Abstract
Background
Black men in the US have substantially higher prostate cancer incidence rates than the general population. The extent to which the incidence disparity is due to prostate cancer being more prevalent, more aggressive, and/or more frequently diagnosed in black men is unknown.
Methods
We estimated three independently developed models of prostate cancer natural history in black men and in the general population using an updated reconstruction of PSA screening, based on the National Health Interview Survey in 2005, and prostate cancer incidence from the Surveillance, Epidemiology, and End Results program in 1975–2000. Using the estimated models, we compared prostate cancer natural history in black men and in the general population.
Results
The models projected that 30–43% (range across models) of black men develop preclinical prostate cancer by age 85 years, a risk that is (relatively) 28–56% higher than in the general population. Among men who have had preclinical disease onset, black men have a similar risk of diagnosis (35–49%) compared with the general population (32–44%), but their risk of progression to metastatic disease by the time of diagnosis is 44–75% higher than in the general population.
Conclusions
Prostate cancer incidence patterns implicate higher incidence of preclinical disease and higher risk of metastatic progression among black men. The findings suggest screening black men earlier than white men and support further research into the benefit-harm tradeoffs of more aggressive screening policies for black men.
Keywords: cancer epidemiology, mass screening, natural history, prostatic neoplasms, prostate-specific antigen, racial disparities, statistical methods and models
INTRODUCTION
Prostate cancer is the most frequent cancer diagnosis and the second leading cause of cancer death in US men. Prostate cancer incidence among black men in the US is 60% higher and mortality is more than double the rate observed in white men.1
There is an extensive literature exploring likely drivers of the racial disparity in prostate cancer observed in the US. Regarding mortality, a persistent question concerns the relative contributions of differential access to care versus biology. Some studies2, 3 have suggested that differential access to care may partially explain the greater burden of adverse outcomes in black men. Others have identified differences in germline and tumor genetics between black and white men.1, 4–6 Black race has been identified as an independent prognostic factor for disease recurrence in multiple reports,7, 8 supporting a biologically more aggressive disease phenotype but also raising questions about disparities in surgery quality.9 In general, black men are less likely to receive primary surgery,10–12 but the extent to which this observation is related to demographics, access to care, or personal preference is unclear.
At least one major driver behind the higher rate of prostate cancer deaths among black is their higher incidence of the disease. Taksler et al. concluded that the majority (76%) of the disparity in prostate cancer mortality may be explained by higher prostate cancer incidence in black men.13 The objective of the present study is to investigate and explain the higher observed incidence in black men. Whether this arises from a higher risk of disease onset or faster progression to an aggressive or symptomatic state is unclear. In their multi-ethnic study of UK men, Metcalfe et al.14 suggest that the latter is unlikely; however, they do not formally interrogate this hypothesis.
Understanding whether and how natural history might be different in black men is important because, if black men have a higher susceptibility to prostate cancer and/or a greater tendency to develop aggressive disease, it may be of value to consider different screening policies for them. This issue was first raised by Powell et al.,15 following observation of more aggressive disease characteristics and more frequent recurrence among black men following radical prostatectomy, and again16 based on a narrowing of prostate cancer survival disparities observed following the adoption of PSA screening in the US.
We previously studied the natural history of prostate cancer in the general population via statistical and computer modeling of latent disease onset and progression to clinical and metastatic states.17–19 By calibrating the models to observed population patterns of prostate cancer incidence before and after the advent of PSA screening, we estimated the risks of critical events in disease natural history and used these results to make inferences about potential impacts of different screening policies.20, 21
In this article, we develop versions of our natural history models that pertain to black men and calibrate these using incidence trends in the Surveillance, Epidemiology, and End Results (SEER) program under updated PSA screening frequencies estimated specifically among the black population. We use the calibrated models to produce estimates of disease onset, progression, and diagnosis risks that pertain to the black population. We compare these risks with estimates for the general population (i.e., all races) to determine the extent to which the increased incidence among black men is explained by higher risks of disease onset, progression, or diagnosis. Finally, we use our results to examine differential incidence of clinically relevant disease, motivating further research into differential screening policies among black men.
METHODS
In this section, we describe the data and three models that we use to examine evidence of differential prostate cancer natural history in black men. We also describe a test for differences in black natural history relative to the general population and quantify the models’ goodness-of-fit after re-estimating key components of natural history.
PSA screening and prostate cancer incidence data
Because population-based PSA screening utilization was not tracked in real time, we retrospectively reconstructed PSA screening patterns in the US separately for black and white men in a previous study.22 Briefly, this reconstruction used responses to the National Health Interview Survey (NHIS) in 2000 to estimate the age at first PSA test, and longitudinal claims data from the linked SEER-Medicare database to estimate the distribution of inter-screening intervals. The present version of the PSA screening model updates the original version, incorporating responses to the 2005 NHIS as well as information on changes in disease-specific incidence following the advent of PSA (see the online supplemental information for a comparison of the original and updated PSA screening models).
We extracted prostate cancer incidence data from the SEER database before and after the introduction of PSA screening. Specifically, we extracted prostate cancer incidence for ages 50–84, years 1975–2000, SEER historic stages local-regional and distant, tumor grade well or moderately differentiated (low grade) versus poorly differentiated or undifferentiated (high grade), and race categories “black” or “all races.” Missing information on stage, grade, and race was assumed to be missing at random and imputed as the most frequent combination of 20 logistic regression imputations using the mice package in R.23
Three models of prostate cancer natural history
We estimated three models of prostate cancer natural history using PSA screening and prostate cancer incidence data separately for black men and for all races. The three models were previously used to study effects of PSA screening on incidence and mortality trends in the general US population.24, 25
Briefly, the FHCRC model is a microsimulation model that links individual PSA growth and cancer progression. In this model, higher and increasing PSA levels are associated with the presence of latent cancer and shorter intervals to metastatic spread and clinical presentation. The MISCAN model is a microsimulation model that tracks progression through combinations of cancer stages and grades. In this model, advanced stages and higher grades are associated with potentially higher screening test sensitivity and shorter intervals to clinical presentation. The UMICH model is an integrated suite of analytic models that estimates transition probabilities from earlier to later stages and from lower to higher grades during the preclinical detectable phase. In this model, a later stage at onset, a higher grade at onset, and faster progression are each associated with shorter intervals to clinical presentation. In each model, screening potentially detects latent cancer at an earlier stage and/or grade. Key differences between models are the length of the preclinical detectable phase, how much early detection improves tumor characteristics, and how both natural history and screening effects depend on age. Detailed descriptions of the models are given in the online supplemental information.
A framework to explain incidence disparities
Sequential estimation
We first re-estimated natural history in all races using the SEER incidence and updated PSA screening data. Then, we substituted PSA screening patterns for black men and re-estimated natural history in black men following a systematic sequence of steps. Specifically, we re-estimated components of disease natural history, each containing a specific block of parameters. The blocks of parameters governed (a) risk of disease onset and initial tumor features, (b) risks of progression to metastasis and/or high-grade disease, and (c) risk of clinical diagnosis. At each step, the re-estimation involved identifying values of the natural history parameters that allowed the models to most closely match SEER prostate cancer incidence in black men. All models proceeded in this sequential fashion until final versions of the models were obtained that re-estimated all natural history parameters for black men. To evaluate sensitivity to this sequence of re-estimated parameters, a model selection exercise examined alternative sequences.
Natural history summary measures
Given the final versions of the models for black men and for all races, we summarized natural history in terms of the risks of preclinical onset, clinical diagnosis, and metastatic clinical diagnosis; mean ages at these natural history events; and mean years between consecutive events. Because all models were calibrated using data up to age 85 years, the summary measures were truncated at this age.
Testing and quantifying contributions to incidence disparities
We used a likelihood ratio test to evaluate whether re-estimating components of disease natural history significantly improved the models’ fits to the incidence data for black men. The likelihood used age at diagnosis as a survival time and was fit via a customized age-period approach.19 To calculate likelihood ratio statistics, two likelihood functions were fit, one with and one without re-estimation of the component. While we report the likelihood ratio test results, we anticipate that, given the large sample size in the SEER registry, all tests will be highly significant at a traditional threshold. Therefore, we also report the improvement in the goodness-of-fit achieved by re-estimating components of natural history, with goodness-of-fit expressed as the sum across years of the squared difference between annual model-projected and observed age-adjusted incidence rates.
RESULTS
Figure 1 illustrates the annual percentage of men ages 50–84 who received at least 1 PSA test by race and age group over the period 1988–2000 using responses from the NHIS in 2005. Relative to previous estimates using responses from the NHIS in 2000,22 we find that younger men received fewer tests; these differences were similar among black men and all races. The updated screening patterns indicate that, relative to the general population, modestly lower percentages of black men received at least 1 PSA test in all but the youngest ages throughout the 1990s. The greatest racial disparities in PSA testing were in the oldest ages.
Figure 1.
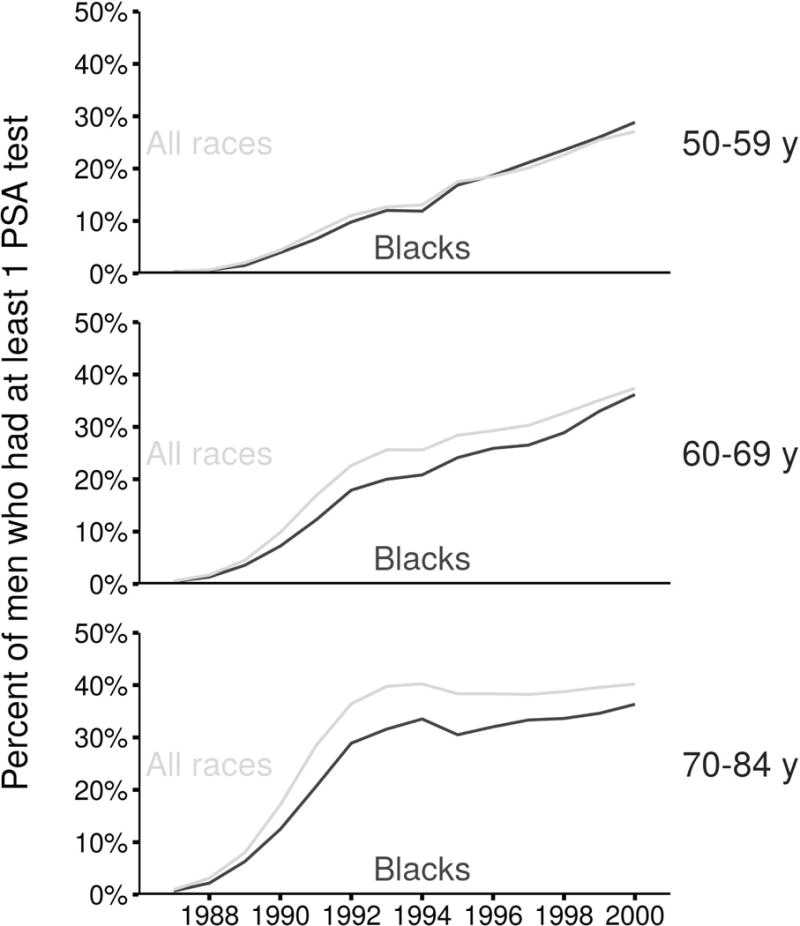
Annual percentage of men receiving at least 1 PSA test based on the updated reconstruction of PSA screening patterns in the US.
Figure 2 shows the results of re-estimating natural history for all races. The figure shows age-adjusted prostate cancer incidence rates per 100,000 men ages 50–84 reported in SEER by historic stage and corresponding model-projected incidence rates. Figure 2 also shows SEER incidence rates for black men and results of the sequential estimation of the models’ natural history components. The sequential estimation found that allowing the risk of disease onset to be different for black men provided an immediate improvement in the models’ fits to incidence in this population. Allowing the risk of progression to distant stage to be different produced higher distant-stage but similar local-regional stage incidence projections. Also allowing the risk of clinical diagnosis to differ in black men provided modest improvements to the fit in some cases (e.g., distant-stage incidence in the FHCRC model). Results of the model selection exercise described in the online supplemental information shows that this sequence for re-estimating parameter blocks reflects descending importance in the improvements in fits.
Figure 2.
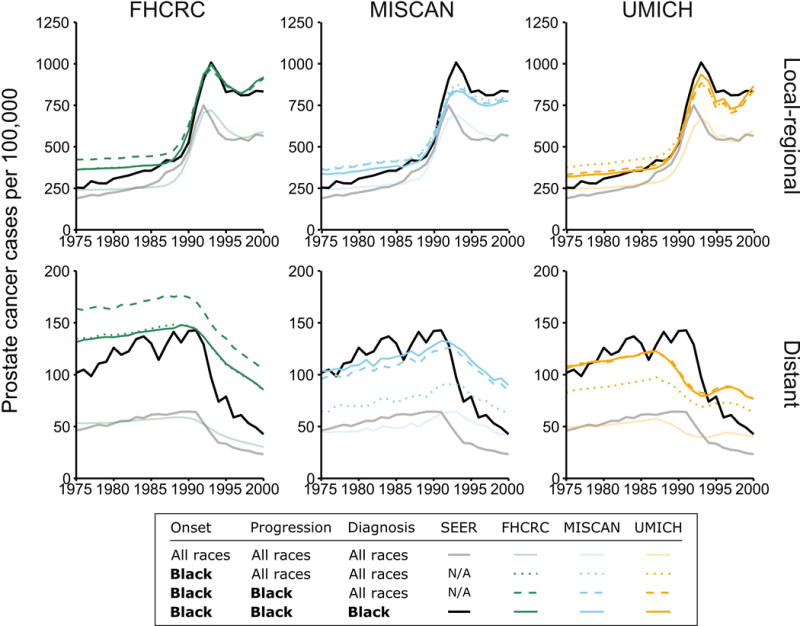
Age-adjusted prostate cancer incidence rates per 100,000 men ages 50–84 years for black men (black line) and all races (gray line) and corresponding projections by three models (colored lines). Model projections are based on the models estimated for all races combined with PSA screening in black men and sequentially re-estimating components of natural history to allow differential risk of onset of preclinical cancer (“Onset”), risk of progression to metastasis and/or higher grade (“Progression”), and risk of clinical diagnosis (“Diagnosis”).
Improvements from re-estimating each block of natural history parameters were highly statistically significant from likelihood ratio tests (all P < 0.0001), and the final models’ fits to stage-specific incidence were substantially improved by re-estimation of the natural history components. The online supplemental information shows that sums of squared differences between observed and projected age-adjusted incidence rates declined dramatically once disease onset was re-estimated, confirming the relative importance of disease onset risk in explaining incidence disparities. All models obtained the best fits (i.e., smallest errors) when all parameter blocks were re-estimated.
Table 1 summarizes natural history measures among black men and for all races up to age 85 years estimated by the three final models. In the general population, the risk of developing preclinical disease is 24–29% (range across models). In black men, however, these risks rise to 30–43%, reflecting risks that are (relatively) 28–56% higher than the general population. According to the models, the risks of clinical diagnosis in black men are (relatively) 33–70% higher than the general population; the corresponding observed risk in SEER prior to the advent of PSA screening was 53% higher in black men than white men (range 42%–62% higher) over the period 1975 to 1986. Among men who have had disease onset, the risk of clinical diagnosis is comparable for blacks (35–49% across models) and all races (32–44% across models), and this translates into sojourn times from disease onset to diagnosis that are very similar for black men and the general population within each model. However, among men with preclinical disease, the models estimate a 44–75% higher risk of metastasis before diagnosis among black men, reflecting greater risk of progression in this population.
Table 1.
Summary measures for natural history events occurring within a man’s lifetime, up to age 85 years, projected by the three models. Ratios are for black men relative to all races and are calculated using 4 significant digits for all measures.
| Measure | FHCRC | MISCAN | UMICH | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Black men | All races | Ratio | Black men | All races | Ratio | Black men | All races | Ratio | |
| Risk of onset, % | 43 | 28 | 1.56 | 30 | 24 | 1.28 | 37 | 29 | 1.29 |
| Risk of clinical diagnosis, % | 15 | 9 | 1.70 | 13 | 10 | 1.33 | 18 | 13 | 1.44 |
| Risk of metastatic clinical diagnosis, % | 4 | 2 | 2.53 | 4 | 2 | 1.84 | 4 | 2 | 2.26 |
| Risk of clinical diagnosis given onset, % | 35 | 32 | 1.09 | 45 | 43 | 1.04 | 49 | 44 | 1.12 |
| Risk of metastatic clinical diagnosis given onset, % | 9 | 6 | 1.63 | 13 | 9 | 1.44 | 12 | 7 | 1.75 |
| Mean age at onset, y | 57 | 59 | 0.97 | 64 | 67 | 0.96 | 65 | 66 | 0.99 |
| Mean age at clinical diagnosis, y | 71 | 72 | 0.99 | 70 | 72 | 0.98 | 75 | 76 | 0.98 |
| Mean age at metastatic clinical diagnosis, y | 70 | 72 | 0.98 | 71 | 73 | 0.98 | 74 | 74 | 0.99 |
| Mean time from onset to clinical diagnosis, y | 18 | 18 | 1.02 | 10 | 10 | 0.98 | 17 | 18 | 0.92 |
| Mean time from onset to metastatic clinical diagnosis, y | 16 | 15 | 1.03 | 12 | 13 | 0.94 | 16 | 21 | 0.79 |
| Risk of PSA or clinical diagnosis*, % | 16 | 10 | 1.65 | 20 | 16 | 1.23 | 20 | 14 | 1.45 |
| Risk of PSA or clinical diagnosis given onset*, % | 38 | 36 | 1.06 | 66 | 68 | 0.97 | 53 | 47 | 1.12 |
| Mean age at PSA or clinical diagnosis*, y | 70 | 71 | 0.99 | 69 | 71 | 0.98 | 73 | 74 | 0.98 |
| Mean time from PSA to clinical diagnosis*, y | 7 | 7 | 1.03 | 8 | 9 | 0.95 | 7 | 7 | 0.96 |
These measures are in the presence of modeled PSA screening patterns in 1987–2000 and are included for reference.
DISCUSSION
The observation that prostate cancer diagnosis is more common and more lethal among black men than among white men has never been fully explained. Our study uses three models17–19 previously calibrated to US population incidence trends to learn about features of underlying disease onset and progression unaffected by differential practice patterns around prostate cancer screening and diagnosis. The model results consistently showed that the risk of onset of a preclinical prostate cancer explains a large majority of the observed incidence disparities. Further, in addition to the risk of onset, the risk of progression to metastatic disease before clinical diagnosis was higher among black men, but the risk of clinical diagnosis following disease onset was similar to the general population. The models cannot identify whether these apparent differences in disease natural history are driven by biology, behavioral, or environmental factors, but they are of value in generating hypotheses about underlying mechanisms and their implications for screening policies.
Based on these results, we conclude that black men have more preclinical and progressive prostate cancer than the general population. They are more likely to develop prostate cancer at a younger age, and they are more likely to progress to a metastatic state and/or higher grade before clinical diagnosis. Their higher risk of progression agrees with a previous study based on autopsy and surgical pathology data26 that concluded that black men had an earlier transformation to clinically significant cancer than white men. This study found similar age-specific prevalence of prostate cancer among autopsies conducted in black and white men from the Detroit metropolitan area between 1992 and 2001. The study also found evidence of more aggressive disease in radical prostatectomy specimens from black men, consistent with their markedly higher incidence of metastatic disease at diagnosis. These findings led the authors to conclude that the risk of prostate cancer initiation did not differ by race, but the risk of disease progression was higher among black men. However, similar latent prevalence and greater metastatic clinical incidence of disease among black men is in fact only possible if latent incidence is also higher in this subgroup. For, if latent incidence is similar among black men but progression is faster, this would actually lead to lower latent prevalence at autopsy. Therefore, we conclude that the prior study results are in fact consistent with our finding that the risks of latent incidence and progression are likely both higher among black men.
Our findings motivate considering more intensive screening, e.g., beginning earlier and/or screening more frequently, among black men than among the general US population. To illustrate this, Figure 3 shows the cumulative incidence of “relevant disease,” i.e., disease fated to present before other-cause death. At all ages, the cumulative incidence is higher for black men than for all races. At ages 46–52 (range across models), the cumulative incidence among black men reaches the value estimated at age 55 among all races. Thus, if it is agreed that prostate cancer screening is worthwhile, and starting at age 55 is determined for the general population, our results suggest starting 3–9 years earlier for black men.
Figure 3.
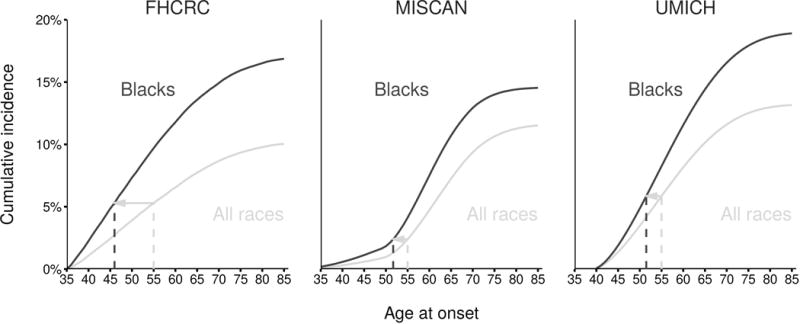
Cumulative incidence of onset of preclinical prostate cancer that would be clinically diagnosed in black men and all races projected by the models. Line segments show ages at which black men have incidence corresponding to levels estimated at age 55 in all races.
We recognize that a consensus about general population screening is still lacking. The US Preventive Services Task Force27 recommends against routine prostate cancer screening in men of average risk, while the American Cancer Society28 recommends shared decision making around prostate cancer screening beginning at age 50 and the American Urological Association29 provides similar guidance with a starting age of 55. However, black men are not average risk and the benefit-harm tradeoffs of screening are likely to be different for this population.30
While starting at an earlier age is unlikely to impact overdiagnosis, other more aggressive screening policies, e.g., shortening intervals between screens or lowering the PSA threshold for biopsy referral, could increase risks of overdiagnosis.31 A comprehensive policy development process addressing whether and how best to screen black men will have to carefully weigh benefit-harm tradeoffs of candidate policies. Understanding race-specific natural history will be a critical pre-requisite for this important work. At this point, however, our findings support considering screening beginning at an earlier age in black men than in the general population. Powell et al.16 also recommend aggressive early prostate cancer PSA testing of African American men. Our work adds to theirs by illustrating one quantitative justification for an age to initiate screening in black men.
In practice, the policy development process will require going beyond examining incidence patterns to projecting mortality in the presence and absence of screening. Since screening benefit is contingent on access to efficacious therapies, benefits of screening in different race groups may be affected by any disparities in access to treatment and any racial differences in treatment efficacy. Future work will extend the models used in this article to project the downstream outcomes of different screening policies in black men under race-specific treatment distributions and efficacies.
Limitations of this study relate primarily to modeling assumptions and data limitations. While the use of multiple models provides some sense of robustness to the specific assumptions made, all models assume that disease is progressive. Thus, none of the models explicitly includes an indolent subpopulation, although each allows heterogeneity of disease progression with some cases progressing rapidly and others slowly. The FHCRC model allows the likelihood of developing high-grade disease to vary with age but does not model grade progression; the other models allow both grade and stage progression. Other differences across models are also driven by differences in the conceptualization of onset and how the risk of onset depends on age. In the FHCRC model, onset refers to the latent incidence of disease that would be detectable by biopsy, which can occur as early as age 35, while in the MISCAN and UMICH models onset refers to the latent incidence of disease that can be detected based on elevated PSA and diagnostic workup, and this rarely occurs before age 40. Finally, the PSA screening rates used by all models are based on a retrospective reconstruction rather than a prospective tracking of prostate cancer screening dissemination in the US population.
In conclusion, this study represents the first examination of how prostate cancer natural history must differ in black men to account for racial variation in patterns of disease incidence before and after the advent of PSA screening. We use observed patterns of disease incidence and screening to learn about key events in the latent process of disease by race. Our results provide quantitative information about the prostate cancer natural history that may support prior suggestions to explore different screening policies among white and black men.13, 15
Supplementary Material
Precis.
Three models were used to study differences in prostate cancer natural history between black men and the general US population. We found black men have higher prevalence of preclinical disease at all ages and higher risk of metastasis, supporting further study into different screening policies for black men.
Acknowledgments
Funding:
This work was made possible by Grant Numbers U01 CA157224 and U01 CA199338 from the National Cancer Institute as part of the Cancer Intervention and Surveillance Modeling Network (CISNET). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Disclosures:
EAMH and HJK received a research grant from Beckman Coultier. All other authors declare no potential conflict of interest.
References
- 1.Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. Journal of Urology. 2007;177:444–449. doi: 10.1016/j.juro.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Underwood W, De Monner S, Ubel P, Fagerlin A, Sanda MG, Wei JT. Racial/ethnic disparities in the treatment of localized/regional prostate cancer. Journal of Urology. 2004;171:1504–1507. doi: 10.1097/01.ju.0000118907.64125.e0. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz K, Powell IJ, Underwood W, 3rd, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology. 2009;74:1296–1302. doi: 10.1016/j.urology.2009.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovannucci E, Stampfer MJ, Krithivas K, et al. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3320–3323. doi: 10.1073/pnas.94.7.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bensen JT, Xu Z, Smith GJ, Mohler JL, Fontham ET, Taylor JA. Genetic polymorphism and prostate cancer aggressiveness: a case-only study of 1,536 GWAS and candidate SNPs in African-Americans and European-Americans. Prostate. 2013;73:11–22. doi: 10.1002/pros.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faisal FA, Sundi D, Tosoian JJ, et al. Racial Variations in Prostate Cancer Molecular Subtypes and Androgen Receptor Signaling Reflect Anatomic Tumor Location. European Urology. 2016;70:14–17. doi: 10.1016/j.eururo.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moul JW, Douglas TH, McCarthy WF, McLeod DG. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. Journal of Urology. 1996;155:1667–1673. [PubMed] [Google Scholar]
- 8.Faisal FA, Sundi D, Cooper JL, et al. Racial disparities in oncologic outcomes after radical prostatectomy: long-term follow-up. Urology. 2014;84:1434–1441. doi: 10.1016/j.urology.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barocas DA, Gray DT, Fowke JH, et al. Racial variation in the quality of surgical care for prostate cancer. Journal of Urology. 2012;188:1279–1285. doi: 10.1016/j.juro.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shavers VL, Brown ML, Potosky AL, et al. Race/ethnicity and the receipt of watchful waiting for the initial management of prostate cancer. Journal of General Internal Medicine. 2004;19:146–155. doi: 10.1111/j.1525-1497.2004.30209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Medical Care. 1998;36:1337–1348. doi: 10.1097/00005650-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Zeliadt SB, Potosky AL, Etzioni R, Ramsey SD, Penson DF. Racial disparity in primary and adjuvant treatment for nonmetastatic prostate cancer: SEER-Medicare trends 1991 to 1999. Urology. 2004;64:1171–1176. doi: 10.1016/j.urology.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Taksler GB, Keating NL, Cutler DM. Explaining racial differences in prostate cancer mortality. Cancer. 2012;118:4280–4289. doi: 10.1002/cncr.27379. [DOI] [PubMed] [Google Scholar]
- 14.Metcalfe C, Evans S, Ibrahim F, et al. Pathways to diagnosis for Black men and White men found to have prostate cancer: the PROCESS cohort study. British Journal of Cancer. 2008;99:1040–1045. doi: 10.1038/sj.bjc.6604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell IJ, Banerjee M, Sakr WA, et al. Should African-American men be tested for prostate carcinoma at an earlier age than white men? Cancer. 1999;85:472–477. [PubMed] [Google Scholar]
- 16.Powell IJ, Vigneau FD, Bock CH, Ruterbusch J, Heilbrun LK. Reducing prostate cancer racial disparity: evidence for aggressive early prostate cancer PSA testing of African American men. Cancer Epidemiology, Biomarkers and Prevention. 2014;23:1505–1511. doi: 10.1158/1055-9965.EPI-13-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulati R, Inoue L, Katcher J, Hazelton W, Etzioni R. Calibrating disease progression models using population data: a critical precursor to policy development in cancer control. Biostatistics. 2010;11:707–719. doi: 10.1093/biostatistics/kxq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: Estimates from the European Randomized Study of Screening for Prostate Cancer. Journal of the National Cancer Institute. 2003;95:868–878. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 19.Tsodikov A, Szabo A, Wegelin J. A population model of prostate cancer incidence. Stat in Med. 2006;25:2846–2866. doi: 10.1002/sim.2257. [DOI] [PubMed] [Google Scholar]
- 20.Gulati R, Gore JL, Etzioni R. Comparative effectiveness of alternative prostate-specific antigen-based prostate cancer screening strategies: Model estimates of potential benefits and harms. Annals of Internal Medicine. 2013;158:145–153. doi: 10.7326/0003-4819-158-3-201302050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heijnsdijk EA, de Carvalho TM, Auvinen A, et al. Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data. Journal of the National Cancer Institute. 2015;107:366. doi: 10.1093/jnci/dju366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariotto A, Etzioni R, Krapcho M, Feuer EJ. Reconstructing prostate-specific antigen (PSA) testing patterns among black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109:1877–1886. doi: 10.1002/cncr.22607. [DOI] [PubMed] [Google Scholar]
- 23.van Buuren S, Groothuis-Oudshoorn K. Multivariate imputation by chained equations in R. Journal of Statistical Software. 2011;45:1–67. [Google Scholar]
- 24.Etzioni R, Tsodikov A, Mariotto A, et al. Quantifying the role of PSA screening in the US prostate cancer mortality decline. Cancer Causes and Control. 2008;19:175–181. doi: 10.1007/s10552-007-9083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etzioni R, Gulati R, Tsodikov A, et al. The prostate cancer conundrum revisited: treatment changes and prostate cancer mortality declines. Cancer. 2012;118:5955–5963. doi: 10.1002/cncr.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. Journal of Urology. 2010;183:1792–1796. doi: 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyer VA, Force USPST Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 28.Wolf AM. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 29.Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. Journal of Urology. 2013;190:419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall L, Bullock AD, Brown AL, Colditz G. Prostate Cancer Isn’t Colorblind. New York Times. 2016 [Google Scholar]
- 31.Gulati R, Cheng HH, Lange PH, Nelson PS, Etzioni R. Screening men at increased risk for prostate cancer diagnosis: Model estimates of benefits and harms. Cancer Epidemiology Biomarkers and Prevention. 2016 doi: 10.1158/1055-9965.EPI-16-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


