Supplemental Digital Content is available in the text.
Abstract
Background:
Tumescent anesthesia antibiotic delivery (TAAD) consists of subcutaneous infiltration of antibiotic(s) dissolved tumescent lidocaine anesthesia. Tumescent lidocaine anesthesia contains lidocaine (≤ 1 g/L), epinephrine (≤ 1 mg/L), sodium bicarbonate (10 mEq/L) in 0.9% saline. Our aim was to measure cefazolin and metronidazole concentrations over time in subcutaneous tumescent interstitial fluid (TISF) after TAAD, in serum after TAAD and after intravenous antibiotic delivery (IVAD). We hypothesize that the pharmacokinetic/pharmacodynamic profiles of TAAD + IVAD are superior to IVAD alone for the prevention of surgical site infections and biofilms.
Methods:
Concentrations of cefazolin and metronidazole in TISF and serum following TAAD and in serum following IVAD were compared in 5 female volunteers. Subjects received cefazolin or cefazolin plus metronidazole by IVAD alone and by TAAD alone. One subject also received concomitant IVAD and TAAD of these 2 antibiotics. Sequential samples of serum or subcutaneous TISF were assayed for antibiotic concentration.
Results:
Cefazolin (1 g) by TAAD resulted in an area under the curve of the concentration–time profile and a maximum concentration (Cmax) in subcutaneous tissue that were 16.5 and 5.6 times greater than in serum following 1 g by IVAD. Metronidazole (500 mg) by TAAD resulted in an area under the curve and Cmax that were 8.1 and 24.7 times greater in TISF, than in serum after 500 mg by intravenous delivery. IVAD + TAAD resulted in superior antibiotic concentrations to IVAD alone.
Conclusions:
TAAD + IVAD produced superior antibiotic bioavailability in both subcutaneous interstitial fluid and serum compared with IVAD alone. There was no evidence that TAAD of cefazolin and metronidazole poses a significant risk of harm to patients.
INTRODUCTION
Surgical site infection (SSI) and bacterial drug resistance remain major problems.1–3 Intravenous antibiotic delivery (IVAD) may not reliably achieve adequate subcutaneous antibiotic concentrations.4 There is a need for improved antibiotic delivery for the prevention of SSIs and biofilms.5–8
Tumescent anesthesia antibiotic delivery (TAAD) consists of a subcutaneous infiltration antibiotics dissolved in a large volume (1–2 L) of tumescent lidocaine anesthesia (TLA). TLA consists of the subcutaneous infiltration of dilute lidocaine (≤ 1 g/L), epinephrine (≤ 1 mg/L), and sodium bicarbonate (10 mEq/L) in a liter bag of 0.9% physiologic saline.
Subcutaneous periincisional injections of antibiotics, dissolved in saline, before incision reduce the risk of SSI.9–14 TAAD is a novel mode of drug delivery that delays systemic drug absorption and prolongs local subcutaneous drug effects.
This research was an exploratory phase 1 pharmacokinetic clinical trial comparing the subcutaneous and systemic bioavailability of antibiotics following TAAD or IVAD. After TAAD, subcutaneous interstitial fluid is designated tumescent interstitial fluid (TISF).
The principal aim of this research was to measure concentrations of cefazolin and metronidazole over time in subcutaneous tissue and serum following TAAD and in serum following intravenous (IV) delivery. We hypothesize that, at equal doses, TAAD provides uniformly greater subcutaneous antibiotic concentrations, area under the curve (AUC∞), maximum concentrations (Cmax), and T > minimum inhibitory concentration (MIC; duration of time that drug concentration exceeds MIC) compared with IV delivery.
A secondary research aim was to determine the correlation between the antibiotic concentration (mg/L) in a TAAD solution and the resulting antibiotic concentration (mg/L) in TISF immediately after tumescent delivery. We hypothesized that these 2 concentrations are highly correlated and nearly equal.
Another secondary research aim was to observe the concentration–time profiles of cefazolin and metronidazole in serum and TISF after subcutaneous tumescent infiltration. We hypothesize that, at equal antibiotic doses and equal concentration in TAAD solution, concentration–time profiles of cefazolin and metronidazole in TISF are virtually identical. Further, we hypothesize that systemic antibiotic absorption following TAAD has a concentration–time profile in serum that resembles a slow constant IV infusion.
METHODS
The authors funded this study. The protocol received institutional review board approval, and written informed consent was obtained before each research procedure. We compared TAAD and IVAD with respect to concentration–time profile, AUC∞, and Cmax.
Only after requesting tumescent liposuction totally by local anesthesia was a person offered the opportunity to participate in this research. Subjects were offered liposuction at no cost. Eligibility requirements were good health, American Society of Anesthesiologists physical status classification 1 (ASA 1), good candidate for liposuction, at least 18 years of age, not pregnant, no history of allergy to lidocaine, cefazolin or metronidazole, and good venous access. Exclusion criteria included use of drugs that impair hemostasis or drugs that impair lidocaine metabolism.
Standard solution TLA solution consisted of 1 g lidocaine, 1 mg epinephrine in 100 mL and 10 mEq of sodium bicarbonate (10 mL) in a 1,000 mL bag of saline (1 g lidocaine in 1,110 mL = 0.09%). For subject 3, the lidocaine and cefazolin concentrations in the TAAD solution were 877 mg/L for infiltration into bilateral hips and outer thighs to not exceed 45 mg/kg of lidocaine.
Adding cefazolin to a TAAD solutions involved withdrawing 10 mL from the TLA solution and injecting it into a vial of cefazolin powder then injecting the solubilized cefazolin into the bag of TLA solution. Adding metronidazole to a TAAD solution involved transferring 500 mg in 100 mL into 1,110 mL of a TLA solution.
Subjects 1, 2, and 3 received cefazolin once by IV infusion and then twice by tumescent infiltration.
Subject 4 received cefazolin and metronidazole dissolved in a single IV bag on 1 occasion by IVAD and by TAAD into abdomen on another occasion.
Subject 5 received concurrent cefazolin and metronidazole, once by IVAD, once by TAAD, and once by concomitant IVAD + TAAD. Procedures for individuals were at least 7 days apart to assure complete antibiotic clearance before a subsequent study.
Subcutaneous tumescent infiltration was accomplished using a peristaltic pump, infiltration tubing, and Monty infiltration cannulas (HKSurgical.Com, San Clemente, Calif.). All patients were fully awake and received no parenteral sedation (see videos, Supplemental Digital Content 10, which demonstrates the preferred technique for subcutaneous infiltration of TAAD solution using a multiholed plastic subcutaneous catheter, HK SubQKath, http://links.lww.com/PRSGO/A443 and Supplemental Digital Content 11, which demonstrates the technique for painless subcutaneous infiltration of large volumes of TLA using multiholed stainless steel cannulas, http://links.lww.com/PRSGO/A444).
Video Graphic 1.
See video, Supplemental Digital Content 10, which demonstrates the preferred technique for subcutaneous infiltration of TAAD solution using a multiholed plastic subcutaneous catheter, HK SubQKath. This video is available in the Related Videos section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A443.
Video Graphic 2.

See video, Supplemental Digital Content 11, which demonstrates the technique for painless subcutaneous infiltration of large volumes of TLA using multiholed stainless steel cannulas. This video is available in the Related Videos section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A444.
IV antibiotic infusion was accomplished over 5 minutes via an antecubital vein. Serum samples were obtained via an indwelling IV catheter in a contralateral antecubital vein. After IVAD, only serum antibiotics concentrations were measured. After TAAD, serum and TISF concentrations were measured.
Blood sampling technique first removed 2 mL of blood, which was discarded; then 10 mL of blood was obtained in a second syringe for drug assay. Finally, the IV catheter was flushed with 2 mL saline and then heparin (5 units/0.5 mL). Beginning at time t = 0 immediately after antibiotic delivery, sequential blood samples were obtained at hours 0, 1, 2, 3, and 4 hours and then every 2 hours. Serum samples were frozen.
Sequential samples of tumescent adipose tissue (10–20 mL) were obtained by hand-held syringe liposuction at time T0 immediately after TAAD and continued at hours 1, 2, 3, and 4 and then every 2 hours until the subject experienced liposuction-associated pain. Samples of fat aspirate were centrifuged, supernatant fat was discarded, and infranatant TISF was frozen. Both serum and interstitial fluid samples were assayed for antibiotic concentration by high pressure liquid chromatography.
The correlation (R2) between the antibiotic concentration (mg/L) in TAAD solution and Cmax (mg/L) in liposuctioned aspirate (TISF) was determined.
The bioavailability of a drug in a tissue was measured by calculating AUC, the area under the concentration-time curve C(t) over the interval (0,T0), where t = 0 is the time immediately after drug delivery and time t = T0 is the time when the drug concentration in the targeted tissue has returned to zero.
Suppose time t = T is the time of the last measurement of the drug concentration in the tissue, and that C(T) > 0, then time T < T0 and T0 must be estimated by visually extending the curve C(t) to the point where C(t) crosses the horizontal axis. Thus, AUC∞ = AUC(0, T) + AUC(T, T0), where AUC(0, T) was determined using the trapezoid rule. AUC(T, T0) has the shape of a triangular of height C(T), base length (T0-T) and area 1/2 C(T)(T0-T).
RESULTS
The research cohort consisted of 5 healthy adult female volunteers, ages 37–64 years. Each subject participated in 2–3 pharmacokinetic procedures. There were 15 pharmacokinetic procedures: 6 IVAD procedures and 9 TAAD procedures.
The maximum concentrations of cefazolin and metronidazole in a TAAD solution were 900 mg/L and 413 mg/L, respectively. See Figures 1–5 and Tables 1–5 for individual concentration–time profiles. Lidocaine dosages ranged from 20 to 45 mg/kg (see additional figures and supplemental raw data, Supplemental Digital Content 1–9, http://links.lww.com/PRSGO/A442).
Fig. 1.
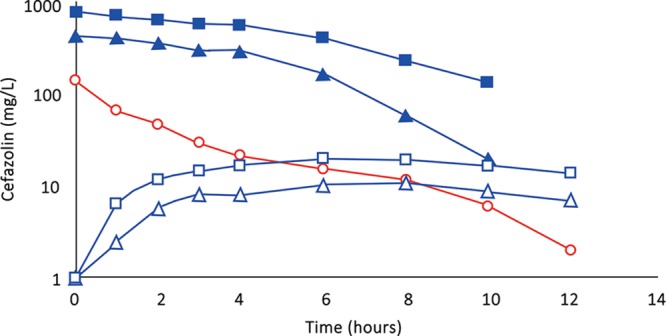
(Subject 1): Comparison of concentration–time profiles of cefazolin by IVAD (red) and TAAD (blue) into subcutaneous abdominal fat of a 74.3-kg female. Square symbols represent 1,000 mg by TAAD1 in a 900 mg/L solution. Triangle symbols represent 1,000 mg by TAAD2 in a 450 mg/L solution. Round red symbols show 1,000 mg by IVAD. Closed symbols represent concentrations in TISF, and open symbols are concentrations in serum.
Fig. 5.
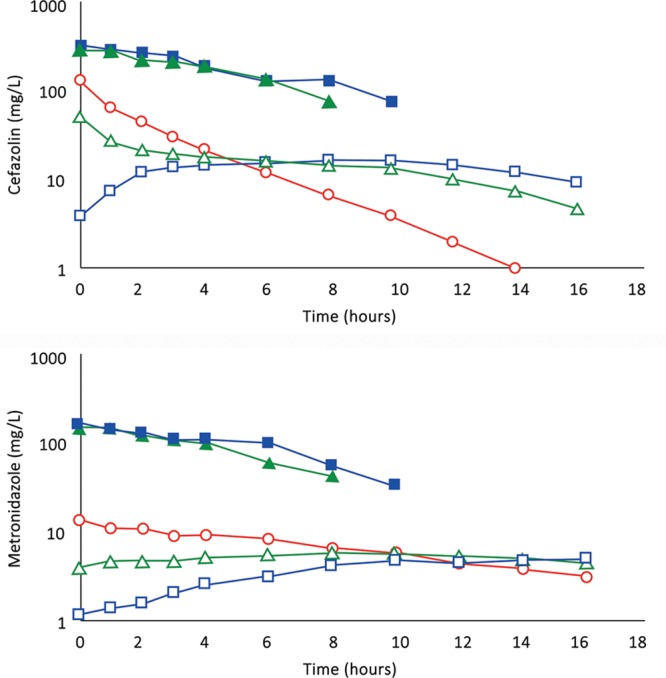
A, (Subject 5): Comparison of concentration–time profiles following 1,200 mg of cefazolin by IVAD1, by TAAD1, and by concomitant IVAD2 + TAAD2. Square blue symbols represent TAAD1 of a 1,200 mg/L solution. Triangle green symbols represent 800 mg by TAAD2 in a 800 mg/L solution with concomitant 400 mg by IVAD2. Round red symbols show 1,200 mg by IVAD1. Closed symbols represent concentrations in TISF, and open symbols are concentrations in serum. B, (Subject 5): Comparison of concentration–time profiles following 600 mg of metronidazole by IVAD1, TAAD1, and IVAD2 + TAAD2. Square blue symbols represent TAAD1 of a 600 mg/L solution. Triangle green symbols represent 400 mg by TAAD2 in a 400 mg/L solution with simultaneous 200 mg by IVAD2. Round red symbols show 600 mg by IVAD1. Closed symbols represent concentrations in TISF, and open symbols are concentrations in serum.
Table 1.
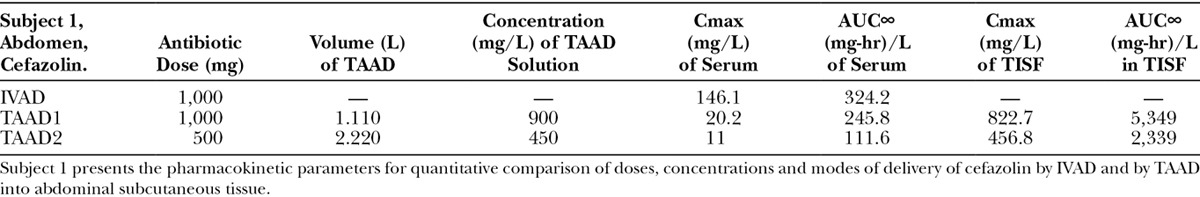
Table 5.
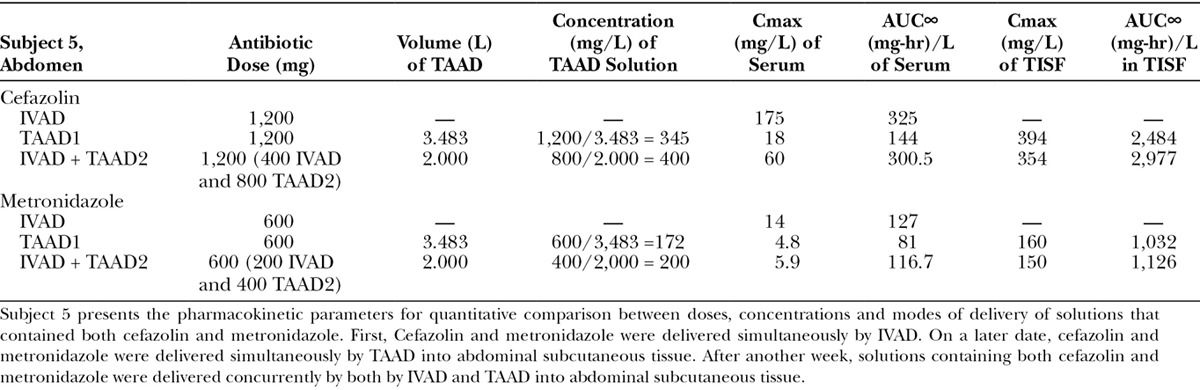
Table 2.
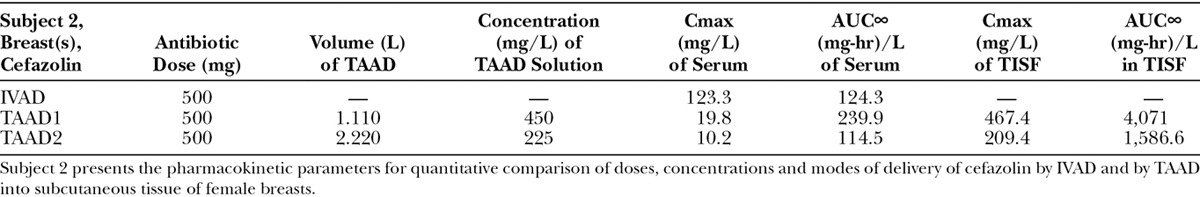
Table 3.
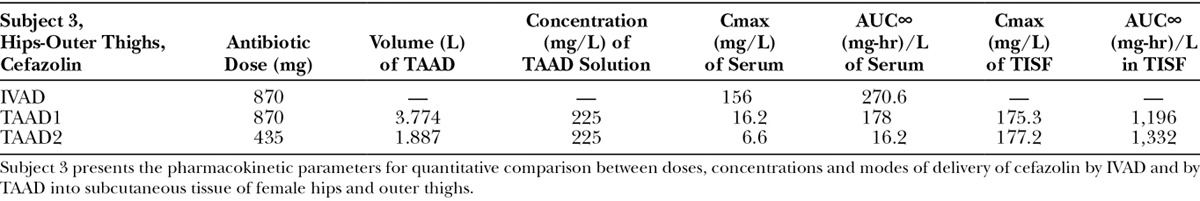
Table 4.
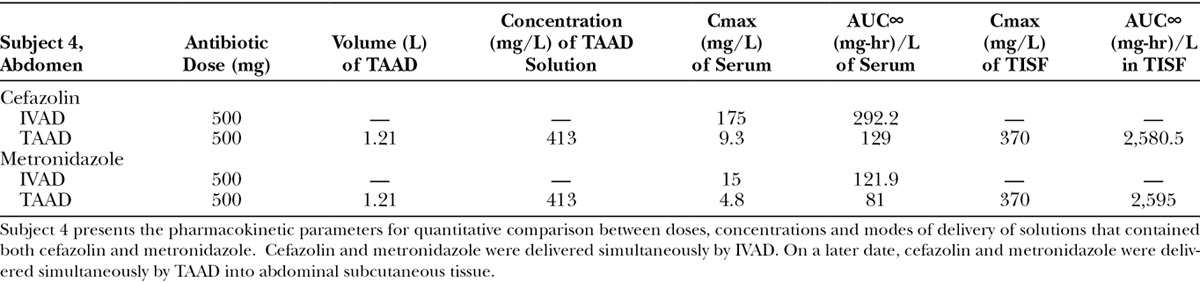
Fig. 2.
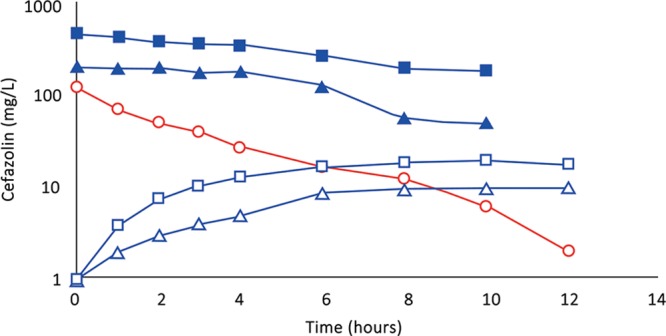
(Subject 2): Comparison of concentration–time profiles of cefazolin by IVAD (red) and TAAD (blue) in the breasts of a 76.4-kg female. Square symbols represent 500 mg by TAAD1 in a 450 mg/L solution (one breast). Triangle symbols represent 500 mg by TAAD2 in a 225 mg/L solution (bilateral breasts). Round red symbols show 1,000 mg by IVAD. Closed symbols represent concentrations in TISF, and open symbols are concentrations in serum.
Fig. 3.
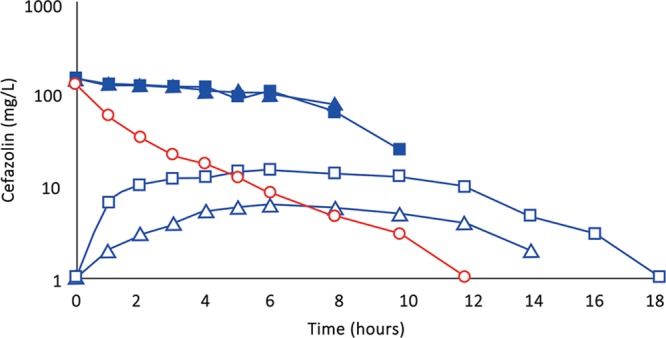
(Subject 3): Comparison of concentration–time profiles of cefazolin by IVAD (red) and TAAD (blue) into the hips and outer thighs of a 66.4-kg female. Square symbols represent 870 mg by TAAD1 in a 228 mg/L solution (bilateral). Triangle symbols represent 435 mg by TAAD2 in a 228 mg/L solution (one side). Round symbols show 1,000 mg by IVAD. Closed symbols represent concentrations in TISF, and open symbols are concentrations in serum.
Fig. 4.
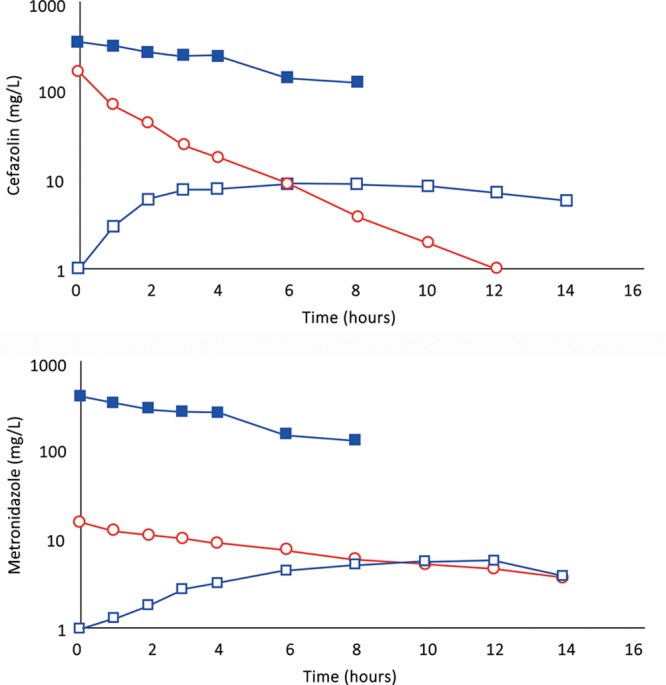
A, (Subject 4): Comparison of cefazolin concentration–time profiles after 500 mg by IVAD (red, round symbols) and 500 mg by TAAD (blue, square symbols) in 413 mg/L solutions into subcutaneous abdominal fat of a 66.3-kg female. Closed symbols represent concentrations in TISF, and open symbols are concentrations in serum. B, (Subject 4): Comparison of metronidazole concentration–time profiles after 500 mg by IVAD (red, round symbols) and 500 mg by TAAD (blue, square symbols) in 413 mg/L solutions into subcutaneous abdominal fat of a 66.3-kg female. Closed symbols represent concentrations in TISF, and open symbols are concentrations in serum.
In TISF, peak antibiotic concentrations (Cmax) occurred at the time of the initial sample, thereafter concentrations declined linearly. TAAD produced a Cmax for both cefazolin and metronidazole in TISF approximately equal to the mg/L concentration in TAAD solution (R2 = 0.97; Fig. 6). In serum after TAAD, antibiotic Cmax occurred after 8–12 hours, thereafter concentrations declined slowly over many hours. In serum after IVAD, Cmax occurred at the time of the initial serum sample, thereafter concentrations declined exponentially.
Fig. 6.
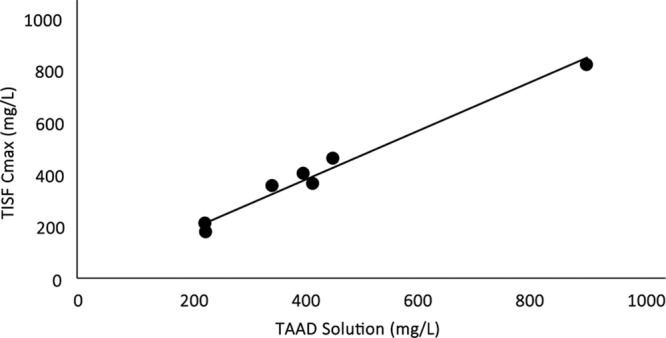
Following TAAD, there is a close correlation between the cefazolin concentration (mg/L) in the TAAD solution and the resulting peak (Cmax) cefazolin (mg/L) concentration in the TISF, with a coefficient of determination of R2 = 0.975.
When antibiotic doses by TAAD and IVAD were equal, the antibiotic concentrations in TISF after TAAD were strictly greater than in serum after IVAD at every time point. It follows that AUC∞, Cmax, and T > MIC (for any hypothetical MIC) in TISF after TAAD were greater than in serum after IVAD.
Subject 1 received 1 g of cefazolin by TAAD and 1 g of cefazolin by IVAD. The AUC∞ and Cmax in interstitial fluid were 16.5 and 5.6 times greater, respectively, after TAAD than in serum after IVAD.
Subject 4 received 500 mg of metronidazole by TAAD and 500 mg metronidazole by IVAD. The AUC∞ and Cmax for metronidazole were 8.1 and 24.7 times greater, respectively, in TISF after TAAD than in serum after IVAD.
After TAAD, the AUC∞ for cefazolin in TISF increased when the mg/L concentration of cefazolin was increased in the TAAD solution (subject 2) and when mg dose of cefazolin was increased in the TAAD solution (subject 3).
As expected, because of differences in volume of distribution and rates of clearance, equal mg doses of cefazolin and metronidazole by IVAD produced considerably different concentration–time profiles in serum. In contrast, TAAD of equal mg doses and equal mg/L concentrations of cefazolin and metronidazole in the TAAD solution resulted in identical concentration–time profiles in TISF (subject 4; Fig. 7).
Fig. 7.
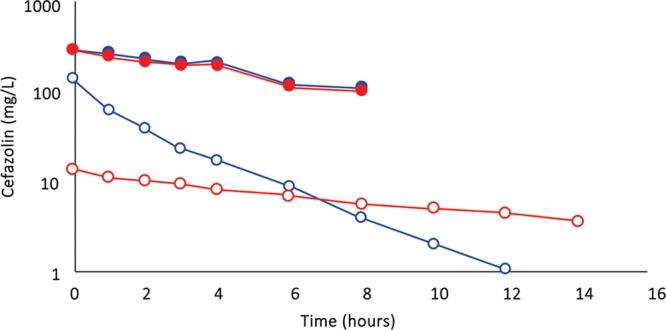
(Subject 4): Comparison of cefazolin 500 mg (blue) and metronidazole 500 mg (red) concentration–time profiles in TISF (closed symbols) by TAAD in 413 mg/L solutions and in serum (open symbols) after 500 mg by IVAD. The pharmacokinetic profiles are distinctly different in serum after IVAD but virtually identical in TISF after TAAD.
For subject 5, when the total doses of cefazolin and metronidazole by IVAD + TAAD equaled the doses by IVAD, IVAD + TAAD resulted in more prolonged serum antibiotic concentration and higher TISF concentrations compared with IVAD alone.
The maximal rates of subcutaneous tumescent infiltration ranged from 125mL/min to 250 mL/min. At this rate the infiltration was virtually painless.
No adverse or unusual systemic or cutaneous reactions (inflammation, tenderness, unusual ecchymosis) were observed following TAAD.
DISCUSSION
For tumescent lidocaine at 28mg/kg in healthy subjects, the risk of mild lidocaine toxicity is 1/5,000,000.15 In patients with significant cardiac, hepatic, or renal impairment, the tumescent lidocaine dosages with TAAD may need to be reduced. Otherwise 28mg/kg is safe with or without general anesthesia. Anesthesiologists commonly provide general anesthesia together with IV lidocaine over 24 hours at total lidocaine dosages that can exceed 28mg/kg. At these dosages of IV lidocaine under general anesthesia toxicity is extremely rare.16
This research was an exploratory phase 1 clinical trial comparing 2 modes of antibiotic delivery: IVAD and TAAD, a novel mode of antibiotic delivery. We compared IVAD and TAAD with respect to bioavailability and maximum tissue antibiotic concentrations (Cmax).
TAAD consists of antibiotics dissolved in a solution of TLA. TLA solutions consist of lidocaine (< 1 g/L), epinephrine (< 1 mg/L), sodium bicarbonate (10 mEq/L) dissolved in 0.9% physiologic saline.
TLA is at least a 10-fold dilution of commercial 1% lidocaine with epinephrine 1:100,000. TLA provides long-lasting (≥ 8 hours) local anesthesia, local subcutaneous vasoconstriction, and profound surgical hemostasis. TLA is used for a wide range of surgical procedures.17–36
This research was intended to be a proof of concept pharmacokinetic study in anticipation of a subsequent randomized clinical trial (RCT) of TAAD + IVAD versus IVAD alone for SSI prevention. To minimize the sample size required for the RCT, the target population ought to have a high risk of SSI. The risk of SSI typically exceeds 15% among open colorectal surgeries in diabetic, obese, immunocompromised, or trauma patients. We chose to study cefazolin and metronidazole because they are generic, effective, inexpensive, widely available, and commonly used for colorectal surgery SSI prophylaxis. When mixed together, cefazolin and metronidazole are stable in solution and are effective after subcutaneous injection for prevention of SSIs.37–42 TAAD may prove useful in reconstructive surgery where SSIs are more common than in cosmetic surgery.
Effective antibiotic prophylaxis of SSIs depends on adequate antibiotic concentrations at the surgical incision site throughout the duration of the surgical procedure.43 The risk of implant surface biofilm formation is correlated with antibiotic concentration in periimplant tissue.44
The antibiotic concentration in TISF immediately after TAAD is virtually identical to the antibiotic concentration in the TAAD solution. Clinicians can use TAAD to deliver a preselected initial subcutaneous antibiotic concentration at a proposed surgical incision site. For some antimicrobials (e.g., aminoglycosides) achieving greater TAAD-like subcutaneous antibiotic concentrations solely by IVAD might not be possible or might pose a significant risk of harm to patients.
At equal antibiotic doses, TAAD provides superior subcutaneous antibiotic bioavailability (AUC∞), Cmax, and T > MIC compared with IVAD. Assuming equal IV doses, the concomitant delivery by TAAD + IVAD is pharmacokinetically superior to IVAD alone.
Tumescent drug delivery has 3 distinct therapeutic properties: local physical effects, local pharmacologic effects, and systemic pharmacologic effects.
Local physical effects of TAAD include mechanical compression of capillaries and veins.
The 0.9% saline component of a tumescent solution provides prolonged local tissue hydration that prevents desiccation of the surgical wound surface. Tumescent tissue acts as a subcutaneous reservoir of 0.9% saline solution at the infiltration (incision) site and can reduce IV fluid requirements.
Local pharmacologic effects of TAAD include epinephrine-induced prolonged and profound capillary vasoconstriction. TAAD takes advantage of the fact that subcutaneous drug absorption is perfusion-rate limited. Reduced subcutaneous blood flow with TAAD effectively isolates the TISF from the systemic circulation resulting in sustained local drug action. The concentration of a TAAD solution of antibiotics that is nontoxic to subcutaneous tissues may far exceed the antibiotic concentration that can be reliably achieved in subcutaneous tissue by IVAD.
Continuous IV infusion of antibiotics may be more effective than intermittent bolus IV infusion in acutely ill patients.44 Continuous IV infusion may reduce the emergence of bacterial resistance and overcome existing resistant bacteria.45,46 Slow systemic absorption of lidocaine and antibiotics following TAAD produces serum concentration–time profiles that resemble a slow constant IV infusion persisting for 12 hours or more.
TAAD maximizes peak drug concentrations in subcutaneous tissue while simultaneously minimizing serum Cmax, compared with IVAD. For surgical procedures confined to subcutaneous tissue, TAAD minimizes the peak antibiotic concentration to which gut microflora are exposed.
Following IVAD, concentrations of cefazolin and metronidazole are typically lower in interstitial fluid than in serum.48–50 Our results show that concentrations in serum after IVAD were always less than in TISF after TAAD, at every time point. Thus, TAAD + IVAD is always superior to IVAD alone with respect to AUC∞, Cmax, and T>MIC in subcutaneous interstitial fluid and in serum, assuming equal IVAD doses. This suggests that TAAD + IVAD ought to improve antibiotic prophylaxis of wound infections.
TAAD + IVAD may be superior for preventing the emergence of bacterial antibiotic resistance. The emergence of bacterial antibiotic resistance is concentration dependent. Resistant mutants proliferate at antibiotic concentrations between the MIC and the (higher) mutant prevention concentration (MPC) but do not proliferate at concentrations below the MIC or above the MPC.51
Antibiotic delivery to a surgical incision site is reduced as a result of decreased local blood flow due to wound cautery, capillary thrombosis, and tissue desiccation. After IVAD, a concentration gradient can develop between an incision site surface (lower concentration) and the vascular compartment (higher concentration).
This concentration gradient may allow resistant mutants at an incision site to evade competition and flourish by invading tissues with higher drug concentrations, where less resistant strains do not survive.52,53 The TAAD concentrations above MPC at a surgical incision site, the site of potential bacterial contamination, may improve SSI prophylaxis and bacterial resistance prevention.54,55
TAAD alone is pharmacokinetically superior to IVAD for achieving high, prolonged subcutaneous antibiotic concentrations in wounds confined to skin and subcutaneous tissue. This suggests the hypothesis that TAAD alone may reduce the risk of incision site wound infection and biofilm formation of subcutaneous implanted devices.
TAAD + IVAD is pharmacokinetically superior to TAAD alone or IVAD alone, assuming equal IVAD doses, for achieving high, prolonged subcutaneous and systemic antibiotic concentrations. This suggests a second hypothesis that TAAD + IVAD is superior to IVAD alone for preventing SSI in wounds involving deep organs and tissues.
Cefazolin and metronidazole have distinctly different pharmacokinetic characteristics after IVAD. But after TAAD, the subcutaneous concentration–time profiles of cefazolin and metronidazole are virtually identical. This suggests that diverse drugs, including antibiotic, antiviral, antifungal, and antitumor drugs, may have similar subcutaneous concentration–time profiles in TISF after tumescent delivery.
We found no adverse effects of tumescent cefazolin, metronidazole, lidocaine, or epinephrine. The subcutaneous injection of cefazolin and metronidazole is “off-label” according to United States Food and Drug Administration–approved package insert labeling. Yet, subcutaneous antibiotic delivery for systemic effect is commonly used for palliative therapy.56–65 These reports and our present data suggest that tumescent delivery of dilute cefazolin and metronidazole represents a nonsignificant risk of harm to patients.
Prolonged open gastrointestinal surgical procedures exceeding 4 hours require redosing with IV antibiotics for optimal SSI prophylaxis.66 There is a high rate of noncompliance with intraoperative redosing.67 Our data suggests that the combination of TAAD + IVAD provides prolonged high antibiotic concentrations in TISF and serum and may reduce the risk of SSI associated with noncompliance to redosing requirements.68
CLINICAL APPLICATION OF TAAD
In clinical practice, we suggest using 1 L or more of TAAD solution consisting of 1 g cefazolin, 500 mg of metronidazole, 1 g of lidocaine with 1 mg epinephrine, and 10 mEq sodium bicarbonate per 1 L bag of 0.9% physiologic saline or Ringer’s lactate.
Stainless steel tumescent infiltration cannulas or plastic tumescent subcutaneous catheters are used for efficient, painless TAAD (Fig. 8, 9).
Fig. 8.

A, There are 3 different types of stainless steel tumescent Monty infiltration cannulas. The Full Monty has holes distributed along nearly its entire length. The Half Monty has holes distributed along the distal half of the cannula. The Tip Monty has holes confined to the distal 2.5 cm of the cannula. The Monty cannulas can be inserted into subcutaneous fat, then withdrawn and reinserted in another direction. B, Over-the-needle subcutaneous catheter for TAAD consists of a 15 cm flexible plastic catheter, with holes distributed longitudinally along the distal 90% of the catheter length, and a sharp-tipped hollow stainless steel stylet, shown assembled and disassembled. The subcutaneous catheters are inserted into subcutaneous tissue and remain in one place throughout the entire process of TAAD infiltration.
Fig. 9.
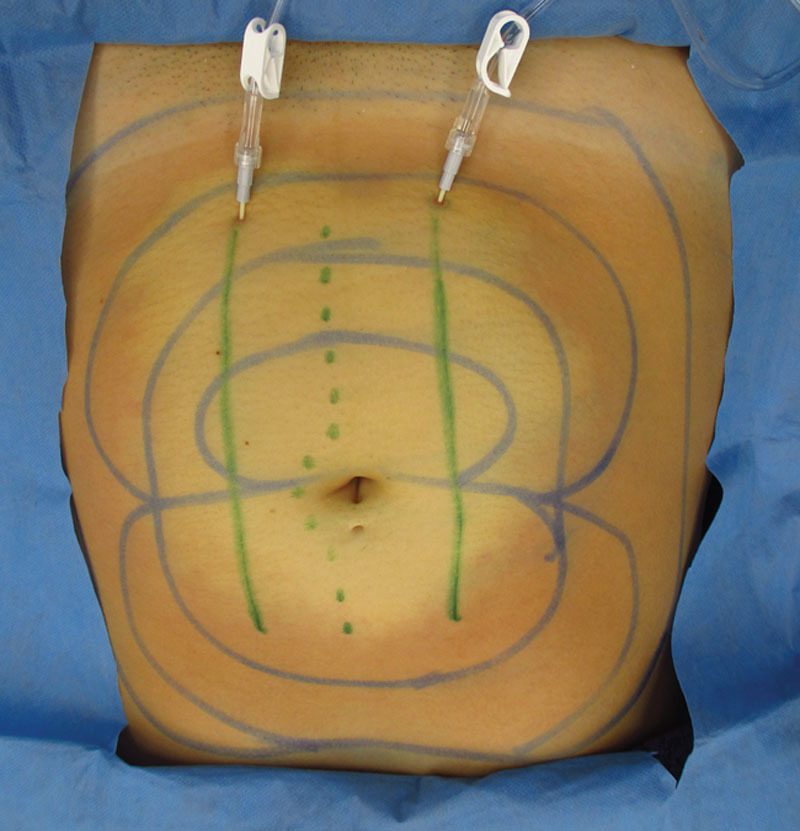
Female abdomen demonstrating the visible blanching and tumescence following TAAD with bilateral tumescent infiltration catheters (solid green lines) inserted into subcutaneous fat parallel to a proposed midline abdominal incision (dotted green line).
A trained nurse can perform TAAD infiltration in the patient’s hospital room or in the preoperative area 1–3 hours before incision, thus reducing the surgeon’s work load in the operating room and allowing time for detumescence and optimal interstitial distribution of the TAAD solution.
The prolonged high tumescent antibiotics concentrations in subcutaneous tissue may reduce the need to precisely give IV antibiotics 30–60 minutes before an incision. With detumescence, tissue remains anesthetic and vasoconstricted for hours while becoming less waterlogged and more easily manipulated.
THERAPEUTIC PROPERTIES OF TUMESCENT LIDOCAINE
The 0.09% lidocaine in the TAAD solution reduces the pain associated with antibiotic injection, provides immediate onset of local surgical anesthesia and prolonged postoperative analgesia. The lidocaine component of a TAAD solution has significant antibacterial, antiplatelet, and systemic antiinflammatory effects.
Lidocaine in vivo is bactericidal at 3 mM (0.09%), the concentration of lidocaine in a TAAD solution.69–72 The lidocaine in a TAAD solution may act synergistically with TAAD antibiotics to kill bacteria. Antibacterial effects of lidocaine have been attributed to disruption of microbial cell membrane permeability by transmembrane anion (Na+, K+, Ca++) channel blockade.73–75 Slow constant systemic absorption of lidocaine prevents SSI in a mouse model.70
Lidocaine impairs platelet function at tumescent lidocaine concentrations (≥ 3 mM) and may reduce the risk of venous thromboembolism.76–80 Although tumescent lidocaine impairs platelet activation, it does not impair surgical hemostasis.81
Lidocaine has significant pharmacologic antiinflammatory properties at clinically safe serum concentrations. Lidocaine may inhibit systemic inflammatory responses to surgical trauma and bacterial infection.81–96 Tumescent infiltration into traumatized or infected tissue engulfs large volumes of damaged tissue, which may prevent or delay the systemic absorption of inflammatory cytokines, chemokines, histones, and pathogens.
The continuous systemic absorption of up to 28 mg/kg of tumescent lidocaine provides predictably safe convenient therapeutic serum lidocaine concentrations, in the range of 1–3 mg/L, for up to 24 hours or more. Systemic lidocaine has been reported to provide preemptive, interoperative, and postoperative analgesia.97 Lidocaine local anesthesia reduces postoperative narcotic use with earlier return of normal bowl function and earlier postoperative ambulation.98–101 Sodium bicarbonate in the TAAD solution raises the pH of the solution and eliminates the stinging pain associated with acidic pH of commercial solutions of lidocaine and epinephrine.102
RISKS OF TUMESCENT DRUG DELIVERY
Human error is the most dangerous aspect of tumescent drug delivery. To avoid miscommunication, legible written physician orders must specify the amount of lidocaine in terms of total milligrams per bag of tumescent solution and total mg/kg dosage permitted for any individual patient. Every bag of TAAD solution must be clearly labeled on both sides with tumescent safety labels that state, “Subcutaneous Tumescent Lidocaine, Not for IV.” Inadvertent IV delivery of a TAAD solution must be avoided.
LIMITATIONS OF THIS STUDY
This research has involved a limited number of patients and is not designed to replicate procedures, test hypotheses, or estimate pharmacokinetic parameters. Our data suggest that TAAD + IVAD is pharmacokinetically superior to IVAD for SSI prevention, but it does not show that TAAD + IVAD is clinically superior to IVAD for SSI prevention.
Some measurements of antibiotic tissue concentrations were discontinued before complete clearance of the antibiotic. In such cases, the last measured antibiotic concentration was > 0, and the estimation of AUC∞ required a subjective visual estimation of the terminal concentration–time curve. Nevertheless, the differences between clinically measured portions of the AUC∞ were so pronounced that the size of the last portion of the AUC∞ did not affect the conclusions.
It is plausible that the transient tumescent vasoconstriction and local tissue hypoxia produced by TLA may adversely affect the incidence of SSIs. However, the incidence of SSIs associated with large volume tumescent liposuction totally by local anesthesia performed under standard aseptic technique is extraordinarily small.103–106
We only investigated the use of cefazolin and metronidazole. The use of other antibiotics for TAAD or IVAD requires clinical investigation to document its safety.
FUTURE RESEARCH
We propose a multicenter RCT of concomitant TAAD + IVAD versus IVAD alone, with the primary endpoint being a reduced incidence of SSI for a variety of surgical procedures. Secondary endpoints are postoperative thromboembolism, surgical blood loss, systemic and local inflammatory response to surgical trauma and surgery, postoperative narcotic requirements, time to postoperative ambulation, and length of stay in hospital. Potential target population include patients undergoing procedures at high risk for SSIs, such those involving prolonged complex plastic surgical procedures, contaminated abdominal wounds, diabetes, impaired immunity, combat wounds, burns, and patients within medically indigent communities (Supplemental Digital Content 2, http://links.lww.com/PRSGO/A442).
CONCLUSIONS
Subcutaneous antibiotic bioavailability using TAAD is superior to that of IVAD. The hypothesis that combined TAAD + IVAD is more effective than IVAD alone for SSI prevention, assuming equal IV antibiotic doses, remains to be tested with RCT. There was no evidence that TAAD of cefazolin and metronidazole poses a significant risk of harm to patients.
ACKNOWLEDGMENTS
Atoussa Cameron, RN, NP, and JoAnn Coker, RN, NP, assisted in caring for patients and obtaining the serum samples. Jonathan Good, Toxicology/Drug Monitoring Lab, Mayo Clinic, Rochester, Minn., performed the cefazolin high-performance liquid chromatography assays. Paytra Klein proof read drafts of the article and checked the accuracy of reference citations. Susan Ruffalo edited an early version of the article.
Supplementary Material
Footnotes
Disclosure: Dr. Klein owns patents on devices for tumescent drug delivery by subcutaneous infiltration. Dr. Klein’s wife, Kathleen Hutton-Klein, MD, owns HK Surgical, Inc., a company that sells devices for tumescent drug delivery. Dr. Langman has no conflicts of interest to report. This was investigator-initiated research, funded entirely by the authors. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Boehm O, Baumgarten G, Hoeft A. Epidemiology of the high-risk population: perioperative risk and mortality after surgery. Curr Opin Crit Care. 2015;21:322–327.. [DOI] [PubMed] [Google Scholar]
- 2.Aiken AM, Karuri DM, Wanyoro AK, et al. Interventional studies for preventing surgical site infections in sub-Saharan Africa—a systematic review. Int J Surg. 2012;10:242–249.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viet Hung N, Anh Thu T, Rosenthal VD, et al. Surgical site infection rates in seven cities in Vietnam: findings of the International Nosocomial Infection Control Consortium. Surg Infect (Larchmt). 2016;17:243–249.. [DOI] [PubMed] [Google Scholar]
- 4.Toma O, Suntrup P, Stefanescu A, et al. Pharmacokinetics and tissue penetration of cefoxitin in obesity: implications for risk of surgical site infection. Anesth Analg. 2011;113:730–737.. [DOI] [PubMed] [Google Scholar]
- 5.Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137:1659–1669.. [DOI] [PubMed] [Google Scholar]
- 6.Deva AK, Adams WP, Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg. 2013;132:1319–1328.. [DOI] [PubMed] [Google Scholar]
- 7.Giormezis N, Kolonitsiou F, Foka A, et al. Coagulase-negative staphylococcal bloodstream and prosthetic-device-associated infections: the role of biofilm formation and distribution of adhesin and toxin genes. J Med Microbiol. 2014;63:1500–1508.. [DOI] [PubMed] [Google Scholar]
- 8.Franchelli S, Pesce M, Savaia S, et al. Clinical and microbiological characterization of late breast implant infections after reconstructive breast cancer surgery. Surg Infect (Larchmt). 2015;16:636–644.. [DOI] [PubMed] [Google Scholar]
- 9.Bhargava P, Mehrotra N, Kumar A. Wound infection after metronidazole infiltration. Trop Doct. 2006;36:37–38.. [DOI] [PubMed] [Google Scholar]
- 10.Shubing W, Litian Z. Preventing infection of the incision after appendectomy by using metronidazole preoperatively to infiltrate tissues at the incision. Am J Surg. 1997;174:422–424.. [DOI] [PubMed] [Google Scholar]
- 11.Quendt J, Blank I, Seidel W. [Peritoneal and subcutaneous administration of cefazolin as perioperative antibiotic prophylaxis in colorectal operations. Prospective randomized comparative study of 200 patients]. Langenbecks Arch Chir. 1996;381:318–322.. [DOI] [PubMed] [Google Scholar]
- 12.el-Sefi TA, el-Awady HM, Shehata MI, et al. Systemic plus local metronidazole and cephazolin in complicated appendicitis: a prospective controlled trial. J R Coll Surg Edinb. 1989;34:13–16.. [PubMed] [Google Scholar]
- 13.Dixon JM, Armstrong CP, Duffy SW, et al. A randomized prospective trial comparing the value of intravenous and preincisional cefamandole in reducing postoperative sepsis after operations upon the gastrointestinal tract. Surg Gynecol Obstet. 1984;158:303–307.. [PubMed] [Google Scholar]
- 14.Pollock AV, Evans M, Smith GM. Preincisional intraparietal Augmentin in abdominal operations. Ann R Coll Surg Engl. 1989;71:97–100.. [PMC free article] [PubMed] [Google Scholar]
- 15.Klein JA, Jeske DR. Estimated maximal safe dosages of tumescent lidocaine. Anesth Analg. 2016;122:1350–1359.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn LK, Durieux ME. Perioperative Use of Intravenous Lidocaine. Anesthesiology. 2017;126:729–737.. [DOI] [PubMed] [Google Scholar]
- 17.Klein JA. The tumescent technique for liposuction surgery. J Am Acad Cosmetic Surg. 1987;4:263–267.. [Google Scholar]
- 18.Shimizu Y, Nagasao T, Taneda H, et al. Combined usage of intercostal nerve block and tumescent anaesthesia: an effective anaesthesia technique for breast augmentation. J Plast Surg Hand Surg. 2014;48:51–55.. [DOI] [PubMed] [Google Scholar]
- 19.Rusciani A, Pietramaggiori G, Troccola A, et al. The outcome of primary subglandular breast augmentation using tumescent local anesthesia. Ann Plast Surg. 2016;76:13–17.. [DOI] [PubMed] [Google Scholar]
- 20.Sleth JC, Servais R, Saizy C. [Tumescent infiltrative anaesthesia for mastectomy: about six cases]. Ann Fr Anesth Reanim. 2008;27:941–944.. [DOI] [PubMed] [Google Scholar]
- 21.Orgill DP. Excision and skin grafting of thermal burns. N Engl J Med. 2009;360:893–901.. [DOI] [PubMed] [Google Scholar]
- 22.Gümüş N. Tumescent infiltration of lidocaine and adrenaline for burn surgery. Ann Burns Fire Disasters. 2011;24:144–148.. [PMC free article] [PubMed] [Google Scholar]
- 23.Blome-Eberwein S, Abboud M, Lozano DD, et al. Effect of subcutaneous epinephrine/saline/local anesthetic versus saline-only injection on split-thickness skin graft donor site perfusion, healing, and pain. J Burn Care Res. 2013;34:e80–e86.. [DOI] [PubMed] [Google Scholar]
- 24.Cohn MS, Seiger E, Goldman S. Ambulatory phlebectomy using the tumescent technique for local anesthesia. Dermatol Surg. 1995;21:315–318.. [DOI] [PubMed] [Google Scholar]
- 25.Vuylsteke ME, Mordon SR. Endovenous laser ablation: a review of mechanisms of action. Ann Vasc Surg. 2012;26:424–433.. [DOI] [PubMed] [Google Scholar]
- 26.Haines WY, Deets R, Lu N, et al. Tumescent anesthesia reduces pain associated with balloon angioplasty of hemodialysis fistulas. J Vasc Surg. 2012;56:1453–1456.. [DOI] [PubMed] [Google Scholar]
- 27.Behroozan DS, Goldberg LH. Dermal tumescent local anesthesia in cutaneous surgery. J Am Acad Dermatol. 2005;53:828–830.. [DOI] [PubMed] [Google Scholar]
- 28.Girard C, Debu A, Bessis D, et al. Treatment of Gorlin syndrome (nevoid basal cell carcinoma syndrome) with methylaminolevulinate photodynamic therapy in seven patients, including two children: interest of tumescent anesthesia for pain control in children. J Eur Acad Dermatol Venereol. 2013;27:e171–e175.. [DOI] [PubMed] [Google Scholar]
- 29.Ramon Y, Barak Y, Ullmann Y, et al. Pharmacokinetics of high-dose diluted lidocaine in local anesthesia for facelift procedures. Ther Drug Monit. 2007;29:644–647.. [DOI] [PubMed] [Google Scholar]
- 30.Abramson DL. Tumescent abdominoplasty: an ambulatory office procedure. Aesthetic Plast Surg. 1998;22:404–407.. [DOI] [PubMed] [Google Scholar]
- 31.Narita M, Sakano S, Okamoto S, et al. Tumescent local anesthesia in inguinal herniorrhaphy with a PROLENE hernia system: original technique and results. Am J Surg. 2009;198:e27–e31.. [DOI] [PubMed] [Google Scholar]
- 32.Locke M, Windsor J, Dunbar PR. Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ J Surg. 2009;79:235–244.. [DOI] [PubMed] [Google Scholar]
- 33.Mizukami T, Hamamoto M. Tumescent local anesthesia for a revascularization of a coronary subclavian steal syndrome. Ann Thorac Cardiovasc Surg. 2007;13:352–354.. [PubMed] [Google Scholar]
- 34.Prasetyono TO, Koswara AF. Linear hand burn contracture release under local anesthesia without tourniquet. Hand Surg. 2015;20:484–487.. [DOI] [PubMed] [Google Scholar]
- 35.Bashir MM, Qayyum R, Saleem MH, et al. Effect of time interval between tumescent local anesthesia infiltration and start of surgery on operative field visibility in hand surgery without tourniquet. J Hand Surg Am. 2015;40:1606–1609.. [DOI] [PubMed] [Google Scholar]
- 36.Lalonde D, Martin A. Tumescent local anesthesia for hand surgery: improved results, cost effectiveness, and wide-awake patient satisfaction. Arch Plast Surg. 2014;41:312–316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivers TE, McBride HA, Trang JM. Stability of cefazolin sodium and metronidazole at 8 degrees C for use as an i.v. admixture. J Parenter Sci Technol. 1993;47:135–137.. [PubMed] [Google Scholar]
- 38.Meyer NL, Hosier KV, Scott K, et al. Cefazolin versus cefazolin plus metronidazole for antibiotic prophylaxis at cesarean section. South Med J. 2003;96:992–995.. [DOI] [PubMed] [Google Scholar]
- 39.Morris WT, Innes DB, Richardson RA, et al. The prevention of post-appendicectomy sepsis by metronidazole and cefazolin: a controlled double blind trial. Aust N Z J Surg. 1980;50:429–433.. [DOI] [PubMed] [Google Scholar]
- 40.Brown GR, Clarke AM. Therapeutic interchange of cefazolin with metronidazole for cefoxitin. Am J Hosp Pharm. 1992;49:1946–1950.. [PubMed] [Google Scholar]
- 41.Hospenthal DR, Murray CK, Andersen RC, et al. ; Infectious Diseases Society of America; Surgical Infection Society. Guidelines for the prevention of infections associated with combat-related injuries: 2011 update: endorsed by the Infectious Diseases Society of America and the Surgical Infection Society. J Trauma. 2011;71:S210–S234.. [DOI] [PubMed] [Google Scholar]
- 42.Cho MJ, Kurtz RR, Lewis C, et al. Metronidazole phosphate—a water-soluble prodrug for parenteral solutions of metronidazole. J Pharm Sci. 1982;71:410–414.. [DOI] [PubMed] [Google Scholar]
- 43.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132; quiz 133.. [PubMed] [Google Scholar]
- 44.Bergamini TM, Peyton JC, Cheadle WG. Prophylactic antibiotics prevent bacterial biofilm graft infection. J Surg Res. 1992;52:101–105.. [DOI] [PubMed] [Google Scholar]
- 45.Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016;42:1535–1545.. [DOI] [PubMed] [Google Scholar]
- 46.De Waele JJ, Lipman J, Akova M, et al. Risk factors for target non-attainment during empirical treatment with β-lactam antibiotics in critically ill patients. Intensive Care Med. 2014;40:1340–1351.. [DOI] [PubMed] [Google Scholar]
- 47.Abdul-Aziz MH, Lipman J, Mouton JW, et al. Applying pharmacokinetic/pharmacodynamic principles in critically ill patients: optimizing efficacy and reducing resistance development. Semin Respir Crit Care Med. 2015;36:136–153.. [DOI] [PubMed] [Google Scholar]
- 48.Brill MJ, Houwink AP, Schmidt S, et al. Reduced subcutaneous tissue distribution of cefazolin in morbidly obese versus non-obese patients determined using clinical microdialysis. J Antimicrob Chemother. 2014;69:715–723.. [DOI] [PubMed] [Google Scholar]
- 49.Hoffstedt B, Walder M. Influence of serum protein binding and mode of administration on penetration of five cephalosporins into subcutaneous tissue fluid in humans. Antimicrob Agents Chemother. 1981;20:783–786.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badia JM, de la Torre R, Farré M, et al. Inadequate levels of metronidazole in subcutaneous fat after standard prophylaxis. Br J Surg. 1995;82:479–482.. [DOI] [PubMed] [Google Scholar]
- 51.Strukova EN, Portnoy YA, Zinner SH, et al. Predictors of bacterial resistance using in vitro dynamic models: area under the concentration-time curve related to either the minimum inhibitory or mutant prevention antibiotic concentration. J Antimicrob Chemother. 2016;71:678–684.. [DOI] [PubMed] [Google Scholar]
- 52.Hermsen R, Deris JB, Hwa T. On the rapidity of antibiotic resistance evolution facilitated by a concentration gradient. Proc Natl Acad Sci U S A. 2012;109:10775–10780.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hol FJ, Hubert B, Dekker C, et al. Density-dependent adaptive resistance allows swimming bacteria to colonize an antibiotic gradient. ISME J. 2016;10:30–38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zelenitsky SA, Ariano RE, Harding GK, et al. Antibiotic pharmacodynamics in surgical prophylaxis: an association between intraoperative antibiotic concentrations and efficacy. Antimicrob Agents Chemother. 2002;46:3026–3030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen EI, Friberg LE. Pharmacokinetic-pharmacodynamic modeling of antibacterial drugs. Pharmacol Rev. 2013;65:1053–1090.. [DOI] [PubMed] [Google Scholar]
- 56.Azevedo EF, Barbosa LA, DeBortoli Cassiani SH. Administration of antibiotics subcutaneously: an integrative literature review. Acta Paul Enferm. 2012;25:817–22.. [Google Scholar]
- 57.Robelet A, Caruba T, Corvol A, et al. [Antibiotics given subcutaneously to elderly]. Presse Med. 2009;38:366–376.. [DOI] [PubMed] [Google Scholar]
- 58.Frasca D, Marchand S, Petitpas F, et al. Pharmacokinetics of ertapenem following intravenous and subcutaneous infusions in patients. Antimicrob Agents Chemother. 2010;54:924–926.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker P, Neuhauser MN, Tam VH, et al. Subcutaneous administration of cefepime. J Pain Symptom Manage. 2005;30:170–174.. [DOI] [PubMed] [Google Scholar]
- 60.Melin-Coviaux F, Hary L, Hurtel AS, et al. Etude pharmaco-clinique comparative de la ceftriaxone par voie sous-cutanee et intraveineuse chez la personne agee. Revue Geriatr. 2000;25:337–347.. [Google Scholar]
- 61.Bricaire F, Castaing JL, Pocidalo JJ, et al. [Pharmacokinetics and tolerance of ceftriaxone after subcutaneous administration]. Pathol Biol (Paris). 1988;36:702–705.. [PubMed] [Google Scholar]
- 62.Borner K, Lode H, Hampel B, et al. Comparative pharmacokinetics of ceftriaxone after subcutaneous and intravenous administration. Chemotherapy. 1985;31:237–245.. [DOI] [PubMed] [Google Scholar]
- 63.Gauthier D, Schambach S, Crouzet J, et al. Subcutaneous and intravenous ceftriaxone administration in patients more than 75 years of age. Med Mal Infect. 2014;44:275–280.. [DOI] [PubMed] [Google Scholar]
- 64.Barbot A, Venisse N, Rayeh F, et al. Pharmacokinetics and pharmacodynamics of sequential intravenous and subcutaneous teicoplanin in critically ill patients without vasopressors. Intensive Care Med. 2003;29:1528–1534.. [DOI] [PubMed] [Google Scholar]
- 65.Champoux N, Du Souich P, Ravaoarinoro M, et al. Single-dose pharmacokinetics of ampicillin and tobramycin administered by hypodermoclysis in young and older healthy volunteers. Br J Clin Pharmacol. 1996;42:325–331.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeong SJ, Ann HW, Kim JK, et al. Incidence and risk factors for surgical site infection after gastric surgery: a multicenter prospective cohort study. Infect Chemother. 2013;45:422–430.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goede WJ, Lovely JK, Thompson RL, et al. Assessment of prophylactic antibiotic use in patients with surgical site infections. Hosp Pharm. 2013;48:560–567.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adembri C, Ristori R, Chelazzi C, et al. Cefazolin bolus and continuous administration for elective cardiac surgery: improved pharmacokinetic and pharmacodynamic parameters. J Thorac Cardiovasc Surg. 2010;140:471–475.. [DOI] [PubMed] [Google Scholar]
- 69.Stratford AF, Zoutman DE, Davidson JS. Effect of lidocaine and epinephrine on Staphylococcus aureus in a guinea pig model of surgical wound infection. Plast Reconstr Surg. 2002;110:1275–1279.. [DOI] [PubMed] [Google Scholar]
- 70.Lu CW, Lin TY, Shieh JS, et al. Antimicrobial effect of continuous lidocaine infusion in a Staphylococcus aureus-induced wound infection in a mouse model. Ann Plast Surg. 2014;73:598–601.. [DOI] [PubMed] [Google Scholar]
- 71.Parr AM, Zoutman DE, Davidson JS. Antimicrobial activity of lidocaine against bacteria associated with nosocomial wound infection. Ann Plast Surg. 1999;43:239–245.. [DOI] [PubMed] [Google Scholar]
- 72.Igarashi T, Suzuki T, Mori K, et al. The effects of epidural anesthesia on growth of Escherichia coli at pseudosurgical site: the roles of the lipocalin-2 pathway. Anesth Analg. 2015;121:81–89.. [DOI] [PubMed] [Google Scholar]
- 73.Lee S, Goodchild SJ, Ahern CA. Local anesthetic inhibition of a bacterial sodium channel. J Gen Physiol. 2012;139:507–516.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodrigues AA, Pina-Vaz C, Mårdh PA, et al. Inhibition of germ tube formation by Candida albicans by local anesthetics: an effect related to ionic channel blockade. Curr Microbiol. 2000;40:145–148.. [DOI] [PubMed] [Google Scholar]
- 75.Johnson SM, Saint John BE, Dine AP. Local anesthetics as antimicrobial agents: a review. Surg Infect (Larchmt). 2008;9:205–213.. [DOI] [PubMed] [Google Scholar]
- 76.Nachmias V, Sullender J, Asch A. Shape and cytoplasmic filaments in control and lidocaine-treated human platelets. Blood. 1977;50:39–53.. [PubMed] [Google Scholar]
- 77.Huang GS, Lin TC, Wang JY, et al. Lidocaine priming reduces ADP-induced P-selectin expression and platelet-leukocyte aggregation. Acta Anaesthesiol Taiwan. 2009;47:56–61.. [DOI] [PubMed] [Google Scholar]
- 78.Tobias MD, Pilla MA, Rogers C, et al. Lidocaine inhibits blood coagulation: implications for epidural blood patch. Anesth Analg. 1996;82:766–769.. [DOI] [PubMed] [Google Scholar]
- 79.Grant GJ, Ramanathan S, Patel N, et al. The effects of local anesthetics on maternal and neonatal platelet function. Acta Anaesthesiol Scand. 1989;33:409–412.. [DOI] [PubMed] [Google Scholar]
- 80.Pinto LM, Pereira R, de Paula E, et al. Influence of liposomal local anesthetics on platelet aggregation in vitro. J Liposome Res. 2004;14:51–59.. [DOI] [PubMed] [Google Scholar]
- 81.Klein JA. Tumescent technique for local anesthesia improves safety in large-volume liposuction. Plast Reconstr Surg. 1993;92:1085–1098; discussion 1099.. [PubMed] [Google Scholar]
- 82.Hahnenkamp K, Theilmeier G, Van Aken HK, et al. The effects of local anesthetics on perioperative coagulation, inflammation, and microcirculation. Anesth Analg. 2002;94:1441–1447.. [DOI] [PubMed] [Google Scholar]
- 83.Wright JL, Durieux ME, Groves DS. A brief review of innovative uses for local anesthetics. Curr Opin Anaesthesiol. 2008;21:651–656.. [DOI] [PubMed] [Google Scholar]
- 84.Hatakeyama N, Matsuda N. Alert cell strategy: mechanisms of inflammatory response and organ protection. Curr Pharm Des. 2014;20:5766–5778.. [DOI] [PubMed] [Google Scholar]
- 85.Berger C, Rossaint J, Van Aken H, et al. Lidocaine reduces neutrophil recruitment by abolishing chemokine-induced arrest and transendothelial migration in septic patients. J Immunol. 2014;192:367–376.. [DOI] [PubMed] [Google Scholar]
- 86.Wang HL, Liu YY, Yan HD, et al. Intraoperative systemic lidocaine inhibits the expression of HMGB1 in patients undergoing radical hysterectomy. Int J Clin Exp Med. 2014;7:3398–3403.. [PMC free article] [PubMed] [Google Scholar]
- 87.Wang HL, Zhang WH, Lei WF, et al. The inhibitory effect of lidocaine on the release of high mobility group box 1 in lipopolysaccharide-stimulated macrophages. Anesth Analg. 2011;112:839–844.. [DOI] [PubMed] [Google Scholar]
- 88.Van Der Wal S, Vaneker M, Steegers M, et al. Lidocaine increases the anti-inflammatory cytokine IL-10 following mechanical ventilation in healthy mice. Acta Anaesthesiol Scand. 2015;59:47–55.. [DOI] [PubMed] [Google Scholar]
- 89.Lee PY, Tsai PS, Huang YH, et al. Inhibition of toll-like receptor-4, nuclear factor-kappaB and mitogen-activated protein kinase by lignocaine may involve voltage-sensitive sodium channels. Clin Exp Pharmacol Physiol. 2008;35:1052–1058.. [DOI] [PubMed] [Google Scholar]
- 90.Kiyonari Y, Nishina K, Mikawa K, et al. Lidocaine attenuates acute lung injury induced by a combination of phospholipase A2 and trypsin. Crit Care Med. 2000;28:484–489.. [DOI] [PubMed] [Google Scholar]
- 91.Nishina K, Mikawa K, Takao Y, et al. Intravenous lidocaine attenuates acute lung injury induced by hydrochloric acid aspiration in rabbits. Anesthesiology. 1998;88:1300–1309.. [DOI] [PubMed] [Google Scholar]
- 92.Benlier E, Eskiocak S, Puyan FO, et al. Effect of lidocaine on reducing injury in a rat electrical burn model. Ann Plast Surg. 2012;69:152–156.. [DOI] [PubMed] [Google Scholar]
- 93.Liu J, Zhang H, Qi Z, et al. Lidocaine protects against renal and hepatic dysfunction in septic rats via downregulation of Toll–like receptor 4. Mol Med Rep. 2014;9:118–124.. [DOI] [PubMed] [Google Scholar]
- 94.Peiró JR, Barnabé PA, Cadioli FA, et al. Effects of lidocaine infusion during experimental endotoxemia in horses. J Vet Intern Med. 2010;24:940–948.. [DOI] [PubMed] [Google Scholar]
- 95.Gallos G, Jones DR, Nasr SH, et al. Local anesthetics reduce mortality and protect against renal and hepatic dysfunction in murine septic peritonitis. Anesthesiology. 2004;101:902–911.. [DOI] [PubMed] [Google Scholar]
- 96.Schmidt W, Schmidt H, Bauer H, et al. Influence of lidocaine on endotoxin-induced leukocyte-endothelial cell adhesion and macromolecular leakage in vivo. Anesthesiology. 1997;87:617–624.. [DOI] [PubMed] [Google Scholar]
- 97.De Oliveira GS, Jr, Fitzgerald P, Streicher LF, et al. Systemic lidocaine to improve postoperative quality of recovery after ambulatory laparoscopic surgery. Anesth Analg. 2012;115:262–267.. [DOI] [PubMed] [Google Scholar]
- 98.Fierheller EE, Caulkett NA, Haley DB, et al. Onset, duration and efficacy of four methods of local anesthesia of the horn bud in calves. Vet Anaesth Analg. 2012;39:431–435.. [DOI] [PubMed] [Google Scholar]
- 99.Katz J, Clarke H, Seltzer Z. Review article: preventive analgesia: quo vadimus? Anesth Analg. 2011;113:1242–1253.. [DOI] [PubMed] [Google Scholar]
- 100.Rosaeg OP, Bell M, Cicutti NJ, et al. Pre-incision infiltration with lidocaine reduces pain and opioid consumption after reduction mammoplasty. Reg Anesth Pain Med. 1998;23:575–579.. [DOI] [PubMed] [Google Scholar]
- 101.Cui W, Li Y, Li S, et al. Systemic administration of lidocaine reduces morphine requirements and postoperative pain of patients undergoing thoracic surgery after propofol-remifentanil-based anaesthesia. Eur J Anaesthesiol. 2010;27:41–46.. [DOI] [PubMed] [Google Scholar]
- 102.McKay W, Morris R, Mushlin P. Sodium bicarbonate attenuates pain on skin infiltration with lidocaine, with or without epinephrine. Anesth Analg. 1987;66:572–574.. [PubMed] [Google Scholar]
- 103.Coldiron BM, Healy C, Bene NI. Office surgery incidents: what seven years of Florida data show us. Dermatol Surg. 2008;34:285–291; discussion 291.. [DOI] [PubMed] [Google Scholar]
- 104.Grazer FM, de Jong RH. Fatal outcomes from liposuction: census survey of cosmetic surgeons. Plast Reconstr Surg. 2000;105:436–446; discussion 447.. [DOI] [PubMed] [Google Scholar]
- 105.Lehnhardt M, Homann HH, Daigeler A, et al. Major and lethal complications of liposuction: a review of 72 cases in Germany between 1998 and 2002. Plast Reconstr Surg. 2008;121:396e–403e.. [DOI] [PubMed] [Google Scholar]
- 106.Habbema L. Safety of liposuction using exclusively tumescent local anesthesia in 3,240 consecutive cases. Dermatol Surg. 2009;35:1728–1735.. [DOI] [PubMed] [Google Scholar]


