50,000 | 101 | 18.7 | 94 | 15.6 | |
| 50,000–99,999 | 190 | 35.2 | 181 | 30.0 | |
| ≥100,000 | 234 | 43.3 | 313 | 51.8 | |
| Missing | 15 | 2.8 | 16 | 2.6 | |
| Marital Status | 0.054 | ||||
| Single | 164 | 30.4 | 153 | 25.3 | |
| Married | 373 | 69.1 | 449 | 74.3 | |
| Missing | 3 | 0.6 | 2 | 0.3 | |
| Extent of Disease | 0.97 | ||||
| Local | 392 | 72.6 | 439 | 72.7 | |
| Regional | 148 | 27.4 | 165 | 27.3 | |
| Histology* | |||||
| DCIS | 408 | 75.6 | 403 | 66.7 | 0.001 |
| LCIS | 74 | 13.7 | 85 | 14.1 | 0.86 |
| Invasive | 383 | 70.9 | 430 | 71.2 | 0.92 |
| Comorbidities | 0.42 | ||||
| 0 | 14 | 1.82.6 | 11 | 1.8 | |
| 1 | 468 | 86.7 | 538 | 89.1 | |
| 2–3 | 58 | 10.7 | 55 | 9.1 | |
| Chemotherapy | |||||
| Before | 57 | 10.5 | 99 | 16.4 | 0.005 |
| After | 202 | 37.4 | 218 | 36.1 | 0.65 |
| Radiation | |||||
| Before | 37 | 6.8 | 45 | 7.4 | 0.78 |
| After | 117 | 21.7 | 128 | 21.2 | 0.85 |
Using Chi-Square test of independence or Fisher’s exact test without unknown group
Some patients had more than one histopathologic abnormality at the time of the initial diagnosis.
Complications after reconstruction were categorized as minor or major [Tables 2 (Supplemental Digital Content 2), 3 and 4]. Compared with unilateral implant procedures, bilateral implant reconstructions were associated with higher complication rates (25.7% vs 18.4%, p=0.013, Figure 2) and a higher major complication rate (17.2% vs 11.3%, p=0.027). Bilateral autologous procedures were also associated with higher complication rates relative to unilateral autologous reconstructions (55.7% vs 42.6%, p=0.023, Figure 2). A trend toward a higher rate of major complications after bilateral autologous reconstruction was also observed (39.3% vs 30.5%, p=0.10). Controlling for demographic and clinical covariates, bilateral autologous (OR 1.81, 95% CI 1.10–3.00, p=0.020) and bilateral implant reconstructions (OR 1.76, 95% CI 1.21–2.54, p=0.003) were independently associated with a greater odds of complications compared to similar unilateral reconstructions (Table 5).
Table 2.
Rates of Minor Complications for Unilateral and Bilateral Autologous and Implant Breast Reconstruction. (Supplemental Digital Content 2)
| Autologous n=319 | Implant n=825 | |||
|---|---|---|---|---|
| Unilateral | Bilateral | Unilateral | Bilateral | |
| Minor Complication | n=197 (%) | n=122 (%) | n=343 (%) | n=482 (%) |
| Any Minor Complication* | 53 (26.9) | 36 (29.5) | 32 (9.3) | 62 (12.9) |
| Wound dehiscence | 10 (5.1) | 7 (5.7) | 1 (0.3) | 11 (2.3) |
| Wound infection requiring oral antibiotics | 11 (5.6) | 7 (5.7) | 13 (3.8) | 28 (5.8) |
| Mastectomy skin flap necrosis | 22 (11.2) | 6 (4.9) | 15 (4.4) | 29 (6.0) |
| Hypertrophic or keloid scarring | 0 (0) | 2 (1.6) | 1 (0.3) | 1 (0.2) |
| Wound dehiscence at donor site | 19 (9.6) | 9 (7.4) | – | – |
| Wound infection requiring oral antibiotics at donor site | 9 (4.6) | 9 (7.4) | – | – |
| Hypertrophic or keloid scarring at donor site | 2 (1.0) | 1 (0.8) | – | – |
| Abdominal wall bulge, laxity or hernia requiring surgical repair | 0 | 1 (0.8) | – | – |
| Donor site necrosis | 0 | 1 (0.8) | – | – |
| Other Minor Complications | 4 (2.0) | 5 (4.1) | 3 (0.9) | 3 (0.6) |
Some patients had more than one complication- for this evaluation complications in individual patients were only counted once.
Figure 2.
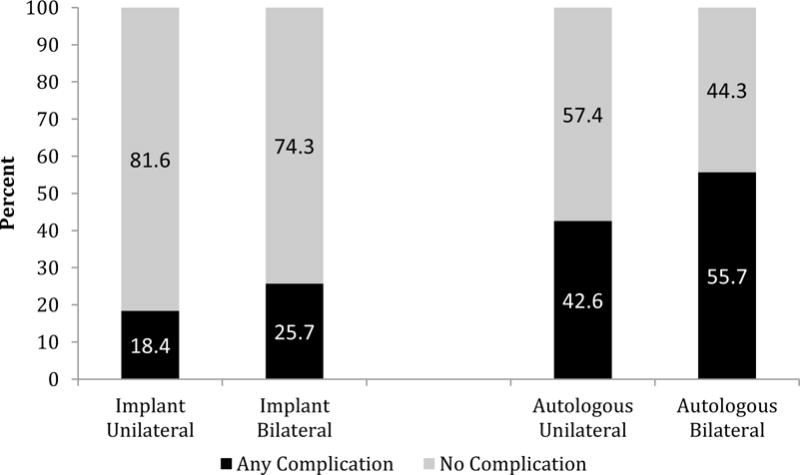
Rates of any postoperative complication (*) following implant and autologous breast reconstruction by laterality
*Some patients had more than one complication- for this evaluation complications in individual patients were only counted once.
Table 5.
Multivariable model of any complication for implant and autologous reconstruction
| Implant (n=823) | ||
|---|---|---|
| Variable | Odds Ratio (95% CI) | p-value |
| Laterality | 0.003 | |
| Unilateral | -Reference- | |
| Bilateral | 1.76 (1.21–2.54) | |
| Age | 1.02 (0.99–1.06) | 0.21 |
| BMI | 1.05 (1.02–1.08) | 0.002 |
| Disease Extent | 0.07 | |
| Local | -Reference- | |
| Regional | 0.67 (0.44–1.03) | |
| Radiation | 0.0001 | |
| None | -Reference- | |
| Ever | 2.25 (1.49–3.39) | |
| Autologous (n=309) | ||
| Variable | Odds Ratio (95% CI) | p-value |
| Laterality | 0.020 | |
| Unilateral | -Reference- | |
| Bilateral | 1.81 (1.10–3.00) | |
| BMI | 1.06 (1.01–1.11) | 0.015 |
| Age | 1.02 (0.99–1.05) | 0.26 |
| Income | 0.025 | |
| <50,000 | -Reference- | |
| 50,000–99,999 | 1.96 (1.07–3.59) | |
| ≥100,000 | 1.00 (0.54–1.86) | |
| Charlson Comorbidity Index | 0.28 | |
| 0 | -Reference- | |
| 1 | 2.13 (0.60–7.51) | |
| 2–3 | 3.11 (0.75–12.95) | |
| Disease Extent | 0.015 | |
| Local | -Reference- | |
| Regional | 2.02 (1.15–3.57) |
PROs for the cohort are recorded in Table 6. Baseline BREAST-Q scores for patients undergoing unilateral or bilateral mastectomy with reconstruction were similar. However, baseline anxiety was greater in women who chose bilateral compared to unilateral implant reconstructions as measured by both the GAD (5.95 vs 4.77, p=0.001) and PROMIS (60.19 vs 58.48, p=0.005). Baseline anxiety was not significantly different in patients who chose unilateral or bilateral mastectomy with autologous reconstruction. At one-year postoperatively, multivariable analysis demonstrated that women who chose CPM with implant reconstructions were more satisfied with their breasts than those who chose similar unilateral reconstructions (p=0.0009). There was no difference in satisfaction with breasts at one year between unilateral and bilateral autologous reconstructions. Anxiety scores at one year (GAD and PROMIS) did not differ significantly between unilateral and bilateral mastectomies with reconstruction.
Table 6.
Comparison of Patient Reported Outcome Scores at Baseline and One Year Post Reconstruction (N=1144)
| Outcome | Subset | N Missing | Mean Score at Baseline | Mean Change (Score at 1 year-Score at BL) | Bilateral vs. Unilateral | Multivariable p-value* | ||
|---|---|---|---|---|---|---|---|---|
| Bilateral | Unilateral | Bilateral | Unilateral | |||||
| Breast-Q Satisfaction with Breast | Implant | 274 | 63.69 | 65.83 | −4.48 | −10.14 | 5.67 | 0.0009 |
| Autologous | 76 | 57.37 | 59.68 | 8.53 | 6.97 | 1.56 | 0.35 | |
| Breast-Q Physical Well-Being Abdomen | Implant | 91.55 | 90.28 | |||||
| Autologous | 83 | 86.74 | 87.62 | −6.76 | −5.57 | −1.19 | 0.99 | |
| Breast-Q Psycho-Social Well being | Implant | 278 | 71.73 | 72.45 | −8.68 | −7.38 | −1.30 | 0.70 |
| Autologous | 75 | 66.46 | 70.05 | −0.89 | 2.83 | −3.72 | 0.37 | |
| Breast-Q Physical Well Being Chest/Upper Body | Implant | 270 | 79.41 | 80.02 | −13.19 | −10.91 | −2.28 | 0.10 |
| Autologous | 75 | 74.07 | 77.11 | −6.31 | −5.07 | −1.23 | 0.76 | |
| Breast-Q Sexual Well Being | Implant | 315 | 59.70 | 58.63 | −9.05 | −6.46 | −2.59 | 0.92 |
| Autologous | 91 | 51.54 | 55.46 | 0.87 | 1.31 | −0.45 | 0.49 | |
| GAD7 | Implant | 280 | 5.95 | 4.77 | −0.50 | 0.01 | −0.51 | 0.85 |
| Autologous | 77 | 5.52 | 5.31 | 0.55 | −0.40 | 0.95 | 0.17 | |
| PROMIS- Anxiety Score | Implant | 295 | 60.19 | 58.48 | −5.86 | −6.52 | 0.66 | 0.45 |
| Autologous | 82 | 58.66 | 58.15 | −4.88 | −6.35 | 1.47 | 0.21 | |
P-value from multivariable model controlling for age, BMI, race (white, black or other) and prior chemotherapy
Negative Effect indicates the score decreased over time
DISCUSSION
The oncologic benefit of CPM in women with early stage unilateral breast cancer is uncertain with multiple studies reporting no survival advantage.11–14 The five year risk of developing a second primary tumor in the contralateral breast is relatively low, estimated at 0.5–1% per year in the average breast cancer survivor.15 This risk is greater in select groups such as patients with genetic predispositions to developing breast cancer, strong personal or family histories of cancer, and prior chest wall radiation; however, the observed rise in the rate of CPM use has been driven primarily by patients who are not at high risk for contralateral breast cancer.16 Interestingly, the sociodemographic profile of the young, white, educated patients who choose CPM is consistent across studies. Associations between these factors and the decision to undergo CPM are unclear, but highlight the influence of sociocultural aspects on surgical decision-making.
Though the HR-QOL benefits of post-mastectomy breast reconstruction compared to mastectomy alone are well documented,17 reconstruction is not without potential for significant morbidity. The complications associated with breast reconstruction take on an even greater significance in the context of an elective procedure such as prophylactic mastectomy. The potential for increased morbidity with bilateral, as opposed to unilateral breast reconstruction, has not been evaluated prospectively in this patient population. One recent single institution retrospective study showed that patients undergoing CPM were 2.7 times more likely to have a major complication requiring readmission or additional intervention.18 Overall complication rates of 28.6% were observed in patients undergoing unilateral mastectomy and 41.6% in those who had CPM. Of note 33% of the included patients were not reconstructed and these patients had significantly lower complication rates, potentially lowering the reported complication rates; complications were also not presented by type of reconstruction. An evaluation of 30 day complications using the NSQIP database also revealed greater odds for overall complications in patients undergoing bilateral mastectomy with implant or autologous breast reconstruction.19 Patients undergoing bilateral mastectomy and reconstruction with either technique had significantly longer hospital stays and were more likely to require transfusions. With limited follow up time and a focus on complications that occur in the in-patient setting, the overall complication rates reported for unilateral versus bilateral implants (8.8% vs 10.1%) and autologous reconstruction (14.7% vs 21.2%) were relatively lower than has been published by others.18, 20 Our implant complication rates (18.4%, unilateral and 25.7%, bilateral) and autologous complication rates (42.6%, unilateral and 55.7%, bilateral) are on the higher end of what is reported in the literature. This is likely explained by the prospective nature of this study with rigorous documentation of all complications encountered over the course of a year. Many such complications tend to be overlooked or entirely missed in retrospective studies. In the current cohort, after adjusting for confounders, rates of complications were significantly greater in women undergoing CPM with either autologous or implant based reconstruction (Table 3 and 4). Although greater complication rates and side-effects can be anticipated following any bilateral compared to unilateral procedure, women may continue to choose CPM for a variety of reasons. One consideration is that although complications may be significantly higher from a statistical standpoint, the rate may be acceptable to patients and/or physicians. An alternate interpretation is that women remain willing to accept complications for other perceived benefits of CPM.
Table 3.
Rates of Major Complications for Unilateral and Bilateral Autologous Breast Reconstructions
| Major Complication | Autologous | |
|---|---|---|
| Unilateral | Bilateral | |
| N=197 (%) | N=122 (%) | |
| Any Major Complication | 60 (30.5) | 48 (39.3) |
| Post-operative bleeding or hematoma requiring needle aspiration or re-operation | 12 (6.1) | 10 (8.2) |
| Wound infection requiring IV antibiotics | 7 (3.6) | 2 (1.6) |
| Wound infection requiring surgical drainage | 2 (1.0) | 3 (2.5) |
| Acute partial flap necrosis within 30 days | 9 (4.6) | 5 (4.1) |
| Flap loss | 0 (0) | 3 (2.5) |
| Chronic flap necrosis | 18 (9.1) | 14 (11.5) |
| Seroma | 4 (2.0) | 1 (0.8) |
| Post-operative bleeding or hematoma requiring needle aspiration or re-operation at donor site | 4 (2.0) | 2 (1.6) |
| Wound infection at donor site requiring IV antibiotics | 0 | 3 (2.5) |
| Wound infection at the donor site requiring surgical or percutaneous drainage of abscess | 0 | 5 (4.1) |
| Donor site necrosis | 5 (2.5) | 10 (8.2) |
| Chronic fat necrosis of the donor site | 3 (1.5) | 2 (1.6) |
| Donor site seroma | 9 (4.6) | 8 (6.6) |
| Abdominal wall bulge, laxity or hernia requiring surgery | 3 (1.5) | 7 (5.7) |
| Deep vein thrombosis | 1 (0.5) | 0 |
| Pulmonary embolus | 0 | 2 (1.6) |
| Other Major Complications | 8 (4.1) | 9 (7.4) |
Table 4.
Rates of Major Complications for Unilateral and Bilateral Implant-Based Breast Reconstructions
| Major Complication | Implant | |
|---|---|---|
| Unilateral | Bilateral | |
| N=343 (%) | N=482 (%) | |
| Any Major Complication | 40 (11.7) | 83 (17.2) |
| Post-operative bleeding or hematoma requiring needle aspiration or re-operation | 9 (2.6) | 19 (3.9) |
| Wound infection requiring IV antibiotics | 11 (3.2) | 25 (5.2) |
| Wound infection requiring surgical drainage | 3 (0.9) | 6 (1.2) |
| Capsular contracture (Baker Class III or IV) | 1 (0.3) | 8 (1.7) |
| Implant malposition | 1 (0.3) | 3 (0.6) |
| Seroma | 12 (3.5) | 18 (3.5) |
| Implant leakage | 3 (0.9) | 2 (0.4) |
| Abdominal wall bulge, laxity or hernia requiring surgery | 0 | 1 (0.2) |
| Tissue expander removal | 20 (5.8) | 47 (9.8) |
| Implant removal | 7 (2.0) | 24 (5.0) |
| Deep vein thrombosis | 3 (0.9) | 0 |
| Pulmonary embolus | 0 | 2 (0.4) |
| Other Major Complications** | 2 (0.6) | 2 (0.4) |
Other major complications include: negative exploration & washout for suspected hematoma, (n=1); nipple flap necrosis (n=2); pneumothorax (n=1).
A number of studies have evaluated factors influencing the decision to proceed with CPM. Broadly these can be broken down into clinical or decisional characteristics.21 Clinical characteristics include, but are not limited to features such as family history of breast cancer, ipsilateral recurrence of breast cancer, history of prior breast biopsy, preoperative Magnetic Resonance Imaging (MRI) testing and availability of reconstruction. Decisional characteristics comprise aspects such as anxiety, fear, or worry of cancer recurrence, desire to avoid future surveillance, desire for reconstructive symmetry, and desire to improve on breast aesthetics. Multiple investigators have cited anxiety as an important factor in the decision making process for women to choose CPM,21, 22, 23 but few have directly measured preoperative anxiety levels using validated PRO instruments.24, 25 A prospective questionnaire based study on the surgical decision-making process for CPM showed that patients with less knowledge about breast cancer and greater worry were more interested in CPM.25 Greater cancer worry ultimately was a significant predictor for patients who went on to have CPM. An evaluation of 45 patients undergoing CPM, using the Hospital Anxiety and Depression Scale, demonstrated no difference in baseline or two-year postoperative anxiety and depression scores when compared to women from the general population.24 By contrast, in evaluating only patients with unilateral breast cancer, the current study showed significantly greater preoperative anxiety in women who chose a bilateral compared to a unilateral implant reconstruction. With an evaluation of changes in anxiety levels in individual patients from base line to one year postoperatively, no significant difference was found. Appreciating the greater baseline anxiety levels of women undergoing CPM with implant reconstruction, it is fair to consider that these patients have a distinct set of concerns that differentiates them from those choosing autologous tissue.26 For example, patients who undergo prosthetic reconstruction tend to be younger, lack the abdominal adiposity necessary for autologous reconstruction (Table 8, Supplemental Digital Content 4) and have aesthetic concerns about the donor site scars associated with autologous reconstruction. These younger women are also potentially at a stage in life when careers, relationships and family life are just beginning so a diagnosis of breast cancer tends to have a profound effect. The differences notwithstanding, this data provides objective evidence of the potential benefit of CPM on relieving anxiety in a select group of patients.
Table 8.
Patient Socio-Demographic and Clinical characteristics by Procedure Type- Implant vs. Autologous. (N=1144) (Supplemental Digital Content 4)
| Variable | Implant n=825 |
Autologous n=319 |
p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age | <.0001 | ||||
| <30 | 29 | 3.5 | 0 | 0.0 | |
| 30–39 | 129 | 15.6 | 29 | 9.1 | |
| 40–49 | 320 | 38.8 | 118 | 37.0 | |
| 50–59 | 216 | 26.2 | 115 | 36.1 | |
| >=60 | 131 | 15.9 | 57 | 17.9 | |
| BMI | <.0001 | ||||
| <=30 | 677 | 82.1 | 196 | 61.4 | |
| >30 | 147 | 17.8 | 123 | 38.6 | |
| Missing | 1 | 0.1 | 0 | 0.0 | |
| Race | 0.047 | ||||
| White | 731 | 88.6 | 268 | 84.0 | |
| Black | 49 | 5.9 | 22 | 6.9 | |
| Other | 36 | 4.4 | 25 | 7.8 | |
| Missing | 9 | 1.1 | 4 | 1.3 | |
| Ethnicity | 0.032 | ||||
| Hispanic | 56 | 6.8 | 11 | 3.5 | |
| Non-Hispanic | 753 | 91.3 | 301 | 94.4 | |
| Missing | 16 | 1.9 | 7 | 2.2 | |
| Education Level | <.0001 | ||||
| College degree | 643 | 77.9 | 205 | 64.3 | |
| No college degree | 179 | 21.7 | 113 | 35.4 | |
| Missing | 3 | 0.4 | 1 | 0.3 | |
| Employed | 0.567 | ||||
| Yes | 574 | 69.6 | 228 | 71.5 | |
| No | 241 | 29.2 | 88 | 27.6 | |
| Missing | 10 | 1.2 | 3 | 0.9 | |
| Income | <.0001 | ||||
| <50,000 | 116 | 14.1 | 79 | 24.8 | |
| 50,000–99,999 | 251 | 30.4 | 120 | 37.6 | |
| >=100,000 | 436 | 52.9 | 111 | 34.8 | |
| Missing | 22 | 2.7 | 9 | 2.8 | |
| Marital status | 0.744 | ||||
| Single | 226 | 27.4 | 91 | 28.5 | |
| Married | 594 | 72.0 | 228 | 71.5 | |
| Missing | 5 | 0.6 | 0 | 0.0 | |
| Extent of Disease | 0.050 | ||||
| Local | 586 | 71.0 | 245 | 76.8 | |
| Regional | 239 | 29.0 | 74 | 23.2 | |
| Histology* | |||||
| DCIS | 583 | 70.7 | 228 | 71.5 | 0.788 |
| LCIS | 132 | 16.0 | 27 | 8.5 | 0.001 |
| Invasive | 605 | 73.3 | 208 | 65.2 | 0.007 |
| Comorbidities | 0.009 | ||||
| 0 | 12 | 1.5 | 13 | 4.1 | |
| 1 | 740 | 89.7 | 266 | 83.4 | |
| 2–3 | 73 | 8.8 | 40 | 12.5 | |
| Chemotherapy | |||||
| Before | 113 | 13.7 | 43 | 13.5 | 0.924 |
| After | 307 | 37.2 | 113 | 35.4 | 0.574 |
| Radiation | |||||
| Before | 36 | 4.4 | 46 | 14.4 | <.0001 |
| After | 182 | 22.1 | 63 | 19.8 | 0.393 |
Some patients had more than one histopathologic abnormality at the time of the initial diagnosis.
Beyond the effect of CPM on anxiety and vice versa, it is important to understand how CPM impacts other aspects of quality of life. Hwang and colleagues approached this question by administering the BREAST-Q to 2,760 patients who had undergone unilateral or bilateral mastectomy with reconstruction. Without evaluating specific reconstruction types, they found that patients undergoing CPM reported higher breast satisfaction scores with lower physical and psychosocial well-being scores.27 In our assessment of reconstruction types, women who chose a unilateral compared to bilateral prosthetic based breast reconstruction were significantly less satisfied with their breasts as measured by the BREAST-Q; this difference did not exist for autologous reconstructions. Such a difference likely reflects the asymmetry noted by women when comparing their native breast to an implant and may underlie the choice made by many for CPM as an alternative to a contralateral symmetry procedure (e.g. mastopexy or reduction).3,26,28 Previous studies demonstrate strong independent associations between the choice for CPM and implant, not autologous, breast reconstruction.30,31 It is likely that the simplicity of prosthetic breast reconstruction, combined with its absent donor site morbidity and ease of removal in cases of complications has served to facilitate recent U.S. trends towards CPM.2
Not surprisingly no difference in satisfaction with breasts was measurable for women who chose unilateral versus bilateral autologous transfer. This makes sense intuitively since in unilateral autologous transfer, the tissue used for reconstruction is very similar in appearance and consistency to breast tissue. It is also interesting to note that women who underwent autologous transfers experienced increased satisfaction with their breasts compared to baseline. This is in contradistinction to women who had either unilateral or bilateral implant reconstructions where postoperative scores were lower than baseline. Taken together, perhaps these findings should be used to advocate for greater numbers of unilateral autologous reconstructions when feasible, since this solution minimizes surgical complications, maximizes chest well-being, and has satisfaction with breasts comparable with bilateral reconstructions. Moreover, health related quality of life data demonstrates greater long-term satisfaction with autologous transfer compared with prosthetic reconstructions.32 An important negative finding was the absence of a difference in sexual well-being between women who chose unilateral mastectomy and those who chose CPM with either method of breast reconstruction.
Strengths of the current study include its prospective design and patient accrual from multiple institutions with a host of reconstructive surgeons which lends to the generalizability of our findings. This is the first study using preoperative BREAST-Q scores as a covariate to adjust for measured differences in postoperative levels of satisfaction and HR-QOL. Because the BREAST-Q is the only condition specific PRO instrument for breast reconstruction, it may more effectively highlight subtle differences between groups than generic instruments used in previous analyses. A fundamental limitation of this study has to do with the fact that data was not collected on first degree relatives with genetic mutations predisposing them to breast cancer, tumor staging and hormone receptor status, variables known to increase the risk for breast cancer recurrence. In comparing patients who chose CPM versus unilateral mastectomy there may be unmeasured confounders which could not be adjusted for between groups in the final multivariate analyses. This study is also limited by the fact that most of the institutions included are major academic medical centers, making it difficult to comment on subtle variations that might be encountered in community based practices and hospitals. Furthermore, a selection bias is possible with a potential for retention of patients with greater satisfaction and fewer complications. Based on our comparison of included patients and those with missing PRO data (Table 7, Supplemental Digital Content 3, demographic and clinical characteristics of patients with complete or missing PRO data), we have no evidence of missing data in a preferential fashion that would support a selection bias.
Table 7.
Demographic and Clinical Characteristics in patients with complete PRO data (n=711) or any missing PRO data (n=433). (Supplemental Digital Content 3)
| Variable | PRO Complete | PRO Missing | ||
|---|---|---|---|---|
| N | % | N | % | |
| Age | ||||
| ≤30 | 20 | 2.8 | 9 | 2.1 |
| 30–39 | 92 | 12.9 | 66 | 15.2 |
| 40–49 | 277 | 39.0 | 161 | 37.2 |
| 50–59 | 212 | 29.8 | 119 | 27.5 |
| ≥60 | 110 | 15.5 | 78 | 18.0 |
| BMI | ||||
| ≤30 | 566 | 79.6 | 307 | 70.9 |
| >30 | 145 | 20.4 | 125 | 28.9 |
| Missing | 0 | 0 | 1 | 0.2 |
| Race | ||||
| White | 643 | 90.4 | 356 | 82.2 |
| Black | 26 | 3.7 | 45 | 10.4 |
| Other | 33 | 4.6 | 28 | 6.5 |
| Missing | 9 | 1.3 | 4 | 0.9 |
| Ethnicity | ||||
| Hispanic | 34 | 4.8 | 33 | 7.6 |
| Non-Hispanic | 661 | 93.0 | 393 | 90.8 |
| Missing | 16 | 2.3 | 7 | 1.6 |
| Education Level | ||||
| College degree | 550 | 77.4 | 298 | 68.8 |
| No college degree | 160 | 22.5 | 132 | 30.5 |
| Missing | 1 | 0.1 | 3 | 0.7 |
| Employed | ||||
| Yes | 514 | 72.3 | 288 | 66.5 |
| No | 191 | 26.9 | 138 | 31.9 |
| Missing | 6 | 0.8 | 7 | 1.6 |
| Income | ||||
| <50,000 | 108 | 15.2 | 87 | 20.1 |
| 50,000–99,999 | 235 | 33.1 | 136 | 31.4 |
| ≥100,000 | 354 | 49.8 | 193 | 44.6 |
| Missing | 14 | 2.0 | 17 | 3.9 |
| Marital Status | ||||
| Single | 177 | 24.9 | 140 | 32.3 |
| Married | 532 | 74.8 | 290 | 67.0 |
| Missing | 2 | 0.3 | 3 | 0.7 |
| Extent of Disease | ||||
| Local | 527 | 74.1 | 304 | 70.2 |
| Regional | 184 | 25.9 | 129 | 29.8 |
| Histology (both sides) | ||||
| DCIS | 509 | 71.6 | 302 | 69.8 |
| IDC | 485 | 68.2 | 328 | 75.8 |
| LCIS | 104 | 14.6 | 55 | 12.7 |
| Comorbidities | ||||
| 0 | 3 | 0.4 | 2 | 0.5 |
| 1 | 5 | 0.7 | 4 | 0.9 |
| 2 or more | 703 | 98.9 | 427 | 98.6 |
| Chemotherapy | ||||
| Before | 88 | 12.4 | 68 | 15.7 |
| After | 257 | 36.2 | 163 | 37.6 |
| Radiation | ||||
| Before | 40 | 5.6 | 42 | 9.7 |
| After | 139 | 19.6 | 106 | 24.5 |
| Complications | ||||
| Minor | 104 | 14.6 | 79 | 18.2 |
| Major | 138 | 19.4 | 93 | 21.5 |
CONCLUSIONS
This prospective study demonstrates subjective benefits to CPM including gains in HR-QOL and psychosocial aspects of disease management. Such findings can be used to substantiate CPM especially in the era of patient centeredness advocated by the Institute of Medicine.33 Despite its higher complications rates, the choice for CPM should remain with patients to decide upon in shared decision-making process with their doctors. Lastly, physicians need to be aware of the current findings in order to present a balanced picture not only to patients considering CPM, but to those considering unilateral mastectomy as well.
Acknowledgments
Support for this study was provided by a grant from the National Cancer Institute (1R01CA152192) to A.L.P. and E.G.W.
Footnotes
To be presented at Plastic Surgery The Meeting, October 16 through 20, in Boston, Massachusetts.
Disclosure Statement: Dr. Pusic is a co-developer of the BREAST-Q, which is owned by Memorial Sloan-Kettering Cancer Center. She receives a portion of licensing fees (royalty payments) when the BREAST-Q is used in industry sponsored clinical trials.
References
- 1.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:5203–9. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 2.Albornoz CR, Matros E, Lee CN, et al. Bilateral Mastectomy versus Breast-Conserving Surgery for Early-Stage Breast Cancer: The Role of Breast Reconstruction. Plastic and reconstructive surgery. 2015;135:1518–26. doi: 10.1097/PRS.0000000000001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koslow S, Pharmer LA, Scott AM, et al. Long-term patient-reported satisfaction after contralateral prophylactic mastectomy and implant reconstruction. Annals of surgical oncology. 2013;20:3422–9. doi: 10.1245/s10434-013-3026-2. [DOI] [PubMed] [Google Scholar]
- 4.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plastic and reconstructive surgery. 2009;124:345–53. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 5.QScore Scoring Software. 2015 Accessed 8/9/15. at https://webcore.mskcc.org/breastq/scoring.html.
- 6.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of internal medicine. 2006;166:1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 7.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Medical care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RD B. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons Inc; 1987. [Google Scholar]
- 9.Imai KK, Gary A, Lau Olivia. Zelig: Everyone’s Statistical Software. 2007 [Google Scholar]
- 10.Imai K, King Gary, Lau Olivia. Toward A Common Frame- work for Statistical Analysis and Development. Journal of Computational and Graphical Statistics. 2008;17:892–913. [Google Scholar]
- 11.Chung A, Huynh K, Lawrence C, Sim MS, Giuliano A. Comparison of patient characteristics and outcomes of contralateral prophylactic mastectomy and unilateral total mastectomy in breast cancer patients. Annals of surgical oncology. 2012;19:2600–6. doi: 10.1245/s10434-012-2299-1. [DOI] [PubMed] [Google Scholar]
- 12.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. The Cochrane database of systematic reviews. 2010:CD002748. doi: 10.1002/14651858.CD002748.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Peralta EA, Ellenhorn JD, Wagman LD, Dagis A, Andersen JS, Chu DZ. Contralateral prophylactic mastectomy improves the outcome of selected patients undergoing mastectomy for breast cancer. American journal of surgery. 2000;180:439–45. doi: 10.1016/s0002-9610(00)00505-5. [DOI] [PubMed] [Google Scholar]
- 14.van Sprundel TC, Schmidt MK, Rookus MA, et al. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. British journal of cancer. 2005;93:287–92. doi: 10.1038/sj.bjc.6602703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. The New England journal of medicine. 2002;347:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 16.Hawley ST, Jagsi R, Morrow M, et al. Social and Clinical Determinants of Contralateral Prophylactic Mastectomy. JAMA surgery. 2014 doi: 10.1001/jamasurg.2013.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eltahir Y, Werners LL, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plastic and reconstructive surgery. 2013;132:201e–9e. doi: 10.1097/PRS.0b013e31829586a7. [DOI] [PubMed] [Google Scholar]
- 18.Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Annals of surgical oncology. 2013;20:4113–20. doi: 10.1245/s10434-013-3108-1. [DOI] [PubMed] [Google Scholar]
- 19.Silva AK, Lapin B, Yao KA, Song DH, Sisco M. The effect of contralateral prophylactic mastectomy on perioperative complications in women undergoing immediate breast reconstruction: a NSQIP analysis. Ann Surg Oncol. 2015;22(11):3474–80. doi: 10.1245/s10434-015-4628-7. [DOI] [PubMed] [Google Scholar]
- 20.Crosby MA, Garvey PB, Selber JC, et al. Reconstructive outcomes in patients undergoing contralateral prophylactic mastectomy. Plast Reconstr Surg. 2011;128(5):1025–33. doi: 10.1097/PRS.0b013e31822b6682. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SM, Sepucha K, Ruddy KJ, et al. Local Therapy Decision-Making and Contralateral Prophylactic Mastectomy in Young Women with Early-Stage Breast Cancer. Annals of surgical oncology. 2015 doi: 10.1245/s10434-015-4572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borstelmann NA, Rosenberg SM, Ruddy KJ, et al. Partner support and anxiety in young women with breast cancer. Psycho-oncology. 2015 doi: 10.1002/pon.3780. [DOI] [PubMed] [Google Scholar]
- 23.Rendle KA, Halley MC, May SG, Frosch DL. Redefining risk and benefit: understanding the decision to undergo contralateral prophylactic mastectomy. Qual Health Res. 2015;25(9):1251–9. doi: 10.1177/1049732314557085. [DOI] [PubMed] [Google Scholar]
- 24.Unukovych D, Sandelin K, Liljegren A, et al. Contralateral prophylactic mastectomy in breast cancer patients with a family history: a prospective 2-years follow-up study of health related quality of life, sexuality and body image. European journal of cancer. 2012;48:3150–6. doi: 10.1016/j.ejca.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Parker PA, Peterson SK, Bedrosian I, et al. Prospective study of surgical decision-making process for contralateral prophylactic mastectomy in women with breast cancer. Ann Surg. 2016;263(1):178–83. doi: 10.1097/SLA.0000000000001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craft RO, Colakoglu S, Curtis MS, et al. Patient satisfaction in unilateral and bilateral breast reconstruction [outcomes article] Plastic and reconstructive surgery. 2011;127:1417–24. doi: 10.1097/PRS.0b013e318208d12a. [DOI] [PubMed] [Google Scholar]
- 27.Hwang ES, Locklear TD, Rushing CN, et al. Patient-reported outcomes after choice for contralateral prophylactic mastectomy. J Clin Oncol. 2016 Mar 7; doi: 10.1200/JCO.2015.61.5427. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.McCarthy CM, Klassen AF, Cano SJ, et al. Patient satisfaction with postmastectomy breast reconstruction: a comparison of saline and silicone implants. Cancer. 2010;116:5584–91. doi: 10.1002/cncr.25552. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal S, Kidwell KM, Kraft CT, et al. Defining the relationship between patient decisions to undergo breast reconstruction and contralateral prophylactic mastectomy. Plastic and reconstructive surgery. 2015;135:661–70. doi: 10.1097/PRS.0000000000001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cemal Y, Albornoz CR, Disa JJ, et al. A paradigm shift in U.S. breast reconstruction: Part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plastic and reconstructive surgery. 2013;131:320e–6e. doi: 10.1097/PRS.0b013e31827cf576. [DOI] [PubMed] [Google Scholar]
- 32.Hu ES, Pusic AL, Waljee JF, et al. Patient-reported aesthetic satisfaction with breast reconstruction during the long-term survivorship Period. Plastic and reconstructive surgery. 2009;124:1–8. doi: 10.1097/PRS.0b013e3181ab10b2. [DOI] [PubMed] [Google Scholar]
- 33.Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): 2001. [PubMed] [Google Scholar]


