Summary:
Keloids are a manifestation of a fibroproliferative scarring disorder of the skin and develop in response to dermal injury in patients with a susceptible background. Local, systemic, and genetic factors contribute to keloid susceptibility. These factors include tension on the edges of the wound, hormonal influences, and ethnicity, respectively. Castleman’s disease is a rare lymphoproliferative disorder that is characterized by the unregulated overproduction of interleukin-6, which leads to systemic lymphadenopathy and constitutional inflammatory symptoms. This case report shows that the bilateral auricular keloids of an adult woman were greatly exacerbated by the onset of Castleman’s disease. We present our multimodal management algorithm for auricular keloids, which involves core excision and radiation therapy and achieves excellent aesthetic outcomes. The current treatment pathway for auricular keloids and the possible relationship between interleukin-6 and keloid progression will be discussed.
Keloids are unpleasant scars that are the result of an intrinsically dysfunctional healing process that commences after dermal injury.1 The formation of these pathological scars involves chronic inflammation of the reticular dermis that is characterized histologically by accelerated angiogenesis and excessive collagen deposition.1 Many local, systemic, and genetic factors are associated with the development and progression of keloids. The local factors include mechanical stretch tension on the wound edges and repeated irritation of the wound. The systemic factors include increased levels of circulating estrogen and androgens and hypertension. The genetic factors include certain single nucleotide polymorphisms and an Asian or Afro-Caribbean genetic background.1,2 Given that keloids are caused by persistent inflammation, it is highly likely that circulatory inflammatory mediators may shape keloid pathogenesis and progression.
Castleman’s disease (also known as angiofollicular lymph node dysplasia) was first described in 1954. It is a group of rare lymphoproliferative disorders.3 It is characterized by distinctive architectural anomalies in nodal compartments that are also seen in autoimmune conditions, including germinal center hyperplasia, immunoblast accumulation, and hypervascularity.4 Strikingly, these lymph nodes demonstrate an isolated overproduction of interleukin-6 (IL-6). The serum levels of this circulating cytokine correlate with symptom severity and the degree of lymphadenopathy.4 Because various lines of evidence show that IL-6 may play an important role in keloid pathogenesis and progression, it is possible that the systemic inflammation in Castleman’s disease exacerbates keloid scars.
Here, we show for the first time that the onset of Castleman’s disease greatly exacerbated the auricular keloids of an adult patient. We also describe the treatment approach that we adopted with this patient. Because it continues to be difficult to treat keloids effectively, and this patient was at particular risk of recurrence because of her high circulating levels of IL-6, we used a multifaceted and structured approach to treat her auricular keloids, namely, core excision followed by radiotherapy. This strategy achieved an excellent outcome. The potential implications of this case in terms of future directions in keloid research will be discussed.
CASE
Shortly after being diagnosed with Castleman’s disease, a 37-year-old Japanese woman with a history of keloid scarring was referred to our specialist service because of worsening of the bilateral keloids on her ears. These unusually severe keloids involved large portions of the posterolateral components of both the cartilaginous and lobular auricle. At the first examination, it was unclear whether the lesions consisted of keloids alone or whether the keloids were combined with lymphoma (Figs. 1, 2). The patient said that before the onset of Castleman’s disease, the keloids were small and localized to the earlobes. She had first developed these keloids at the age of 18 years after having her ears pierced.
Fig. 1.
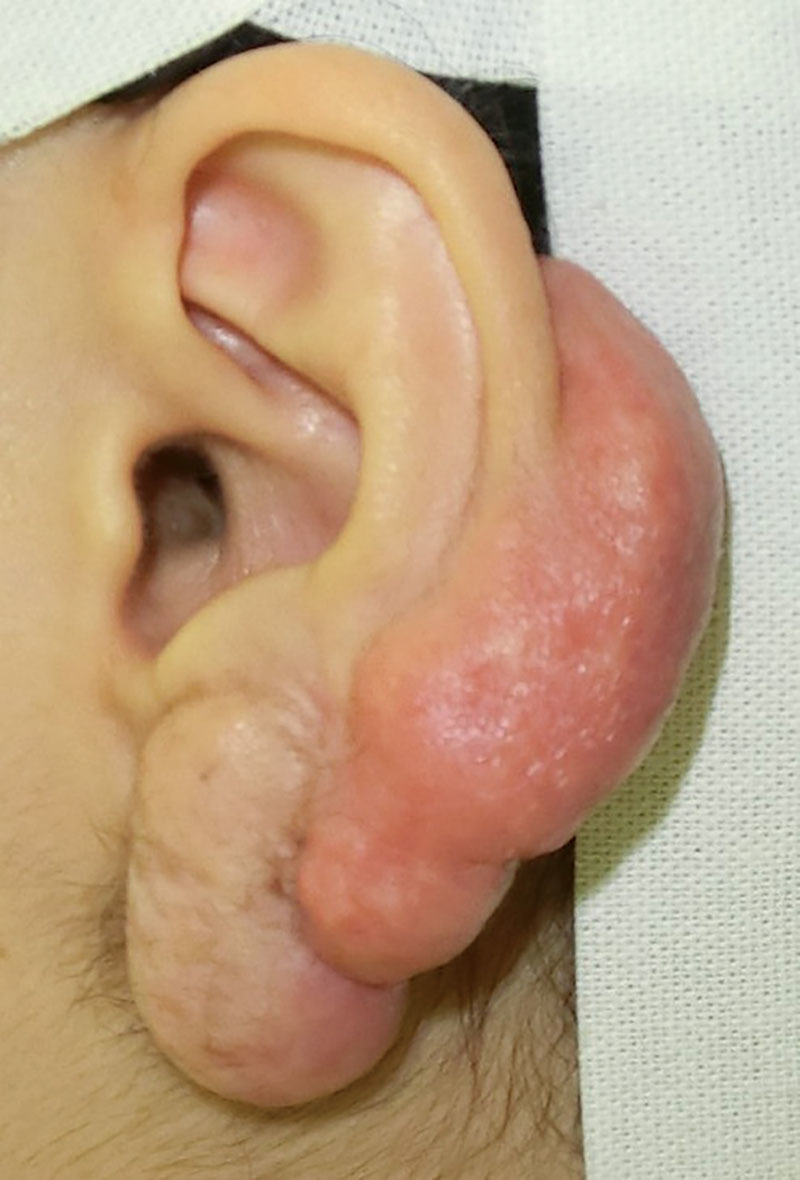
A preoperative view of the anterior ear. The keloid involved large portions of the posterolateral components of both the cartilaginous and lobular auricle.
Fig. 2.
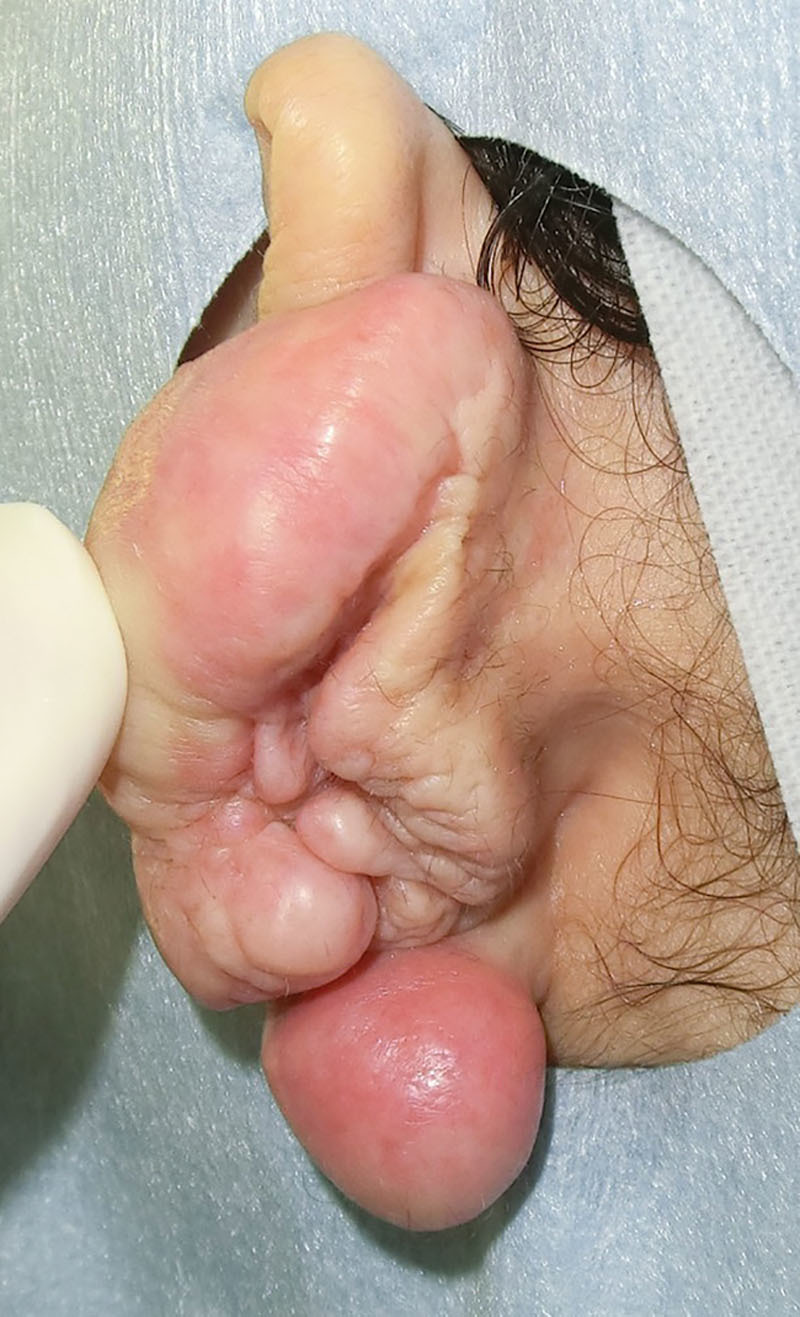
A preoperative view of the postauricular sulcus. The border of the keloid and normal skin were ill-defined.
The patient was diagnosed with multicentric type Castleman’s disease by lymph node biopsy at the age of 29 years. At that time, enlarged lymph nodes were detected in her neck, mediastinum, abdomen, and groin, and her serum levels of IL-6 were higher than the normal range (7.9 pg/mL). She was treated with systemic administration of steroid, but the treatment did not improve her keloids. Therefore, the patient sought more effective treatments and came to our service.
We removed the auricular keloids under general anesthesia by a core excision method, where the epidermis and papillary dermis are preserved and only the pathological hypertrophic reticular dermis is removed (Fig. 3). The retained skin was sutured to the wound bed with 6-0 polypropylene sutures that remained in place for 10 days. On postoperative days 1, 2, and 3, the patient received a total radiation dose of 15 Gy in 3 fractions over 3 days. The radiation was delivered by a 4 MeV electron beam.
Fig. 3.
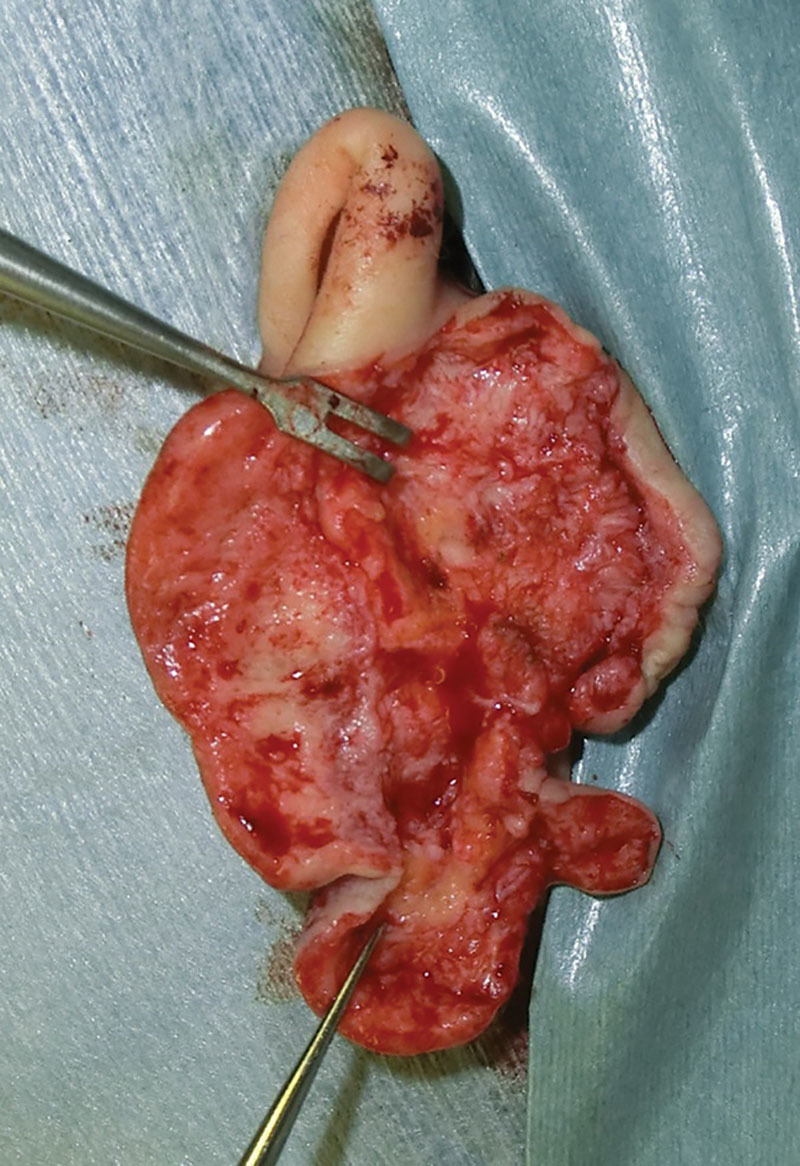
An intraoperative view. Only the pathological hypertrophic reticular dermis was resected.
Over the next 6 months, the patient applied fixation tapes on her ears to prevent friction from the bedding during sleep. Three years have passed since the surgery, which appears to have been curative as there are no signs of recurrence (Fig. 4).
Fig. 4.
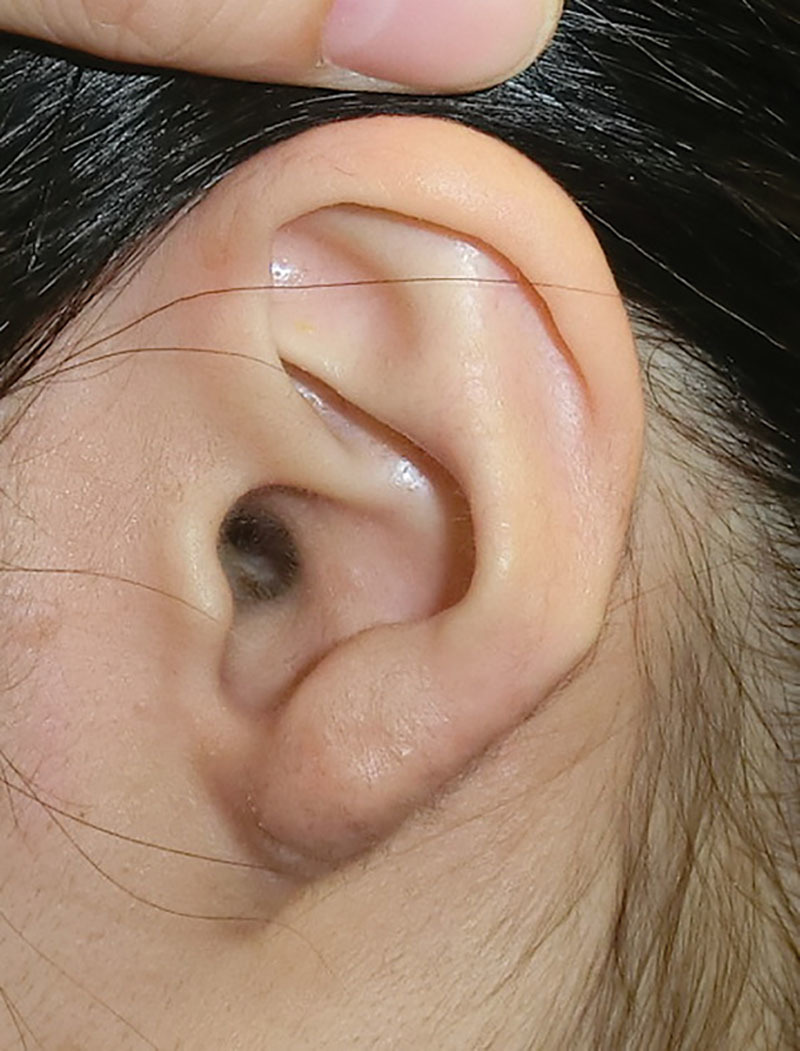
A postoperative view 3 years after surgery and radiation therapy. There was no recurrence of pathologic scarring.
The histopathological examination of the resected tissues showed the absence of abnormal lymphocytes or plasma cell infiltration. Consequently, the auricular lesions were diagnosed definitively as keloids.
DISCUSSION
This adult woman exhibited marked exacerbation of her auricular keloids at the onset of Castleman’s disease. We achieved pleasing results by using the core excision procedure, postoperative radiotherapy, and fixation tape. We have described this protocol previously and recommend it as a preferred approach due to its effectiveness.5–7 The ability of postoperative radiotherapy to prevent recurrence has also been supported by many studies.8,9 Although it could be suggested that the benefits of postoperative radiotherapy may be offset by a possible risk of carcinogenesis, this risk has not yet been proven to exist. Thus, this therapeutic strategy is promising, especially, given its pleasing outcome in even a patient whose keloids were complicated by the presence of Castleman’s disease.
This is the first time that the onset of the rare lymphoproliferative disorder that is Castleman’s disease has been shown to associate with keloid progression. This finding is not entirely surprising, given that Castleman’s disease results in a proinflammatory systemic state due to high serum levels of IL-6 and keloids appear to be driven by persistent inflammation. Moreover, there are numerous lines of evidence showing that IL-6, which is a potent immunomodulator, is a common causative factor of keloid pathogenesis. In particular, the stem cells in keloids exhibit features of benign tumor cells, namely, uncontrolled self-renewal and proliferation, and these features are driven by IL-6 via an autocrine/paracrine axis.10 This dysregulation of growth and persistent expansion are key characteristics of keloids. Moreover, black patients with keloids exhibit significantly higher circulating levels of IL-6 than black control subjects.11 In addition, a single nucleotide polymorphism in the gene encoding IL-6 associates with susceptibility to keloid formation and the development of severe keloids.
Although keloid pathogenesis is due to multifactorial interactions between local, systemic, and genetic factors, the studies described above and the current case indicate that IL-6 may play a key role in this interplay. The precise mechanism is unclear. However, it is known that immediately after dermal injury, fibroblasts express IL-6, which then recruits inflammatory cells and stimulates collagen deposition.12 Moreover, although mature scars bear low levels of IL-6, keloid fibroblasts continue to express IL-6 and its receptors moderately to strongly long after the initial injury.12 The reasons for this are unclear. Further research into how IL-6 shapes keloid development and progression is warranted because this information may aid the development of IL-6 antagonistic drugs that may prevent, ameliorate, or cure keloids.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Ogawa R, Okai K, Tokumura F, et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20:149–157.. [DOI] [PubMed] [Google Scholar]
- 2.Huang C, Murphy GF, Akaishi S, et al. Keloids and hypertrophic scars: update and future directions. Plast Reconstr Surg Glob Open. 2013;1:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dham A, Peterson BA. Castleman disease. Curr Opin Hematol. 2007;14:354–359.. [DOI] [PubMed] [Google Scholar]
- 4.Paratore DA, Herts BR. Castleman disease. J Urol. 2015;194:529–530.. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa R, Akaishi S, Dohi T, et al. Analysis of the surgical treatments of 63 keloids on the cartilaginous part of the auricle: effectiveness of the core excision method. Plast Reconstr Surg. 2015;135:868–875.. [DOI] [PubMed] [Google Scholar]
- 6.Ragoowansi R, Cornes PG, Glees JP, et al. Ear-lobe keloids: treatment by a protocol of surgical excision and immediate postoperative adjuvant radiotherapy. Br J Plast Surg. 2001;54:504–508.. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa R, Akaishi S, Kuribayashi S, et al. Keloids and hypertrophic scars can now be cured completely: recent progress in our understanding of the pathogenesis of keloids and hypertrophic scars and the most promising current therapeutic strategy. J Nippon Med Sch. 2016;83:46–53.. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa R, Yoshitatsu S, Yoshida K, et al. Is radiation therapy for keloids acceptable? The risk of radiation-induced carcinogenesis. Plast Reconstr Surg. 2009;124:1196–1201.. [DOI] [PubMed] [Google Scholar]
- 9.Caccialanza M, Piccinno R, Schiera A. Postoperative radiotherapy of keloids: a twenty-year experience. Eur J Dermatol. 2002;12:58–62.. [PubMed] [Google Scholar]
- 10.Zhang Q, Yamaza T, Kelly AP, et al. Tumor-like stem cells derived from human keloid are governed by the inflammatory niche driven by IL-17/IL-6 axis. PLoS One. 2009;4:e7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCauley RL, Chopra V, Li YY, et al. Altered cytokine production in black patients with keloids. J Clin Immunol. 1992;12:300–308.. [DOI] [PubMed] [Google Scholar]
- 12.Murao N, Seino K, Hayashi T, et al. Treg-enriched CD4+ T cells attenuate collagen synthesis in keloid fibroblasts. Exp Dermatol. 2014;23:266–271.. [DOI] [PubMed] [Google Scholar]


