Abstract
CTLA-4, PD-1, and PD-L1 monoclonal antibodies, commonly known as immune checkpoint inhibitors, are used for the treatment of various malignancies. The mechanism of action involves the inhibition of negative regulators of immune activation, which results in many patients developing immune-related adverse events (irAEs) including endocrinopathies, pneumonitis, colitis, hepatitis and dermatologic events. Dermatologic irAEs include maculopapular rash, pruritus, vitiligo, blistering disorders, mucocutaneous lichenoid eruptions, rosacea, and exacerbation of psoriasis. Alopecia secondary to immune checkpoint inhibitors has been reported in 1.0-1.6% of patients. We characterize four cases of alopecia areata (AA) secondary to immune checkpoint inhibitors with images, histology, and concomitant nail findings, including the first report of anti-PD-L1 therapy induced AA, and a review of the literature. Patients treated with immune checkpoint inhibitors, singly or in combination, who developed partial or complete alopecia (areata and universalis-type) during treatment for their underlying cancer were analyzed (N=4). Three (75%) patients had AA, while one (25%) had universalis-type. Two patients had resolution after topical, oral, or intralesional therapies and one patient had resolution after discontinuation of immunotherapy; all regrown hair exhibited poliosis. One (25%) patient had coincident onychodystrophy. This report describes a series of four patients who developed partial or complete alopecia (i.e. areata and universalis-type) during treatment with immune-checkpoint inhibitor therapies for cancer. Recognition and management of hair-related irAEs are important for pretherapy counseling and interventions that would contribute to maintaining optimal health-related quality of life.
Keywords: alopecia areata, CTLA-4, PD-1, PD-L1, VEGF
INTRODUCTION
Immune checkpoint inhibitors in oncology result in distinct adverse events (AEs) compared to cytotoxic chemotherapy or targeted therapies. These novel agents consist of monoclonal antibodies (mABs) that target immune ‘checkpoints’ including the cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) and the programmed cell death protein (PD-1) receptors and its ligand (PD-L1). Given their activity in durable responses, they are being explored for a growing number of solid and hematologic malignancies.
The mechanism of action involves the inhibition of negative regulators of immune activation, which results in many patients developing immune-related AEs (irAEs) including endocrinopathies, pneumonitis, colitis, hepatitis and dermatologic events.1 The pathophysiology of dermatologic irAEs is unknown, but it is hypothesized that reactivated CD4+/CD8+ T cells target unidentified cutaneous antigens resulting in an inflammatory process after cross-reacting with normal antigens.2 Dermatologic irAEs include maculopapular rash, pruritus, vitiligo, blistering disorders, mucocutaneous lichenoid eruptions, rosacea, and exacerbation of psoriasis.2-4 Alopecia is a known side effect of PD-1 receptor inhibitors and anti-CTLA-4 agents with a prevalence of 1.0-1.6%; however, it has not been reported with PD-L1 inhibitors nor been characterized with images, histology, and concomitant nail findings.5-8 We report a case series of four patients who developed partial or complete alopecia after treatment with immune checkpoint inhibitors for solid tumors and a review of the literature.
CASE SERIES
Case 1
A 67-year-old female diagnosed with BRAF-mutant metastatic melanoma received two cycles of combination immunotherapy with CTLA-4 and PD-1 receptor inhibitors. Complications included grade 3 colitis requiring multiple hospitalizations and treatment with steroids and infliximab, of which two doses were given with the last dose being 6 months prior to the onset of alopecia; immunotherapy was stopped after colitis. The patient was referred to dermatology for alopecia. Examination of the scalp revealed well-defined areas of alopecia grade 1. Laboratory investigations were remarkable for mild anemia and an elevated ESR, hypervitaminoses (B12, E) and markedly elevated levels of manganese. A biopsy obtained from a patch on the posterior scalp revealed a non-scarring alopecia with peri-infundibular lymphocytic infiltrate (Figure 1A). The patient was started on clobetasol foam, biotin, and orthosilicic acid. At the follow-up visit 2 months later, there was regrowth with poliosis in the alopecic areas (Figure 2A).
Figure 1. Histology.

A. Case 1. Photomicrograph of a section of the posterior scalp biopsy showing a perifollicular (predominantly peri-infundibular) lymphocytic infiltrate with ‘nanogen’ hair and residual fibrovascular tracts (follicular streamers/stellae), and mild mucin deposition; terminal:vellus (T:V) ratio = 1.2 [hematoxylin and eosin (H&E), 100x objective].
B. Case 3. Sparse peribulbar lymphocytic infiltrate.
Figure 2. Clinical images of alopecia areata.
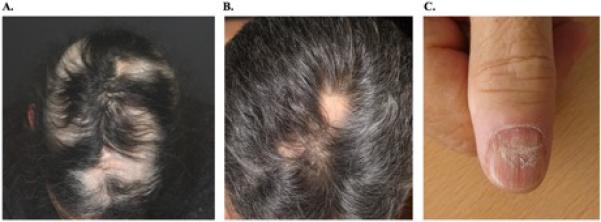
A. Case 1. Regrowth with poliosis on follow-up after 2 months.
B. Case 3. Focal areas of patchy hair loss in the mid-scalp region.
C. Case 4. Superficial proximal onychoschizia associated with Beau's lines of the proximal nails.
Case 2
A 65-year-old female diagnosed with wild type-BRAF metastatic melanoma was treated with CTLA-4 and PD-1 receptor inhibitors with partial response. The immunotherapy was discontinued after four cycles secondary to grade 3 transaminitis. The patient developed alopecia of the scalp 3 months after the last cycle. Examination of the scalp revealed diffuse hair thinning, most prominent in a focal area of poliosis on the vertex. Laboratory investigations were remarkable for mild anemia and low T4 levels. A scalp biopsy obtained from the area revealed a non-scarring, inflammatory pattern of alopecia. Follow-up revealed improvement in alopecia but persistent poliosis.
Case 3
A 61-year-old male diagnosed with clear cell renal cell carcinoma metastatic to the lungs, liver, and pancreas received a vascular endothelial growth factor inhibitor and a PD-L1 inhibitor. After 10 cycles, several round and well-defined areas of alopecia were appreciated on the scalp (Figure 2B). Other adverse events included pruritus and elevated hemoglobin and hematocrit levels. A biopsy obtained from an alopecic patch on the posterior scalp revealed non-scarring alopecia with mild, perifollicular, perivascular, and periadnexal lymphocytic infiltrate (Figure 1B). Intralesional triamcinolone was administered, and topical clobetasol spray daily was prescribed. At follow-up 7 weeks later, there was regrowth in all areas with poliosis.
Case 4
A 62-year-old male with renal cell carcinoma metastatic to the lungs was treated with CTLA-4 and PD-1 receptor inhibitors. Complications included diabetes mellitus and grade 1 rash of the lower limbs. The patient developed alopecia of the scalp and body, along with tender nails changes after the third cycle. Examination revealed alopecia universalis and superficial proximal onychoschizia associated with Beau's lines of the proximal nails of all 10 fingernails along with tenderness (Figure 2C). The patient is maintained on immunotherapy with persistent alopecia and nail changes after 16 cycles.
DISCUSSION
Alopecia is a side effect of CTLA-4 and PD-1 receptor inhibitors with an incidence of 1.0-1.6%, yet no analysis has been dedicated to its characterization with images, histology, and concomitant nail findings nor has it been documented in PD-L1 inhibitors.5-8 CTLA-4 inhibitor induced alopecia was first documented in 2006, whereupon a patient developed nonscarring alopecia of the scalp, eyebrows, face, pubic region, and trunk (incidence of 1.6%; N= 63). Biopsy of the scalp revealed predominant CD4+ T cells and scant CD8+ T cells, consistent with idiopathic AA.5 The phase one study of PD-1 inhibitor nivolumab in 2012 reported 3 patients with alopecia, not otherwise specified (incidence of 1.0%; N= 296).6 A case report in 2013 described one patient who developed alopecia universalis, widespread vitiligo, and hypophysitis secondary to CTLA-4 inhibitor, ipilimumab.7 A retrospective review of PD-1 inhibitor side effects revealed 7 patients with alopecia not otherwise specified, ranging from grade 1 alopecia, loss of eyelashes, to decreased growth of body hair (incidence of 1.4%; N= 496).8
Alopecia areata is a benign autoimmune dermatologic disorder occurring in approximately 1.7% of the US population. It presents with well-circumscribed areas of alopecia affecting hair-bearing areas, most often the scalp. AA may be associated with several other autoimmune diseases such as vitiligo, diabetes, and thyroid disease, while a family history may exist in 20% of patients.9 While the diagnosis of AA is generally clinical and the histopathological findings can be subtle/equivocal, both the clinical presentation and scalp biopsies in our cases were consistent with AA. In addition, hair regrowth in the alopecic areas manifested with poliosis (Cases 1-3), is a relatively well-recognized feature of AA.9 TNF-alpha inhibitors have been associated with alopecia in patients receiving chronic therapy; however, Case 1 presented here received only two doses of infliximab.10 The clinico-morphological pattern and the time of onset, a few months after initiation of immunotherapy, suggest a temporal relationship between the drug and the development of AA.
The histopathology of the acute phase is generally significant for a dense peribulbar lymphocytic inflammation.11 The underlying pathophysiology of AA is unclear, but a CD4+/CD8+ mediated process against the hair follicle12 and the presence of specific IgG autoantibodies in the peripheral blood13 corroborate an autoimmune hypothesis. Additionally, the hair follicle dermal sheath cup cell expresses PD-L1, whereby PD-1 inhibitors may directly induce AA.14 A severe combined immunodeficiency mouse model revealed melanocyte antigens Gp100-derived G9-209, G9-280, and MART-1 (27-35) have been shown to function as the target antigens in experimental AA, raising the possibility of melanocyte-specific cytotoxic T cells in melanoma patients.15 It could be hypothesized that immune checkpoint inhibitors lead to an activation of inflammatory responses causing exposure of hair follicle antigens, such as PD-L1 and melanocyte antigens, in addition to creating an imbalance between the immune system and self-tolerance in susceptible individuals.
With expanding use of immune checkpoint inhibitors, health-care providers must adequately evaluate unique forms of hair loss for underlying pathology, besides the routine chemotherapy-induced alopecia. We recommend clinical evaluation of the scalp, hair pull test, scalp biopsy, and laboratory testing for other causes of alopecia (thyroid dysfunction and deficiencies of zinc, vitamin D, and ferritin). The timely recognition and institution of early dermatologic intervention for alopecia areata are vital to prevent progression, more extensive involvement, and maintain optimal health-related quality of life in patients.
What's already known about this topic?
- Immune checkpoint inhibitors have various immune-related adverse events, including alopecia with an incidence ranging from 1.0 to 1.6%
- The clinical, histologic, associated findings and response to therapy of immune checkpoint inhibitor induced alopecia has not previously been characterized
What does this study add?
- Immune checkpoint inhibitors may be associated with alopecia, with clinical and histologic findings consistent with alopecia areata
- Knowledge and recognition of this type of alopecia is critical for counseling and early intervention, in order maintain optimal health-related quality of life
Acknowledgment
We are indebted to Ms. Bernadette Murphy for the clinical photography.
Funding: This study was supported in part by the NIH/NCI Cancer Center Support Grant P20 CA008748 and the RJR Oncodermatology Fund at Memorial Sloan Kettering Cancer Center. Funding/Sponsors were not involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
ABBREVIATIONS
- AE
adverse event
- AA
alopecia areata
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4
- irAE(s)
immune-related adverse event(s)
- mAb
monoclonal antibody
- PD-1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
Footnotes
Conflicts of Interest Disclosures: Lacouture has consulting agreements with Dignitana and Paxman, and has received research funding from Berg. Postow has had a consulting or advisory role with Amgen and Bristol-Meyers Squibb, and receives research support from Bristol-Meyers Squibb and Novartis (Inst). Sibaud has had a speaking, consultant, or advisory role with Roche, Novartis, GlaxoSmithKline, Pierre Fabre, Merck, Bristol-Myers Squibb, Bayer and Boehringer Ingelheim. Hsieh received consulting fees from Eisai, Chugai, and Novartis; and Research Funding from Novartis, Eisai, CGI, and Pfizer. Motzer has received consulting fees from Pfizer, Novartis and Eisai; and Research funding to Hospital from Pfizer, Novartis, Genentech, BMS, and Eisai.
REFERENCES
- 1.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–91. doi: 10.1093/annonc/mdv383. DOI: 10.1093/annonc/mdw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28(4):254–63. doi: 10.1097/CCO.0000000000000290. DOI: 10.1097/cco.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 3.Naidoo J, Schindler K, Querfeld C, et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383–9. doi: 10.1158/2326-6066.CIR-15-0123. DOI: 10.1158/2326-6066.cir-15-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. DOI: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaber SH, Cowen EW, Haworth LR, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142(2):166–72. doi: 10.1001/archderm.142.2.166. DOI: 10.1001/archderm.142.2.166. [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. DOI: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assi H, Wilson KS. Immune toxicities and long remission duration after ipilimumab therapy for metastatic melanoma: two illustrative cases. Curr Oncol. 2013;20(2):e165–9. doi: 10.3747/co.20.1265. DOI: 10.3747/co.20.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. DOI: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366(16):1515–25. doi: 10.1056/NEJMra1103442. DOI: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- 10.Bene J, Moulis G, Auffret M, et al. Alopecia induced by tumour necrosis factor-alpha antagonists: description of 52 cases and disproportionality analysis in a nationwide pharmacovigilance database. Rheumatology (Oxford) 2014;53(8):1465–9. doi: 10.1093/rheumatology/keu145. DOI: 10.1093/rheumatology/keu145. [DOI] [PubMed] [Google Scholar]
- 11.Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139(12):1555–9. doi: 10.1001/archderm.139.12.1555. DOI: 10.1001/archderm.139.12.1555. [DOI] [PubMed] [Google Scholar]
- 12.Paus R, Slominski A, Czarnetzki BM. Is alopecia areata an autoimmune-response against melanogenesis-related proteins, exposed by abnormal MHC class I expression in the anagen hair bulb? Yale J Biol Med. 1993;66(6):541–54. [PMC free article] [PubMed] [Google Scholar]
- 13.Lu W, Shapiro J, Yu M, et al. Alopecia areata: pathogenesis and potential for therapy. Expert Rev Mol Med. 2006;8(14):1–19. doi: 10.1017/S146239940601101X. DOI: 10.1017/s146239940601101x. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Marr AK, Breitkopf T, et al. Hair follicle mesenchyme-associated PD-L1 regulates T-cell activation induced apoptosis: a potential mechanism of immune privilege. J Invest Dermatol. 2014;134(3):736–45. doi: 10.1038/jid.2013.368. DOI: 10.1038/jid.2013.368. [DOI] [PubMed] [Google Scholar]
- 15.Gilhar A, Landau M, Assy B, et al. Melanocyte-associated T cell epitopes can function as autoantigens for transfer of alopecia areata to human scalp explants on Prkdc(scid) mice. J Invest Dermatol. 2001;117(6):1357–62. doi: 10.1046/j.0022-202x.2001.01583.x. DOI: 10.1046/j.0022-202x.2001.01583.x. [DOI] [PubMed] [Google Scholar]


