Abstract
Bisphosphonates represent the gold-standard pharmaceutical agent for reducing fracture risk. Long-term treatment with bisphosphonates can result in tissue brittleness which in rare clinical cases manifests as atypical femoral fracture. Although this has led to an increasing call for bisphosphonate cessation, few studies have investigated therapeutic options for follow-up treatment. The goal of this study was to test the hypothesis that treatment with raloxifene, a drug that has cell-independent effects on bone mechanical material properties, could reverse the compromised mechanical properties that occur following zoledronate treatment. Skeletally mature male C57Bl/6J mice were treated with vehicle (VEH), zoledronate (ZOL), or ZOL followed by raloxifene (RAL; 2 different doses). At the conclusion of 8 weeks of treatment femora were collected and assessed with microCT and mechanical testing. Trabecular BV/TV was significantly higher in all treated animals compared to VEH with both RAL groups having significantly higher BV/TV compared to ZOL (+21%). All three drug-treated groups had significantly more cortical bone area, higher cortical thickness, and greater moment of inertia at the femoral mid-diaphysis compared to VEH with no difference among the three treated groups. All three drug-treated groups had significantly higher ultimate load compared to VEH-treated animals (+14 to 18%). Both doses of RAL resulted in significantly higher displacement values compared to ZOL-treated animals (+25 to +50%). In conclusion, the current work shows beneficial effects of raloxifene in animals previously treated with zoledronate. The higher mechanical properties of raloxifene-treated animals, combined with similar cortical bone geometry compared to animals treated with zoledronate, suggest the raloxifene treatment is enhancing mechanical material properties of the tissue.
Keywords: bisphosphonate, bone quality, mechanical properties, SERMs
INTRODUCTION
Over the past few decades, bisphosphonates have represented the gold-standard pharmaceutical agent for reducing fracture risk in osteoporosis as well a number of other metabolic conditions that induce skeletal fragility [1,2]. Their potent anti-remodeling effect slows bone loss and thereby stabilizes overall mechanical competence and reduces fracture. The benefits of remodeling suppression, however, are not without cost.
Remodeling serves an important function to the skeleton in the form of removing regions of tissue that are damaged, are void of viable cells, or have tissue-level properties that are suboptimal. Suppression of remodeling with bisphosphonates has been shown to allow microdamage accumulation [3], permit increased regions of non-viable osteocytes [4], and lead to bone that has higher mineralization and non-enzymatic collagen cross-linking [5–7]. These changes are associated with compromised tissue-level mechanical properties [8,9]. Clinical reductions in fracture risk shows that these tissue-level changes do not outweigh the benefits from preservation of bone mass [10], at least in the short term. Preclinical data suggest, however, that with long-term treatment the tissue-level changes may be sufficient in some situations to at least partially negate the benefits [11,12]. An extreme example is atypical femoral fractures, associated with anti-remodeling treatment, that tend to occur when treatment extends beyond five years and are hypothesized to be caused by changes related to remodeling suppression [13,14]. Although atypical femoral fractures are quite rare, their challenging treatment makes them clinically significant.
Bisphosphonates bind to the skeleton and therefore have long-lasting effects [15]. Clinical data show a single dose of zoledronate retains an effect on bone resorption for well beyond 5 years [16,17]. Although this long-lasting effect represents a positive feature of the drug [18], it presents a challenge from the perspective of changes to bone material properties with long-term remodeling suppression. Raloxifene, an FDA approved agent for reducing fracture risk, has recently been shown to affect material-level mechanical properties of bone through both cellular effects on the estrogen receptor as well as non-cellular mechanisms that affect hydration [19,20]. The goal of this study was to test the hypothesis that raloxifene treatment could reverse the compromised mechanical properties that occur following zoledronate treatment. To test this hypothesis, we utilized a mouse model that has previously been shown to manifest bisphosphonate-induced reductions in bone mechanical properties [21].
METHODS
Animals and Drug treatment
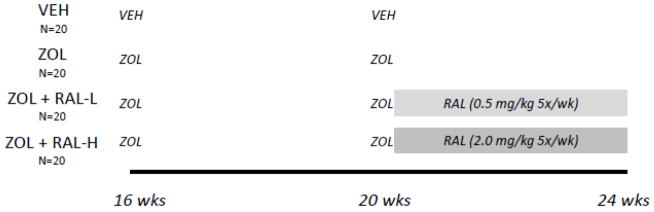
Sixteen week old C57Bl/6J male mice (n=80, from JAX laboratory) were used for this study. Male mice were chosen to build on previous data from our lab and others using this strain and sex [21–23]. Animals received a zoledronic acid (n=60; ZOL, subcutaneous injection of 0.06 mg/kg/body weight (BW)) or saline vehicle (n=20; VEH) at 16 weeks of age, then another at 20 weeks of age (Figure 1). At 20 weeks of age, animals in designated treatment groups received raloxifene (RAL; 5 days/week, subcutaneous injection) at one of two doses: low dose (0.5 mg/kg/BW (n=20; ZOL + RAL-L)) or high dose (2.0 mg/kg/BW (n=20; ZOL + RAL-H)). The dose and duration of ZOL was chosen as it has shown efficacy in suppressing remodeling and affecting mechanical properties in this genetic strain [21]. The lower dose of RAL has been used previously and shown efficacy in vivo [24]; a higher dose was arbitrarily chosen as 4× higher to determine if dose-responses could be quantified. At 24 weeks of age, animals were euthanized and the right femurs were removed, wrapped in saline-soaked gauze, and frozen (−20C) for later analysis. All procedures were approved by the Indiana University School of Medicine Animal Care and Use Committee prior to initiating the study.
Figure 1.
Schematic representation of study design. VEH, saline vehicle; ZOL, zoledronate; RAL, raloxifene. Zoledronate was given as a single injection at 16 and 20 weeks; raloxifene was given as a daily injection, 5 times per week, between 20 and 24 weeks.
μCT Imaging
Right femora were thawed and scanned with microCT (Skyscan 1176) to obtain trabecular and cortical bone morphology as previously described [21]. Scans were conducted using a 9 micron voxel size. Trabecular bone was analyzed in the distal femur metaphysis (~0.5 mm long segment) and cortical bone was assessed at the mid-diaphysis (single slice). All scan reconstruction/analysis was done using manufacturer provided software. Nomenclature is reported in accordance with suggested guidelines [25].
Mechanical Testing
Whole bone mechanical properties were assessed in four-point bending as previously described [21] and in accordance with recommendations [26]. The anterior surface of each femur was placed on two lower supports with a span length of 9 mm and an upper span length of 3 mm. Specimens were loaded to failure at a rate of 2 mm/min, producing a force-displacement curve for each sample. Using a custom MATLAB program [24], structural and apparent material properties were determined. Apparent material properties were derived using standard beam-bending equations for four-point bending and geometric data from microCT analyses.
Statistical Analysis
Comparisons among the four groups were made using a one-way ANOVA. When a significant main effect was present, post-hoc tests (pLSD) were used to determine individual group differences. A p value of < 0.05 was used for all determinations of significance. All data are presented as means +/− standard deviations.
RESULTS
There was no significant difference in baseline body mass among the groups (Table 1). VEH, ZOL and low dose RAL mice all gained body mass during the experiment. Final body mass of the low dose RAL group was significantly lower than VEH (−5%) while high dose RAL was significantly lower than both VEH and ZOL (−7%). There was no difference in femoral bone length among the groups.
Table 1.
Body mass and femur length
| VEH | ZOL | ZOL + RAL-Low | ZOL + RAL High | ANOVA P value | |
|---|---|---|---|---|---|
| Baseline body mass (g) | 29.7 ± 2.0 | 29.6 ± 1.8 | 29.2 ± 3.4 | 29.3 ± 1.9 | 0.8970 |
| Final body mass (g) | 30.9 ± 2.3 | 30.7 ± 2.1 | 29.5 ± 2.2 * | 28.6 ± 2.3 *# | 0.0032 |
| Femur length (mm) | 15.6 ± 0.23 | 15.5 ± 0.43 | 15.4 ± 0.3 | 15.4 ± 0.34 | 0.0830 |
Data presented as means and standard deviations. VEH, vehicle; ZOL, zoledronate; RAL-L, raloxifene 0.5 mg/kg; RAL-H, raloxifene 2.0 mg/kg.
vs VEH;
vs ZOL.
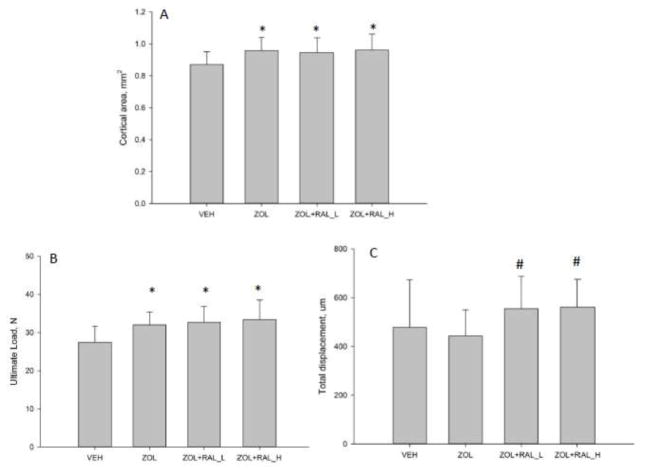
MicroCT-based assessment of trabecular and cortical morphology revealed significant treatment effects. Trabecular BV/TV was significantly higher in all treated animals compared to VEH with both RAL groups having significantly higher BV/TV compared to ZOL (+21%; Table 2). Higher BV/TV with treatment was the result of greater trabecular thickness and number. All three drug-treated groups had significantly more cortical bone area, higher cortical thickness, and greater moment of inertia at the femoral mid-diaphysis compared to VEH with no differences among the three treated groups (Table 2, Figure 2A).
Table 2.
Femoral bone trabecular and cortical architecture
| VEH | ZOL | ZOL + RAL-L | ZOL + RAL-H | ANOVA P value | |
|---|---|---|---|---|---|
| Trabecular BV/TV (%) | 21.6 ± 4.0 | 29.7 ± 4.0 * | 35.9 ± 6.3 *# | 36.1 ± 4.9 *# | 0.0001 |
| Trabecular thickness (μm) | 77 ± 8 | 82 ± 9 | 93 ± 8 *# | 91 ± 8 *# | 0.0001 |
| Trabecular number (#/mm) | 2.8 ± 0.3 | 3.6 ± 0.3* | 3.8 ± 0.4*# | 3.9 ± 0.3*# | 0.0001 |
| Total cross sectional area (mm2) | 2.0 ± 0.2 | 2.2 ± 0.3 | 2.2 ± 0.4 | 2.0 ± 0.2 | 0.1218 |
| Marrow area (mm2) | 1.1 ± 0.2 | 1.2 ± 0.3 | 1.3 ± 0.4 | 1.1 ± 0.2 | 0.1480 |
| Cortical area (mm2) | 0.87 ± 0.08 | 0.96 ± 0.08 * | 0.95 ± 0.09 * | 0.95 ± 0.10 * | 0.0047 |
| Cortical thickness (mm) | 0.20 ± 0.01 | 0.21 ± 0.03 * | 0.21 ± 0.02 * | 0.22 ± 0.02 * | 0.0066 |
| Maximum cross-sectional moment of inertia (mm4) | 0.34 ± 0.07 | 0.40 ± 0.08 * | 0.41 ± 0.10 * | 0.37 ± 0.07 | 0.0475 |
| Minimum cross-sectional moment of inertia (mm4) | 0.14 ± 0.03 | 0.16 ± 0.03 | 0.16 ± 0.04 | 0.15 ± 0.04 | 0.1689 |
Data presented as means and standard deviations. VEH, vehicle; ZOL, zoledronate; RAL-L, raloxifene 0.5 mg/kg; RAL-H, raloxifene 2.0 mg/kg; BV/TV, bone volume per tissue volume.
vs VEH;
vs ZOL.
Figure 2.
Structural geometry and mechanical properties of femoral diaphysis. Treatment with zoledronate (ZOL) or zoledronate followed by raloxifene (RAL) led to significantly more cortical bone (A) in the diaphysis compared to vehicle (VEH) controls. There was no significant difference between the three treatments relative to each other. (B) Ultimate load was also significantly higher in all three drug treated groups. (C) Total displacement was significantly higher in animals given RAL compared to those given only ZOL. P < 0.05 versus (*) VEH or (#) ZOL.
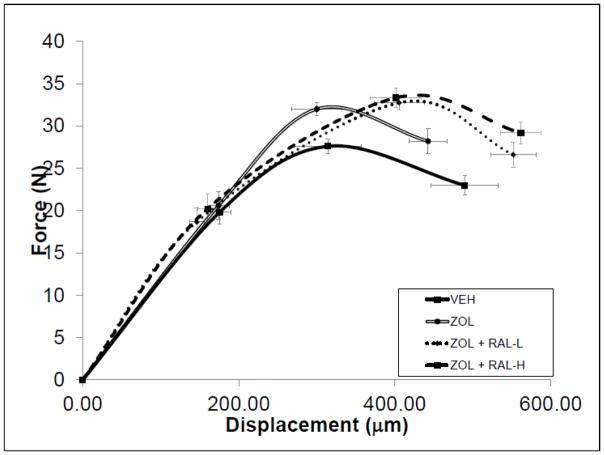
All three drug-treated groups had significantly higher ultimate load compared to VEH-treated animals (+14 to 18%; Figure 2B). While there was no significant effect of ZOL on total or post-yield displacement (−10% and −15%, respectively), both doses of RAL resulted in significantly higher displacement values compared to ZOL-treated animals (+25 to +50%; Figure 2C, Figure 3 and Table 3). Total work and post-yield work were significantly higher in both RAL groups compared to both VEH and ZOL groups. Estimates of apparent mechanical material properties revealed similar patterns of differences among groups with total strain and toughness both being significantly positively affected by RAL treatment compared to ZOL and VEH (Table 3).
Figure 3.
Schematic force-displacement curves for all groups. Error bars represent standard error of the mean for both load (vertical errors) and displacement (horizontal errors).
Table 3.
Mechanical properties of femoral diaphysis
| VEH | ZOL | ZOL + RAL-Low | ZOL + RAL High | ANOVA P value | |
|---|---|---|---|---|---|
| Yield Force (N) | 19 ± 6 | 21 ± 7 | 19 ± 8 | 20 ± 8 | 0.8524 |
| Ultimate Force (N) | 28 ± 4 | 32 ± 3 * | 33 ± 4 * | 33 ± 5 * | 0.0002 |
| Displacement to Yield (mm) | 175 ± 64 | 174 ± 62 | 146 ± 42 | 160 ± 55 | 0.3228 |
| Post-yield Displacement (mm) | 314 ± 193 | 269 ± 144 | 405 ± 144 # | 402 ± 145 # | 0.0162 |
| Total Displacement (mm) | 490 ± 192 | 443 ± 108 | 552 ± 130 # | 561 ± 115 # | 0.0276 |
| Stiffness (N/mm) | 142 ± 28 | 147 ± 26 | 152 ± 29 | 153 ± 24 | 0.5433 |
| Work to Yield (mJ) | 2.0 ± 1.2 | 2.1 ± 1.3 | 1.7 ± 1.0 | 2.0 ± 1.2 | 0.6483 |
| Post-yield Work (mJ) | 7.4 ± 4.2 | 7.4 ± 3.6 | 11.0 ± 3.9 *# | 11.3 ± 4.2 *# | 0.0010 |
| Total Work (mJ) | 9.4 ± 4.2 | 9.5 ± 3.1 | 12.6 ± 3.6 *# | 13.3 ± 3.8 *# | 0.0008 |
| Yield Stress (MPa) | 55 ± 10 | 54 ± 19 | 48 ± 21 | 54 ± 20 | 0.6869 |
| Ultimate Stress (MPa) | 77 ± 10 | 83 ± 11 | 83 ± 11 | 88 ± 9 * | 0.0136 |
| Strain to Yield (με) | 14841 ± 5785 | 15549 ± 5548 | 12992 ± 3637 | 13756 ± 5117 | 0.4010 |
| Total Strain (με) | 41087 ± 15956 | 39714 ± 10518 | 49962 ± 14267 *# | 48146 ± 10511 # | 0.0343 |
| Modulus (GPa) | 4.73 ± 0.9 | 4.28 ± 1.0 | 4.39 ± 1.2 | 4.79 ± 1.0 | 0.3221 |
| Resilience (MPa) | 0.47 ± 0.27 | 0.48 ± 0.27 | 0.38 ± 0.23 | 0.45 ± 0.27 | 0.2871 |
| Toughness (MPa) | 2.25 ± 1.10 | 2.18 ± 0.76 | 2.84 ± 0.77 *# | 3.03 ± 0.86 *# | 0.0050 |
Data presented as means and standard deviations. VEH, vehicle; ZOL, zoledronate; RAL-L, raloxifene 0.5 mg/kg; RAL-H, raloxifene 2.0 mg/kg.
vs VEH;
vs ZOL.
DISCUSSION
The ability of bisphosphonates to significantly reduce fracture risk was a transformative milestone in the treatment of metabolic bone disease [1]. Although this drug class as a whole has a relatively high safety profile, there have emerged significant side effects (such as atypical femoral fracture and osteonecrosis of the jaw) that have been linked to treatment [27,28]. More specifically, the risk of these rare side effects is associated with treatment beyond 5–7 years. This has led to consideration of drug holidays where treatment is stopped for either a set period of time or until some biomarker (turnover markers or BMD) suggests treatment needs to be reinitiated [29]. A challenge to this approach is that bisphosphonates have long-lasting effects due to their binding to the skeleton, exemplified by data showing that after a single dose of zoledronate, bone turnover in patients is reduced for beyond 5 years [16–18]. In many cases this is a positive and suggests that drug holidays can be employed without worry of rapid remodeling recovery. In other cases, such as if there is concern about atypical fractures, simply stopping the bisphosphonate is unlikely to be sufficient to change the risk profile. It is this latter scenario that is the focus of the current work, which asked the question of whether or not dosing with raloxifene could benefit the mechanical properties of bone that had been previously treated with bisphosphonate.
Bending tests of long bones, the most direct way to experimentally assess how intervention affects mechanical integrity, is the modality of choice and yields nearly a dozen outcome variables [26]. These variables provide information on how much load the bone/material can handle (ultimate force/ultimate stress), the bone’s/material’s resistance to bending (stiffness/modulus) and the ability of the bone/material to deform prior to fracture (displacement/strain). Previous work has documented that although some properties are positively affected (such as ultimate load), other properties such as those related to displacement/energy absorption, are reduced following bisphosphonate treatment (alendronate, risedronate, and zoledronate) [8,9,11]. Recently, we extended these findings to a mouse model, documenting that 8 weeks of zoledronate treatment in C57/B6 male mice is sufficient to significantly reduce mechanical properties that are reflective of bone brittleness (total displacement, post-yield displacement, and total strain) [21]. The current study was designed to build on this concept yet ZOL-treatment did not significantly reduce total or post-yield displacement (−10% and −15%, respectively). Effects in the previous study were about twice these (−25% in total displacement) [21]. The reason for these differences between studies is not clear but may simply be a function of the high variability in displacement measures (specifically post-yield properties) from mechanical testing. It is also worth noting that we saw no difference between the two raloxifene doses indicating that more is not necessarily better in terms of enhancing mechanical properties.
Raloxifene is FDA approved for the treatment/prevention of osteoporosis but since its effects on bone mineral density are mild, its clinical use is modest [30]. Despite the minimal effect on BMD, raloxifene has been shown to significantly reduce vertebral fracture risk [31] raising questions as to how such modest BMD changes could reduce fracture risk [32]. Recently, our lab has shown that raloxifene significantly increases bone hydration and this is associated with improved mechanical properties, most notably post-yield parameters [19]. These effects have been noted both in vivo and in vitro, in mice, rats, dogs and humans [19,20,24,33–35]. Since at least some of the effects from raloxifene are not cell-mediated, we hypothesized that treatment with raloxifene could significantly benefit the mechanical properties of bone in conditions such as bisphosphonate-treatment where the tissue becomes brittle. The current work shows clear benefits of raloxifene on mechanical properties compared to ZOL-treatment alone. In addition, benefits of RAL were observed compared to ZOL-treatment in trabecular architecture. There were unexpected effects of RAL on body weight (most notably with the high dose) although there is no clear connection in the literature to such modest body weight differences explaining the effects on mechanical properties. Collectively, these results indicate that raloxifene has the ability to improve both bone mass and mechanics when administered following zoledronate therapy.
A growing number of studies have investigated combination treatment as an approach to treat bone disease [36]. These studies, both clinical and preclinical, are challenging to compare as they have utilized various combinations of agents in various orders (sequential or co-administration). Most studies have combined anti-resorptive (mainly bisphosphonates) and anabolic (mainly PTH) treatments due to their distinctly different mechanisms of action. One clinical study and a few preclinical studies have combined bisphosphonates with raloxifene, although they have done so as co-administration of drugs [37–39]. Results have mainly focused on BMD and biomarkers, with modest benefits of combination treatment. The two reports that have examined mechanics, however, both showed beneficial effects of co-administration compared to either monotherapy. Sequential therapy following bisphosphonate treatment has focused on PTH with the conceptual framework that stimulation of remodeling is going to be key to renewing the skeleton. Results are generally positive although the effects on mechanics have not been evaluated [40]. Our current results lend validity to a unique approach which is to target the existing bone matrix through non-cellular mechanisms (using Raloxifene which has both cellular and non-cellular effects) to make it more mechanically competent while at the same time keeping remodeling low (to preserve bone mass).
A key unanswered question in rodent models showing bisphosphonate-induced embrittlement is how these changes are occurring [21,41]. In humans and larger species (rabbits, dogs, non-human primates), changes in cortical bone mechanics are often attributed to the suppression of intracortical remodeling, resulting in the retention of older tissue that has higher mineralization, accumulation of collagen cross-links, and microdamage development [8]. The lack of normal intracortical remodeling in rodents makes this mechanism implausible. Rather, it suggests that bisphosphonates are having effects on the tissue independent of turnover suppression. We have previously documented that tissue-level BMD is not affected by zoledronate in this mouse model [21], leaving changes in collagen, or perhaps hydration, as potential aspects being directly affected by bisphosphonates.
There are several limitations to the current work. We utilized only one bisphosphonate, zoledronate, and thus do not know if the beneficial effects of raloxifene would occur following treatment with others in this drug class. We also used a single time point of assessment (8 weeks of dosing) and thus do not know if raloxifene could preserve mechanical effects following longer bisphosphonate dosing. Our study utilized male mice with normal bone mass, building on previous work in these mouse strains and, thus, future studies will need to focus on a more clinically relevant model such as ovariectomized female animals. Finally, we did not assess aspects of material properties (changes to collagen, mineral, hydration, etc.) which future work will need to do in order to understand the specific changes responsible for the mechanical effect.
In conclusion, the current work shows beneficial effects of raloxifene in animals previously treated with zoledronate. The higher ultimate load and displacement of raloxifene-treated animals, combined with similar cortical bone geometry compared to animals treated with zoledronate, suggest the raloxifene treatment is enhancing apparent mechanical material properties of the tissue.
Acknowledgments
This work was supported by NIH grants AR62002 (MRA), DK108554 (F32 support for EM), AR65971 (T32 support for MA) and AR067221 (JMW). The microCT utilized in this experiment was purchased through a NIH S10 grant (OD 016208).
Footnotes
Conflict of Interest statement
The authors declare that they have no conflicts of interest related to the current work.
References
- 1.Russell RGG. Bisphosphonates: the first 40 years. Bone. 2011;49:2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Eastell R, Walsh JS, Watts NB, Siris E. Bisphosphonates for postmenopausal osteoporosis. Bone. 2011;49:82–88. doi: 10.1016/j.bone.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Allen M, Iwata K, Phipps R, Burr D. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Allen MR, Burr DB. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. Journal of Oral and Maxillofacial Surgery. 2008;66:987–994. doi: 10.1016/j.joms.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen MR, Gineyts E, Leeming D, Burr DB, Delmas P. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2008;19:329–337. doi: 10.1007/s00198-007-0533-7. [DOI] [PubMed] [Google Scholar]
- 6.Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20:887–894. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gourion-Arsiquaud S, Allen M, Burr D, Vashishth D, Tang S, Boskey A. Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone. 2010;46:666–672. doi: 10.1016/j.bone.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen MR, Burr DB. Bisphosphonate effects on bone turnover, microdamage, and mechanical properties: what we think we know and what we know that we don’t know. Bone. 2011;49:56–65. doi: 10.1016/j.bone.2010.10.159. [DOI] [PubMed] [Google Scholar]
- 9.Acevedo C, Bale H, Gludovatz B, Wat A, Tang SY, Wang M, et al. Alendronate treatment alters bone tissues at multiple structural levels in healthy canine cortical bone. Bone. 2015;81:352–363. doi: 10.1016/j.bone.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Russell RGG, Watts N, Ebetino FH, Rogers M. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 11.Burr DB, Liu Z, Allen MR. Duration-dependent effects of clinically relevant oral alendronate doses on cortical bone toughness in beagle dogs. Bone. 2015;71:58–62. doi: 10.1016/j.bone.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen M, Burr D. Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res. 2007;22:1759–1765. doi: 10.1359/jbmr.070720. [DOI] [PubMed] [Google Scholar]
- 13.Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 14.Ettinger B, Burr DB, Ritchie RO. Proposed pathogenesis for atypical femoral fractures: lessons from materials research. Bone. 2013 doi: 10.1016/j.bone.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Allen MR. Skeletal accumulation of bisphosphonates: implications for osteoporosis treatment. Expert Opin Drug Metab Toxicol. 2008;4:1371–1378. doi: 10.1517/17425255.4.11.1371. [DOI] [PubMed] [Google Scholar]
- 16.Grey A, Bolland MJ, Horne A, Wattie D, House M, Gamble G, et al. Five years of anti-resorptive activity after a single dose of zoledronate — Results from a randomized double-blind placebo-controlled trial. Bone. 2012:1–5. doi: 10.1016/j.bone.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Reid IR, Lyles K, Su G, Brown JP, Walsh JP, del Pino-Montes J, et al. A single infusion of zoledronic acid produces sustained remissions in paget disease: Data to 6.5 years. J Bone Miner Res. 2011;26:2261–2270. doi: 10.1002/jbmr.438. [DOI] [PubMed] [Google Scholar]
- 18.Reid IR. Short-term and long-term effects of osteoporosis therapies. Nature Reviews Endocrinology. 2015:1–11. doi: 10.1038/nrendo.2015.71. [DOI] [PubMed] [Google Scholar]
- 19.Gallant MA, Brown DM, Hammond M, Wallace JM, Du J, Deymier-Black AC, et al. Bone cell-independent benefits of raloxifene on the skeleton: A novel mechanism for improving bone material properties. Bone. 2014;61:191–200. doi: 10.1016/j.bone.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bivi N, Hu H, Chavali B, Chalmers MJ, Reutter CT, Durst GL, et al. Structural features underlying raloxifene’s biophysical interaction with bone matrix. Bioorganic & Medicinal Chemistry. 2015:1–11. doi: 10.1016/j.bmc.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 21.Aref MW, McNerny EMB, Brown D, Jepsen KJ, Allen MR. Zoledronate treatment has different effects in mouse strains with contrasting baseline bone mechanical phenotypes. Osteoporos Int. 2016:1–9. doi: 10.1007/s00198-016-3701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jepsen KJ, Akkus OJ, Majeska RJ, Nadeau JH. Hierarchical relationship between bone traits and mechanical properties in inbred mice. Mamm Genome. 2003;14:97–104. doi: 10.1007/s00335-002-3045-y. [DOI] [PubMed] [Google Scholar]
- 23.Price C, Herman BC, Lufkin T, Goldman HM, Jepsen KJ. Genetic Variation in Bone Growth Patterns Defines Adult Mouse Bone Fragility. J Bone Miner Res. 2005;20:1983–1991. doi: 10.1359/JBMR.050707. [DOI] [PubMed] [Google Scholar]
- 24.Berman AG, Wallace JM, Bart ZR, Allen MR. Raloxifene reduces skeletal fractures in an animal model of osteogenesis imperfecta. Matrix Biology. 2015:1–26. doi: 10.1016/j.matbio.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 26.Jepsen KJ, Silva MJ, Vashishth D, Guo XE, van der Meulen MCH. Establishing Biomechanical Mechanisms in Mouse Models: Practical Guidelines for Systematically Evaluating Phenotypic Changes in the Diaphyses of Long Bones. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khosla S, Burr DB, Cauley J. Bisphosphonate-Associated Osteonecrosis of the Jaw: Report of a Task Force of the American Society for Bone and Mineral Research. Journal of Bone and …. 2007 doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 28.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the american society for bone and mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 29.Brown JP, Morin S, Leslie W, Papaioannou A, Cheung AM, Davison KS, et al. Bisphosphonates for treatment of osteoporosis: expected benefits, potential harms, and drug holidays. Can Fam Physician. 2014;60:324–333. [PMC free article] [PubMed] [Google Scholar]
- 30.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Jama. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 31.Marcus R, Wong M, Heath H, Stock JL. Antiresorptive treatment of postmenopausal osteoporosis: comparison of study designs and outcomes in large clinical trials with fracture as an endpoint. Endocrine Reviews. 2002;23:16–37. doi: 10.1210/edrv.23.1.0453. [DOI] [PubMed] [Google Scholar]
- 32.Riggs B, Melton L., III Bone turnover matters: the raloxifene treatment paradox of dramatic decreases in vertebral fractures without commensurate increases in bone density. J Bone Miner Res. 2002;17:11–14. doi: 10.1359/jbmr.2002.17.1.11. [DOI] [PubMed] [Google Scholar]
- 33.Newman CL, Creecy A, Granke M, Nyman JS, Tian N, Hammond MA, et al. Raloxifene improves skeletal properties in an animal model of cystic chronic kidney disease. Kidney Int. 2016;89:95–104. doi: 10.1038/ki.2015.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen MR, Territo PR, Lin C, Persohn S, Jiang L, Riley AA, et al. In Vivo UTE-MRI Reveals Positive Effects of Raloxifene on Skeletal-Bound Water in Skeletally Mature Beagle Dogs. J Bone Miner Res. 2015;30:1441–1444. doi: 10.1002/jbmr.2470. [DOI] [PubMed] [Google Scholar]
- 35.Aref M, Gallant MA, Organ JM, Wallace JM, Newman CL, Burr DB, et al. In vivo reference point indentation reveals positive effects of raloxifene on mechanical properties following 6 months of treatment in skeletally mature beagle dogs. Bone. 2013;56:449–453. doi: 10.1016/j.bone.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosman F. Combination therapy for osteoporosis: a reappraisal. BoneKEy Reports. 2014;3:1–8. doi: 10.1038/bonekey.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnell O. Additive Effects of Raloxifene and Alendronate on Bone Density and Biochemical Markers of Bone Remodeling in Postmenopausal Women with Osteoporosis. Journal of Clinical Endocrinology & Metabolism. 2002;87:985–992. doi: 10.1210/jcem.87.3.8325. [DOI] [PubMed] [Google Scholar]
- 38.Diab T, Wang J, Reinwald S. Effects of the combination treatment of raloxifene and alendronate on the biomechanical properties of vertebral bone. Journal of Bone and …. 2011 doi: 10.1002/jbmr.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amugongo SK, Yao W, Jia J, Dai W, Lay Y-AE, Jiang L, et al. Effect of sequential treatments with alendronate, parathyroid hormone (1–34) and raloxifene on cortical bone mass and strength in ovariectomized rats. Bone. 2014 doi: 10.1016/j.bone.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ettinger B, Martin SJ, Crans G, Pavo I. Differential effects of Teriparatide on BMD After Treatment With Raloxifene or Alendronate. J Bone Miner Res. 2004;19:745–751. doi: 10.1359/JBMR.040117. [DOI] [PubMed] [Google Scholar]
- 41.Smith ER, Allen MR. Bisphosphonate-induced reductions in rat femoral bone energy absorption and toughness are testing rate-dependent. J Orthop Res. 2013;31:1317–1322. doi: 10.1002/jor.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]