Supplemental Digital Content is available in the text.
Abstract
Background:
Delayed or even lack of healing of a split-thickness skin graft (STSG) donor site is a potential problem with elderly patients or those with poor wound healing capabilities. A proactive solution that may minimize this risk is to regraft that donor site using otherwise discarded skin graft remnants.
Methods:
A prospective, nonrandomized, consecutive study was designed to compare the time to healing of the commonly used anterior thigh STSG donor site in patients who had routine dressings (n = 113) versus those with comorbidities known to adversely affect wound healing and had planned regrafting (n = 204). Those comorbidities included age (≥65 years), diabetes mellitus, peripheral vascular disease, chronic renal disease, and chronic steroid use.
Results:
The average number of comorbidities in the regrafted subgroup versus those not regrafted was 1.41 and 0.31, respectively. This was considered to be a significant difference (P < 0.0001) confirming the validity in predicting patients at risk for adverse donor-site healing that would benefit by regrafting. The mean time required for donor-site reepithelialization of those regrafted was 17.2 days compared with 17.8 days for those not regrafted (P = 0.2395), which was not significantly different.
Conclusions:
Regrafting the STSG donor site of patients with known comorbidities, that is, those expected to have delayed healing in general, had a mean time to reepithelialization comparable with conventionally treated individuals. This was considered a direct consequence of recycling rather than discarding any excess skin graft materials when so indicated and can be a proactive solution to a potentially cumbersome dilemma.
INTRODUCTION
Despite the ubiquitous use of autogenous split-thickness skin grafts (STSG) to successfully treat a variety of wounds, the secondary wound created at the donor site can become a problem often not anticipated. Although healthy patients heal their STSG donor site quickly without incident, this is not always true for patients who have comorbidities that in general affect wound healing. The elderly individual, or those with diabetes mellitus, peripheral vascular disease (venous or arterial), or if immunocompromised can all then be afflicted by the creation of a second festering wound if donor-site reepithelialization were to be impaired.1,2
The plethora of available STSG donor-site treatment options corroborates the fact that a perfect solution for all individuals does not yet exist. These range from vendor manufactured materials [e.g., Xeroform (Covidien, Mansfield, Mass.) or Kaltostat (Convatec, Skillman, N.J.)],3–8 innovative approaches (cultured epithelial grafts,2 noncontact low-frequency ultrasound9), or even the peculiar (aluminum foil, banana leaves, a free flap).10–12 Donor-site location may be just as important a factor, as it is well known that the scalp has an accelerated healing time due to the number of epidermal appendages present that promote reepithelialization.13 On the contrary, systemic comorbidities should be expected to negatively impact any donor-site healing.14
The ideal STSG donor-site dressing must be readily available, economical, hemostatic, not immunogenic, antibacterial, and still able to promote optimum reepithelialization.15–19
Because the best STSG recipient site is obviously its donor site, as a corollary, the autograft would rightfully be its best potential dressing. Fatah and Ward previously showed in a prospective study that advanced age alone delays wound healing when patients are not regrafted.1,20 Our larger prospective study was intended to confirm the benefit of regrafting the STSG donor site in not only the elderly but also those patients where wound healing would be anticipated to be suboptimal.
METHODS
A long-term prospective consecutive patient study from October 2007 through March 2011 of all individuals (n = 317) who had anterior thigh STSG donor sites as part of any reconstructive surgery by the senior author (G.G.H.) were included. There were 144 females and 173 males with an average age of 62.7 (range, 12–96 years). Of these, 37 patients had more than 1 STSG procedure during the 3.5-year study period. However, only their initial surgical event was included to eliminate any later donor-site treatment bias.
The study population was nonrandomized into 2 groups. Before the harvest of an STSG, if it was known or if there was a concern that a patient was at risk for poor donor-site wound healing, the operative plan was often to consider taking a larger amount than necessary but always to replace any leftover meshed STSG remnants back on the donor site (see video, Supplemental Digital Content 1, which summarizes how to use skin graft remnants to expedite skin graft donor-site healing. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A441). Otherwise, all remaining patients were treated with donor-site coverage by Xeroform or Kaltostat according to hospital availability (Table 1).
Table 1.
Summary of Operative Data
Video Graphic 1.
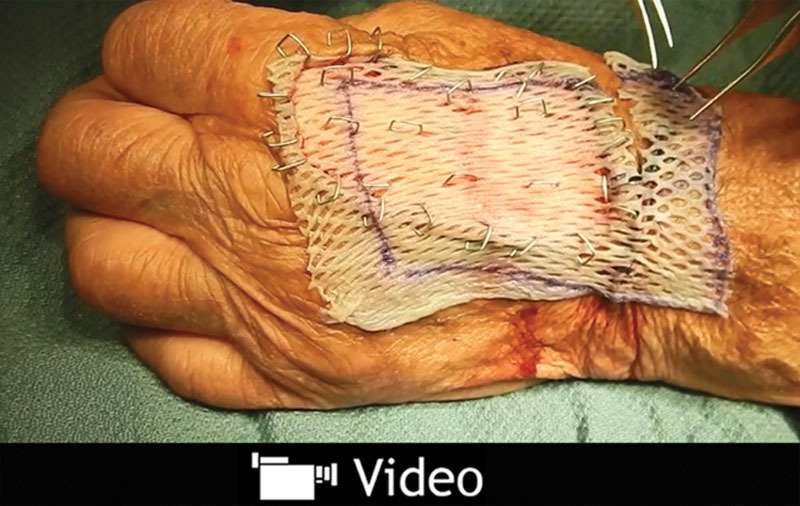
See video, Supplemental Digital Content 1, which summarizes how to use skin graft remnants to expedite skin graft donor-site healing. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A441.
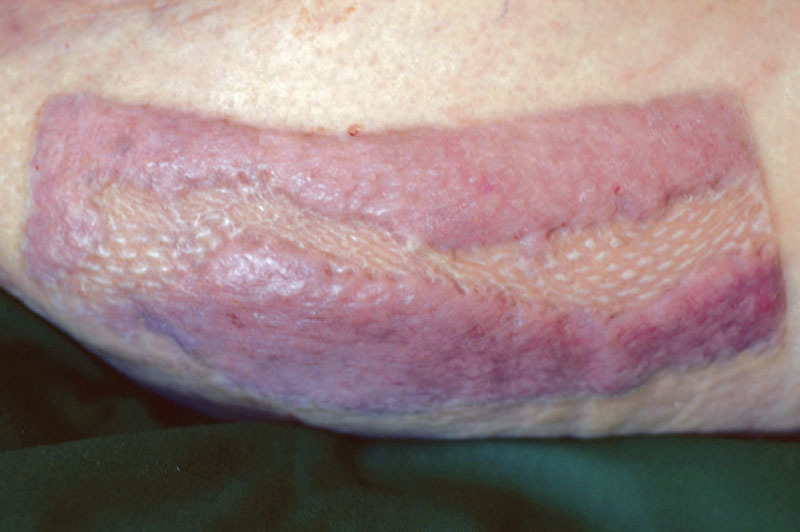
All STSG were harvested from the anterior upper thigh using a standard motorized dermatome. Typical graft thickness was 14,000ths of an inch (14:1,000). Most grafts were meshed 1½:1. Any remaining skin graft, if so chosen to be an advantage, was returned to the donor site and secured with staples (Fig. 1 and Supplemental Digital Content 1). Postoperative donor-site healing was then evaluated at office visits, usually no more than once or twice weekly. A healed donor site was defined as that that had completely reepithelialized.
Fig. 1.
Technique of scattered replacement of STSG remnants in the center of their donor site.
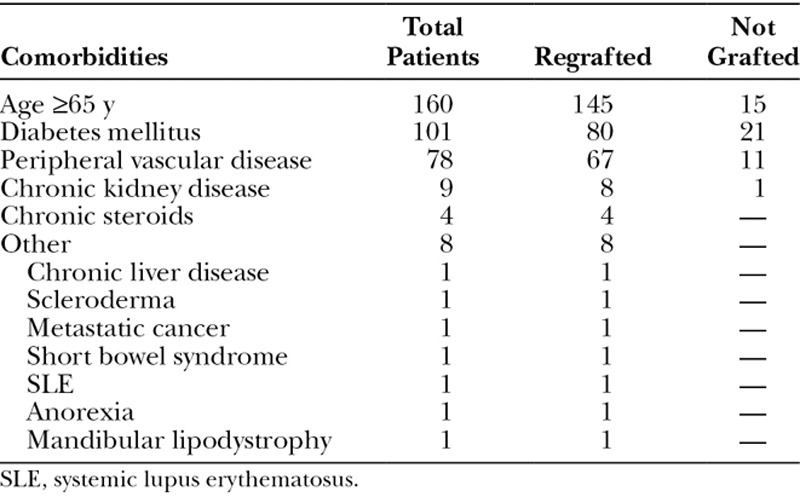
Comorbidities known to affect wound healing were quantified for all patients. The main comorbidities in this population group included age (≥65 years), diabetes mellitus, peripheral vascular disease (venous or arterial), chronic renal disease, or habitual use of steroids (Table 2).
Table 2.
Observed Comorbidities
A statistical analyses of comparison data was performed using the SAS program (the SAS Institute, Inc., Cary, N.C.). Standard t tests (with no assumption of equal variances) were used to test the differences between the 2 subgroups (regrafted versus not regrafted) with regard to the average age, donor-site surface area, number of comorbidities, and time to donor-site healing (Tables 1, 3.). A P value < 0.005 was considered to be significant.
Table 3.
Correlation of Comorbidities to Time of STSG Donor-Site Healing
RESULTS
Of the 317 patients who had an STSG procedure in this study, 204 (64%) were chosen preoperatively to have regrafting of the donor site (87 males and 117 females; Table 1.). Their average age was 72 years. Their average donor-site surface area was 69.1 cm2. Five patients were lost to follow-up or died during the postoperative period. The other group of 113 patients (86 males and 27 females) had only conventional donor-site dressings (Table 1), with 95 patients (84%) treated with Xeroform and 18 patients (16%) with Kaltostat. The average age and donor-site surface area of this group was 46 years and 105.5 cm2, respectively. Four of these patients were also lost to follow-up or died during the postoperative period.
In descending order of frequency, the most common comorbidities encountered overall were the elderly (defined as age ≥65 years; 145 regrafted, 15 not grafted), diabetes mellitus (80 regrafted, 21 not grafted), peripheral vascular disease (67 regrafted, 11 not grafted), chronic renal disease (8 regrafted, 1 not grafted), and chronic steroid use (4 regrafted, 4 not grafted; Table 2). Only 1 patient each had 1 of the following adverse medical conditions: chronic liver disease, scleroderma, metastatic cancer, short bowel syndrome, systemic lupus erythematosus, anorexia, and mandibular lipodystrophy (Table 2.). All these latter patients had their donor sites regrafted, as all were considered at risk for poor donor-site healing.
The average number of comorbidities as listed for those having regrafting was 1.41 versus 0.31 for those treated expectantly (P < 0.0001; Table 3), which confirmed the surgeon’s ability to prospectively reasonably estimate patients at risk. Some with comorbidities were not regrafted intentionally because on a physiological basis these were considered inconsequential for that individual. The mean time for donor-site healing for those regrafted was 17.2 days as opposed to 17.8 for those not grafted (P = 0.2395), a difference found not to be statistically significant, realizing our pragmatic limitation in that daily donor-site observations was impossible as these were usually outpatient procedures.
DISCUSSION
Unexpected complications due to delayed healing of an STSG donor site, sometimes exceeding that of the recipient site, can be a frustrating experience for both the clinician and the patient. Many topical treatment modalities have been developed just to avoid this issue. However, this large prospective study demonstrates instead the benefit of regrafting the STSG donor site to be an even simpler option when possible, specifically in those patients with multiple comorbidities known to affect wound healing.20
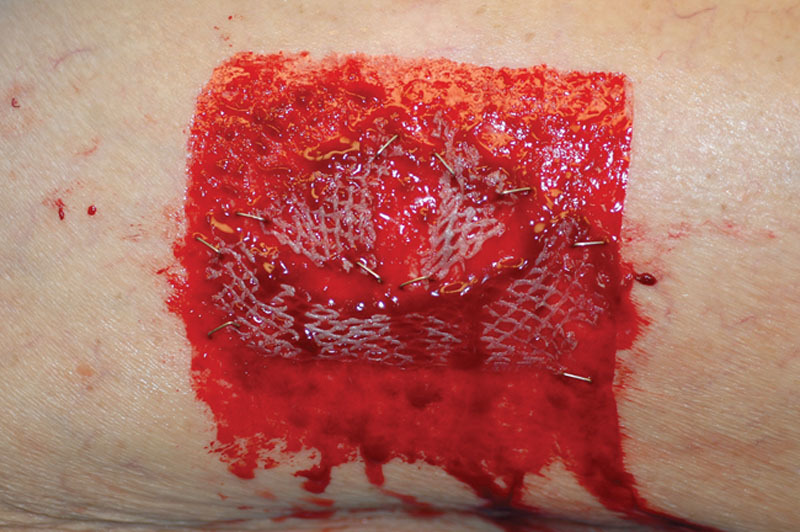
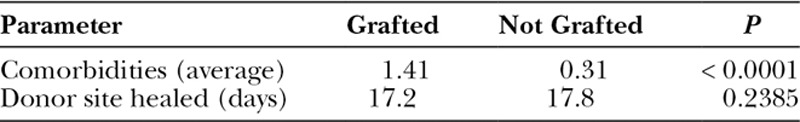
Placing any excess STSG, no matter how trivial an amount, back upon the donor site facilitates the healing process by adding scattered “islands” of tissue that each have an independent reepithelialization potential. Other obvious benefits of using this biologic dressing include immediate availability, minimal expense, and reduction of the inflammatory or pain response inherent with any open wound. There can also be a decreased risk of hypertrophic scarring often found in donor areas where spontaneous reepithelialization only has occurred (Fig. 2).
Fig. 2.
Example of hypertrophic scarring occurring only in split skin graft donor site area where there was no regrafting.
The detrimental impact of age on wound healing of the skin is obvious and well known.2,20–22 The other main comorbidities evaluated in this study—diabetes mellitus, peripheral vascular disease, chronic steroid use, and chronic renal disease—have likewise been previously linked to a decreased wound healing capacity (Tables 2, 3).
A limitation of this study is the lack of an equivalent control group for the patients who had regrafting, relying instead on a historical control that showed the benefit of this maneuver versus use of local dressings only.20 This would have created an ethical concern as a strictly randomized prospective study would have subjected the sickest patients to a potentially suboptimal treatment modality. The ultimate goal at all times was a healed STSG donor site within a reasonable timeframe, and of course, avoidance of an unhealed donor site that sometimes may itself require a repeat STSG (Fig. 3.). It should also be understood that in relatively healthy, and especially younger individuals, timewise there would be no benefit by regrafting, with the concomitant price of an aesthetically displeasing result typical of a meshed skin graft (Fig. 4).
Fig. 3.
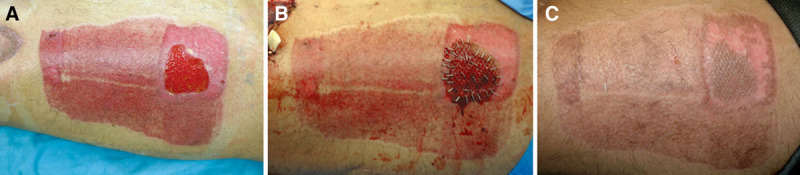
A, Granulating wound months after complete spontaneous STSG donor-site reepithelialization failed, requiring secondary skin grafting (B), with eventual total healing (C).
Fig. 4.
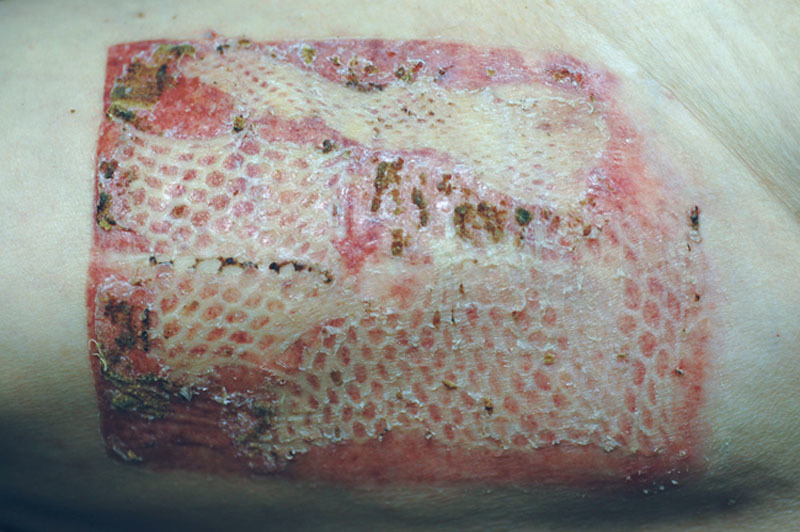
Typical suboptimal aesthetic appearance of the donor site expected after regrafting with meshed skin grafts.
From these observations, it would be difficult to argue against the value of routinely regrafting the STSG donor site in those patients expected to have poor reepithelialization potential. In these same individuals, there could even be more benefit than risk to harvest additional STSG material just for the purpose of regrafting as Wood et al.1 reported in their series with minimal donor-site morbidity and no infections.
Healing of an STSG donor site has often been trivialized but can be complicated. A delay or even lack of healing can be an expensive, time-consuming, and certainly frustrating event for all involved. The experienced surgeon should know in advance which patients are at risk for poor wound healing. A proactive solution to this dilemma is to recycle rather than discard leftover skin graft remnants so as to facilitate STSG donor-site reepithelialization when indicated.
Supplementary Material
Footnotes
Presented at the American Society of Plastic Surgeons Senior Residents Conference, January 18–21, 2012, Tampa, Fla.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The article processing charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Wood RJ, Peltier GL, Twomey JA. Management of the difficult split-thickness donor site. Ann Plast Surg. 1989;22:80–81.. [DOI] [PubMed] [Google Scholar]
- 2.Blight A, Fatah MF, Datubo-Brown DD, et al. The treatment of donor sites with cultured epithelial grafts. Br J Plast Surg. 1991;44:12–14.. [DOI] [PubMed] [Google Scholar]
- 3.Attwood AI. Calcium alginate dressing accelerates split skin graft donor site healing. Br J Plast Surg. 1989;42:373–379.. [DOI] [PubMed] [Google Scholar]
- 4.Grossman AJ. A simplified technique for split-thickness skin graft donor-site care. Plast Reconstr Surg. 2004;113:796–797.. [DOI] [PubMed] [Google Scholar]
- 5.Argirova M, Ognjan H, Victorova A. Acticoat versus allevyn as a split-thickness skin graft donor-site dressing: a prospective comparative study. Plast Reconstr Surg 2009;124:298–306.. [DOI] [PubMed] [Google Scholar]
- 6.Birdsell DC, Hein KS, Lindsay RL. The theoretically ideal donor site dressing. Ann Plast Surg. 1979;2:535–537.. [DOI] [PubMed] [Google Scholar]
- 7.Hermans MH. Results of an internet survey on the treatment of partial thickness burns, full thickness burns, and donor sites. J Burn Care Res. 2007;28:835–847.. [DOI] [PubMed] [Google Scholar]
- 8.Bhatti AZ. Telfa as donor-site dressing. Plast Reconstr Surg. 2005;116:1578. [DOI] [PubMed] [Google Scholar]
- 9.Prather JL, Tummel EK, Patel AB, et al. Prospective randomized controlled trial comparing the effects of noncontact low-frequency ultrasound with standard care in healing split-thickness donor sites. J Am Coll Surg. 2015;221:309–318.. [DOI] [PubMed] [Google Scholar]
- 10.Poole MD, Kalus AM, von Domarus H. Aluminium foil as a wound dressing. Br J Plast Surg. 1979;32:145–146.. [DOI] [PubMed] [Google Scholar]
- 11.Gore MA, Akolekar D. Banana leaf dressing for skin graft donor areas. Burns. 2003;29:483–486.. [DOI] [PubMed] [Google Scholar]
- 12.Kim SW, Choi SH, Kim JT, et al. An additional option for split-thickness skin graft donors: the previous free flap sites. Ann Plast Surg. 2015;75:634–636.. [DOI] [PubMed] [Google Scholar]
- 13.Mimoun M, Chaouat M, Picovski D, et al. The scalp is an advantageous donor site for thin-skin grafts: a report on 945 harvested samples. Plast Reconstr Surg. 2006;118:369–373.. [DOI] [PubMed] [Google Scholar]
- 14.Brady SC, Snelling CF, Chow G. Comparison of donor site dressings. Ann Plast Surg. 1980;5:238–243.. [DOI] [PubMed] [Google Scholar]
- 15.Uysal AC, Alagoz MS, Orbay H, et al. An alternative dressing material for the split-thickness skin graft donor site: oxidized regenerated cellulose. Ann Plast Surg. 2006;57:60–64.. [DOI] [PubMed] [Google Scholar]
- 16.Kilinç H, Sensöz O, Ozdemir R, et al. Which dressing for split-thickness skin graft donor sites? Ann Plast Surg. 2001;46:409–414.. [DOI] [PubMed] [Google Scholar]
- 17.Dornseifer U, Lonic D, Gerstung TI, et al. The ideal split-thickness skin graft donor-site dressing: a clinical comparative trial of a modified polyurethane dressing and aquacel. Plast Reconstr Surg. 2011;128:918–924.. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh K, Ponniah AJ, Jones I, et al. The ideal donor-site dressing: are we clear yet? Plast Reconstr Surg. 2010;126:279e–280e.. [DOI] [PubMed] [Google Scholar]
- 19.Voineskos SH, Ayeni OA, McKnight L, et al. Systematic review of skin graft donor-site dressings. Plast Reconstr Surg. 2009;124:298–306.. [DOI] [PubMed] [Google Scholar]
- 20.Fatah MF, Ward CM. The morbidity of split-skin graft donor sites in the elderly: the case for mesh-grafting the donor site. Br J Plast Surg. 1984;37:184–190.. [DOI] [PubMed] [Google Scholar]
- 21.Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28:321–326.. [DOI] [PubMed] [Google Scholar]
- 22.Holt DR, Kirk SJ, Regan MC, et al. Effect of age on wound healing in healthy human beings. Surgery. 1992;112:293–297; discussion 297.. [PubMed] [Google Scholar]


