Abstract
Background:
The vascularized free fibula epiphyseal transfer provides an option for the preservation of limb lengthening after resection of the proximal humerus in pediatric sarcoma patients. The purpose of this study was to provide a long-term follow-up of longitudinal growth patterns and outcomes after free fibula epiphyseal transfer in upper extremity reconstruction.
Methods:
A retrospective review of 4 patients who underwent free fibula epiphyseal transfer after oncologic resection of the proximal humerus for osteosarcoma was performed. Oncologic details that could affect outcomes were included in the review: primary tumor pathology, location of malignancy, and presence of recurrence. Details on the reconstruction included longitudinal growth of the flap from the time of implantation to the most recently available radiograph and postoperative complications. The length of the fibula over time was measured from the humeral head to the olecranon process.
Results:
All patients were alive at the start of this study. The average longitudinal growth rate of the free fibula epiphyseal transfer was 0.54 ± 0.18 cm/y, and patients demonstrated satisfactory and consistent longitudinal bone growth and hypertrophy over time. All 4 patients suffered from a complication of postoperative fibula graft fracture, and 1 of 4 patients experienced unremitting peroneal nerve damage. All patients demonstrated normal wrist and hand motion with a normal arc of elbow flexion and extension.
Conclusion:
This study demonstrates that the vascularized fibula epiphyseal transfer offers the ability to preserve longitudinal limb growth and hypertrophy throughout adolescence.
INTRODUCTION
Pediatric patients undergoing oncologic resection of the proximal humerus require a durable reconstruction that can facilitate longitudinal growth in the years after surgery. Vascularized free fibula epiphyseal transfer for upper extremity reconstruction is an appealing option in the skeletally immature patient.1–4 By providing cortical bone length with use of the ossification centers at the epiphyseal plate, patients maintain limb continuity with the glenohumeral joint, preserve hand and elbow function, and retain both limb length and growth potential. Although prior literature has described vascularized fibula epiphyseal transfers, few have detailed the longitudinal growth patterns and complications over time in a series of pediatric patients.2–6
The purpose of this study was to provide a follow-up account of 4 skeletally immature patients who underwent oncologic resection of the proximal humerus for osteosarcoma and who underwent reconstruction via vascularized free fibula epiphyseal transfer. The assessment includes details of longitudinal growth patterns, hypertrophy index, and complications. These findings will aid in further defining the ability of vascularized free fibula epiphyseal transfers to preserve bone growth in the pediatric patient population.
METHODS
A retrospective review of 4 patients at Duke University Medical Center who underwent free fibula epiphyseal transfer after oncologic resection of osteosarcoma affecting the proximal humerus was performed. Specific oncologic details reviewed include primary tumor pathology, the presence of local or metastatic recurrence, and the need for adjuvant therapy. Reconstructive outcomes included the longitudinal growth of the graft from the first postoperative image to the most recently available radiograph, graft hypertrophy, and postoperative complications. The length of the graft over time was measured from the humeral head to the olecranon process. Furthermore, the patient’s height was recorded from each follow-up visit to better define overall variations in skeletal growth. Graft hypertrophy was determined by the DeBoer and Wood graft hypertrophy index as previously described.2 The surgical technique utilized for free fibula epiphyseal transfer was previously described by Erdmann et al.6 For fibula harvest, the donor leg is marked from the lateral malleolus to the proximal fibula head at the insertion of the biceps femoris while the patient is supine with the donor leg flexed at the hip and knee. The fibula head is palpable at the proximal lateral aspect of the lower leg. A longitudinal skin incision is made, and the superficial peroneal nerve is identified and protected as the overlying fascia is divided. The deep peroneal nerve and the anterior tibial artery and vein are then dissected between the anterior tibialis and extensor digitorum longus muscles. The deep peroneal nerve is protected while branches of the anterior tibial artery and vein are ligated. However, in some instances, nerve branches to the peroneal musculature must be divided and coapted after fibula harvest. The distal fibula osteotomy is then performed based on the dimensions of the humerus defect. The vascularized fibula graft is then transferred for reconstruction of the proximal humerus.
Statistical Analysis
A random coefficients linear mixed-effects model was fit to the longitudinal data of the patients who underwent free fibula epiphyseal transfer. The assumption is that the mean growth of the reconstructed humerus can be modeled as a line curve for each patient. The model can be expressed in mathematical notation as follows: 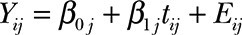 , where Yij is the observed humerus length (measured in mm) for the j-th patient (j = 1, 2, 3, and 4) at the time of the i-th follow-up visit measured in months, tij is the associated time of measurement from the first radiograph measurement, and Eij is a random error term assumed to be normally distributed with zero mean and constant variance, henceforth denoted as
, where Yij is the observed humerus length (measured in mm) for the j-th patient (j = 1, 2, 3, and 4) at the time of the i-th follow-up visit measured in months, tij is the associated time of measurement from the first radiograph measurement, and Eij is a random error term assumed to be normally distributed with zero mean and constant variance, henceforth denoted as 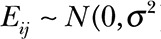 . The intercept
. The intercept 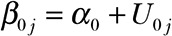 and slope
and slope 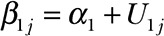 terms associated with each patient curve are both random, parameters α0 and α1 are fixed and need to be estimated from the data, and U0j and U1j are random terms bivariate normally distributed
terms associated with each patient curve are both random, parameters α0 and α1 are fixed and need to be estimated from the data, and U0j and U1j are random terms bivariate normally distributed 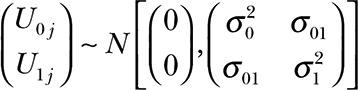 . The model allows us to estimate an overall intercept (α0, humerus length at time zero in mm) and slope (α1, growth rate in mm per month) while accounting for the repeated measurements taken on each patient. The linear mixed model was fit using the restricted maximum likelihood method and the degrees of freedom for testing the fixed effects were obtained using the method proposed by Kenward and Roger.7–9 The association between the observed patients’ height and humeral length was assessed with the Pearson’s product moment correlation coefficient, and the associated asymptotic confidence interval was estimated based on Fisher’s Z transformation. In all cases, the threshold for assessing statistical significance was set to α = 0.05.
. The model allows us to estimate an overall intercept (α0, humerus length at time zero in mm) and slope (α1, growth rate in mm per month) while accounting for the repeated measurements taken on each patient. The linear mixed model was fit using the restricted maximum likelihood method and the degrees of freedom for testing the fixed effects were obtained using the method proposed by Kenward and Roger.7–9 The association between the observed patients’ height and humeral length was assessed with the Pearson’s product moment correlation coefficient, and the associated asymptotic confidence interval was estimated based on Fisher’s Z transformation. In all cases, the threshold for assessing statistical significance was set to α = 0.05.
RESULTS
Case 1
A 10-year-old male presented with right shoulder pain for several weeks. Radiographic findings revealed a destructive bony lesion in the proximal humerus found to be low-grade osteosarcoma upon biopsy (Fig. 1A). After neoadjuvant chemotherapy, the patient underwent oncologic resection of the proximal humerus with free vascularized osteocutaneous fibula flap for immediate reconstruction (Fig. 1B). Approximately 8 months postoperatively, the patient sustained a transverse fracture at the proximal screw that was managed nonoperatively with a hanging arm cast and subsequent Sarmiento brace (Fig. 1C). Five months after the fracture, the patient’s radiographs demonstrated appropriate callus formation and complete bony union at the fracture site (Fig. 1D). At his most recent clinic visit (Fig. 1E), the patient’s flap had grown approximately 39 mm over 11 years and was approximately 4 cm shorter than the contralateral arm. On physical examination, the patient was described to have normal hand and elbow function. Regarding shoulder function, he retained the ability to passively swing his hand to his head.
Fig. 1.
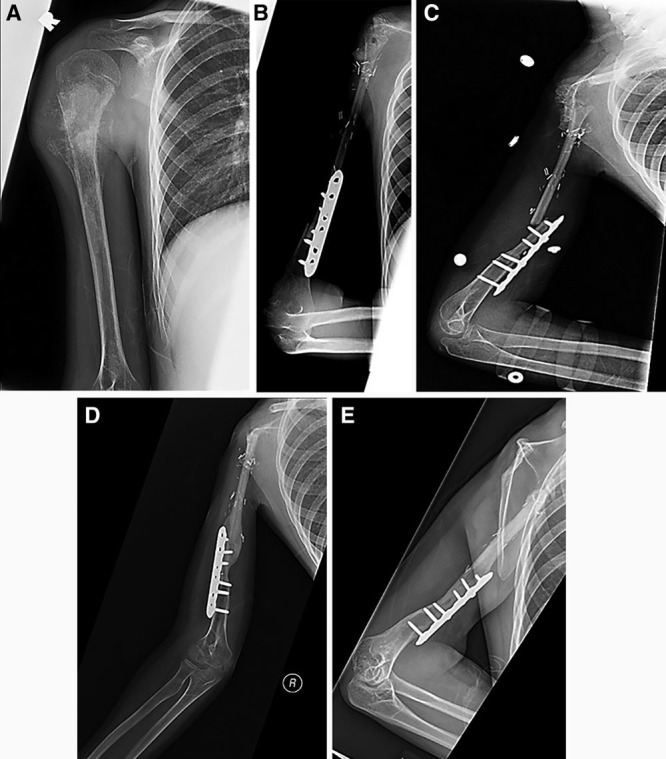
Representative radiographs from case number 1. Preoperative films displaying the oncologic lesions are shown (A). After resection, a vascularized fibula physis transfer was performed on the same operative day (B). Approximately 8 months after the fibula transfer, the patient suffered a fracture through the graft (C). After nonoperative management, sufficient healing bony union and graft survival was observed at 5 months’ post fracture (D) and 11 years postoperatively (E).
Case 2
A 11-year-old male presented for evaluation of right shoulder pain. Initial radiographs revealed a pathologic fracture at the right proximal humerus, with a destructive metaphyseal mass involving the glenohumeral joint. Biopsy of the mass revealed the diagnosis of high-grade osteosarcoma. The patient received 9 weeks of neoadjuvant chemotherapy before undergoing wide resection of the right proximal humerus and reconstruction with vascularized free fibula epiphyseal transfer (Fig. 2A). The postoperative course was uneventful with annual radiographs demonstrating adequate bone remodeling and corticalization. Eight years after the initial operation, the patient suffered trauma to his arm. Radiographs revealed a transverse fracture with separation of the cortical fixation plate and screws from the fibula graft (Fig. 2B). The patient is currently being managed nonoperatively with a Sarmiento brace for 6 months. Before this injury, the patient displayed scant range of motion of the shoulder, with the ability to flex and extend at the elbow. Regarding wrist function, the patient could flex but was unable to extend.
Fig. 2.
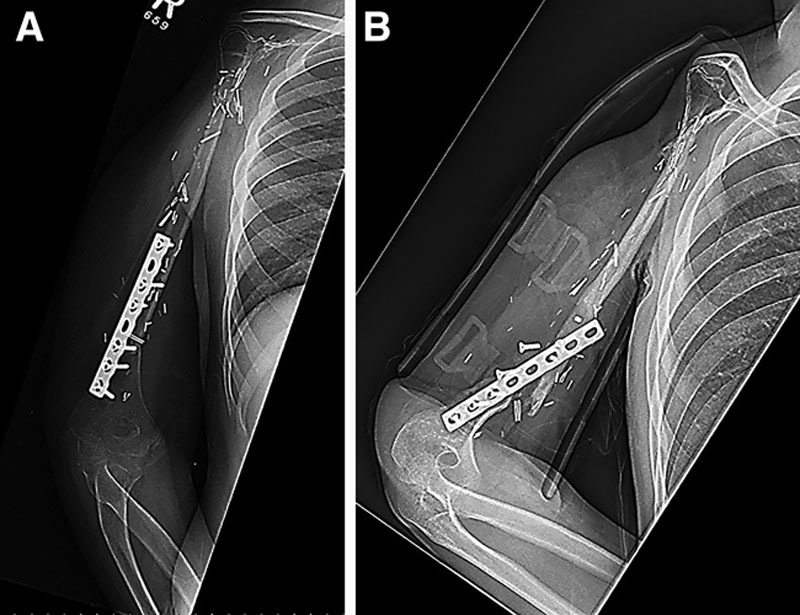
Representative radiographs from case number 2. A, The transplanted fibula flap after oncologic resection of proximal humeral osteosarcoma. B, A transverse fracture suffered 8 years after the initial operation.
Case 3
A 4-year-old man presented with spontaneous shoulder pain and swelling. Radiographs revealed a destructive lesion of the proximal humerus, with biopsy confirming high-grade osteosarcoma. Given the tumor’s rate of growth, oncologic resection of the tumor without immediate reconstruction was elected and a suspension plasty of the humerus was performed (Fig. 3A). Eleven months after resection of the lesion, the patient underwent delayed reconstruction with a left vascularized proximal free fibula flap to the left shoulder joint (Fig. 3B). He then sustained a fracture at the fixation site 6 weeks postoperatively. This was managed with nonoperative observation and sling immobilization (Fig. 3D). Five months after the reconstructive procedure, radiographs demonstrated appropriate callus formation at the fracture site; at 1-year follow-up, there was complete union and remodeling of the prior fracture site (Fig. 3C). At the time of the most recent clinic visit, the patient’s humeral length was noted to be increasing as expected. The patient’s graft has increased in length by about 60 mm over the course of 5 years. On physical examination, the patient exhibited a 90-degree arch of motion through his elbow and normal hand function. He displayed no active motion of the shoulder.
Fig. 3.
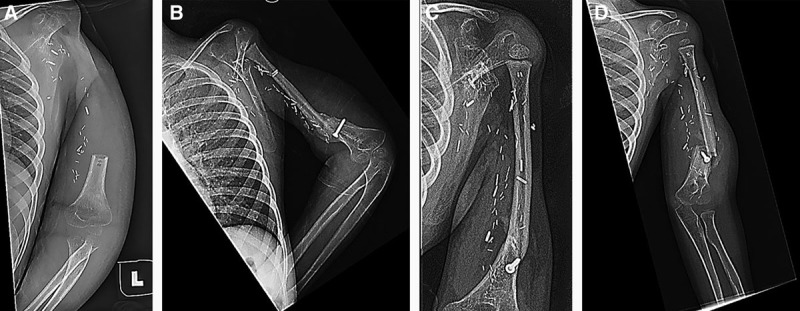
Representative images from case number 3. The patient underwent emergent oncologic resection of proximal humeral osteosarcoma (A), followed by a vascularized fibula physis flap 11 months after the initial oncologic resection (B). After nonoperative management, films demonstrated adequate bone remodeling and callus formation at 1 year (C). Six weeks after humeral reconstruction, the patient sustained a fracture through the fibula graft (D).
Case 4
A 5-year-old woman presented with sudden onset pain in her left arm. Radiographic evaluation revealed an aggressive tumor, involving both the metaphysis and diaphysis with a pathological fracture, and biopsy confirmed the diagnoses as a high-grade osteosarcoma. The patient underwent 6 weeks of neoadjuvant chemotherapy before oncologic resection and immediate reconstruction with a vascularized proximal fibula flap. The postoperative period was uneventful with appropriate corticalization and bone remodeling seen during annual radiographic surveillance. Four years postoperatively the patient sustained a transverse fracture of the fibula graft (Fig. 4). She was managed with nonoperative observation in a Sarmiento brace. The most recent radiographs revealed a healing fibula graft, and the fracture line was no longer clearly visualized (Fig. 4). On physical examination, the patient was noted to have normal elbow and hand function, with no active forward flexion or motion of the shoulder.
Fig. 4.
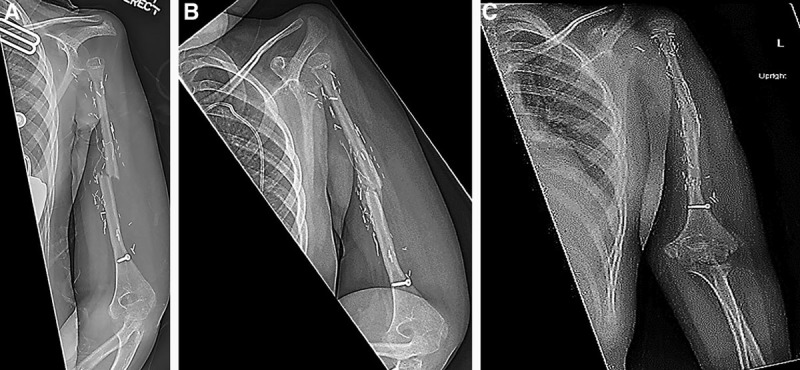
Representative images from case number 4. Four years after the initial operation utilizing the vascularized free fibula physis transfer, the patient sustained a transverse fracture of the mid fibula shaft (A). The patient’s films demonstrated continued healing and callus formation (B). At her most recent clinic appointment, the patient’s films displayed a healed fibula graft, with no evidence of the previously seen fracture line (C).
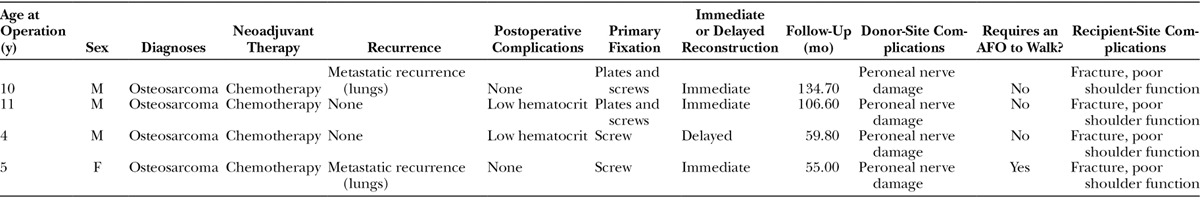
Demographic and Operative Details
As shown in Table 1, 3 male patients and 1 female patient were identified. The mean age at the time of fibula transfer was 7.5 years (range, 4–11). All 4 cases were primary bone tumors and were diagnosed as osteosarcoma. All 4 patients received preoperative chemotherapy, and no patient received radiation treatment. The mean follow-up time was 88.9 months (range, 54.8–134.4). Wide resection of the primary tumor was performed in all cases. Three of 4 reconstructions were performed at the time of tumor resection, whereas 1 reconstruction was delayed. The mean operation time was 690.75 minutes (range, 571–898), and the estimated blood loss during the operation was 412.5 mL (range, 250–700). Osteosynthesis involved the use of plates and screws (n = 2), and screws alone (n = 2).
Table 1.
Demographic Data and Pertinent Medical History
Growth and Hypertrophy of Vascularized Fibula-Based Physis Transfers Over Time
Based on N = 60, measurements unevenly grouped into 4 patients, the rate of growth was estimated to be 0.451 ± 0.150 mm/mo or 0.54 ± 0.18 cm/y (t test; P = 0.0262; Table 2). The variance component estimates from the random coefficients model (Table 3) quantify the variance attributable to each random effect. The estimated variance of the intercept term ( ) reflects the dispersion of humerus length at the first follow-up visit after surgery, which is large in our study mainly due to the age differences among patients. The narrow variance associated with the slope coefficient (
) reflects the dispersion of humerus length at the first follow-up visit after surgery, which is large in our study mainly due to the age differences among patients. The narrow variance associated with the slope coefficient (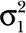 ) suggests that the growth rates across patients are comparable with each other. Geometrically, this would translate into approximately parallel growth lines for the patients. The growth pattern of patients’ humerus length as a function of time (Fig. 5) and the curves described by the patients’ height as a function of time (Fig. 6) are similar. The sample Pearson’s product moment correlation between patients’ height and humerus length (Table 4) indicates the presence of a strong positive association between the skeletal growth and the vascularized fibula transfers over time. Furthermore, the mean graft hypertrophy index increased by more than 10%, with a mean increase of 47.69% (range, 14.5–66.2%).
) suggests that the growth rates across patients are comparable with each other. Geometrically, this would translate into approximately parallel growth lines for the patients. The growth pattern of patients’ humerus length as a function of time (Fig. 5) and the curves described by the patients’ height as a function of time (Fig. 6) are similar. The sample Pearson’s product moment correlation between patients’ height and humerus length (Table 4) indicates the presence of a strong positive association between the skeletal growth and the vascularized fibula transfers over time. Furthermore, the mean graft hypertrophy index increased by more than 10%, with a mean increase of 47.69% (range, 14.5–66.2%).
Table 2.
Fixed Effects Coefficient Estimates for the Linear Mixed-Effects Model, Standard Error, Approximated Degrees of Freedom Using Kenward–Roger Method, t Test Value, and Associated P Value for the Null Hypothesis of No Effect
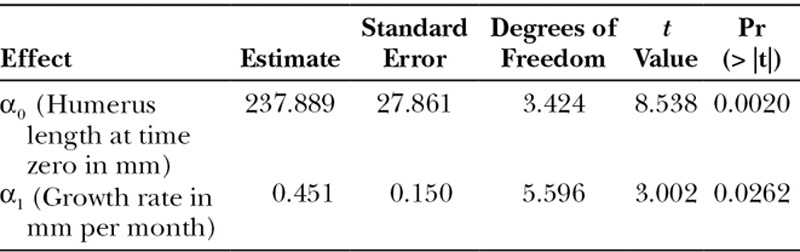
Table 3.
Variance Component Estimates of the Random Coefficients Model
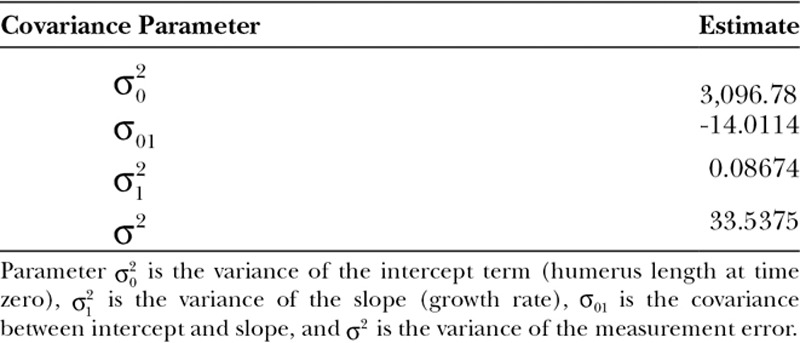
Fig. 5.
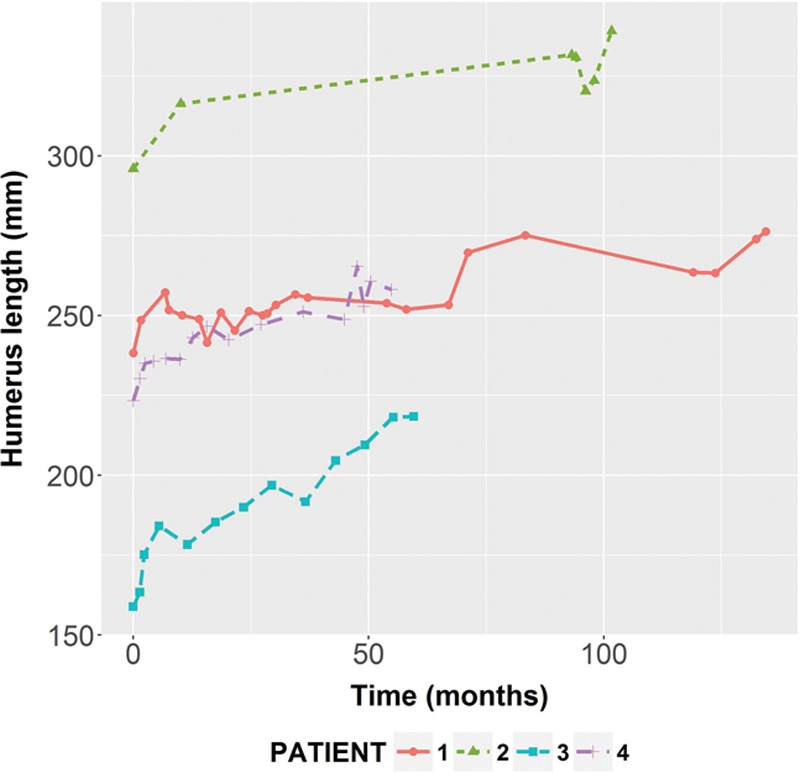
The longitudinal growth of the vascularized fibula epiphyseal transfer for reconstruction of the proximal humerus. Radiographs were obtained at each consecutive year post transplant, and fibula length was measured from the humeral head to the olecranon process. Overall, patients demonstrated steady and continued growth of the transplanted fibula. Patient 1 did not have radiographs available for analysis between years 2 and 8. Patients 3 and 4 were followed until their most recent clinical visit, which was 4 years postoperatively. The average growth rate per year was 0.54 cm.
Fig. 6.
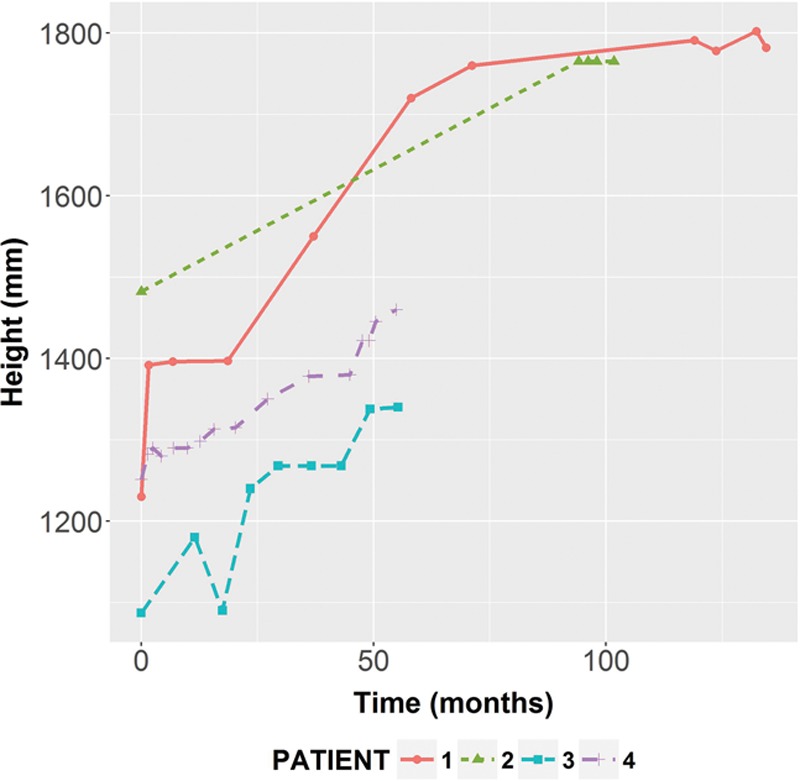
Patients’ height as a function of time. The overall linear association between the patients’ height and the vascularized fibula-based epiphyseal transfer was very strong.
Table 4.
Sample Pearson’s Product Moment Correlation Coefficient between Body Height and Reconstructed Humerus Length for Each Patient in the Study and for All Patients Combined (Overall)

Longitudinal Outcomes and Complications
All patients are still alive and retained a functional salvaged limb during the follow-up period. Fibula graft fracture (n = 4) and peroneal nerve palsy (n = 4) were the most common complications. Two patients experienced a low hematocrit requiring transfusion in the immediate postoperative period. No patients required a reoperation related to the initial procedure. Two patients suffered from the development of metastatic recurrence to the lungs. Complete bony union was noted to be present in all 4 patients after surgery. Of the 4 patients who suffered from a complication of graft fracture, 3 have successfully regained bony union with nonoperative management. One patient continues to have nonunion at the fracture site. All 4 patients suffered from damage to the peroneal nerve after the procedure, described as a lack of sensation on the dorsum of the foot with weakened dorsiflexion. Three of 4 patients have regained the ability to walk without an ankle-foot orthosis (AFO). One patient maintains sensory deficits and requires the use of an AFO for mobility. Regarding upper extremity function, all patients maintain good elbow and hand function. However, all patients lack active motion and forward flexion of the shoulder.
DISCUSSION
Limb lengthening is dependent on the successful transfer of the epiphysis. Although there is an abundance of literature describing the successful transfer of vascularized fibula bone flaps, few discuss the long-term outcomes of bone growth and hypertrophy.10–12 The proximal humeral epiphysis accounts for 40% of longitudinal arm growth and has the highest incidence of osteosarcoma in the upper extremity.13 With the successful microvascular transfer of the fibula epiphyseal plate, it is possible to maintain bone growth over time after oncologic resection of proximal humeral tumors.14 In our series, longitudinal bone growth was demonstrated in all patients who received an epiphyseal transfer. The largest published series of vascularized epiphyseal transplants to the upper extremity by Innocenti et al.12,15 described an annual growth rate of 0.7–1.35 cm/y. In a smaller case series, Zelenski et al.2 demonstrated a growth rate of 1.72 cm/y. In our study, the average growth rate was 0.54 ± 0.18 cm/y (0.45 ± 0.15 mm/mo), and each patient demonstrated satisfactory and consistent longitudinal bone growth over time. Knowing that the majority of epiphyseal closure is completed by 17 years, 2 patients in this study (patients 1 and 2) were able to be followed throughout the time of complete skeletal growth.16 Both of these patients exhibited sustained bone growth, with minor discrepancies in limb length reported at the most recent clinic visits. One patient (patient 3) developed a varus deformity of the transferred fibula. Although the graft continues to grow, the anatomic deformity may result in larger longitudinal growth discrepancies as compared with the other patients in this series. Furthermore, patients demonstrated adequate graft hypertrophy over time consistent with previous studies.2 The growth patterns and bony hypertrophy demonstrated in these patients support the idea that vascularized epiphyseal transfers of the fibula are an ideal option for humeral reconstruction in the pediatric patient population.
As prior studies have demonstrated, graft fracture is one of the most common complications of this operation, occurring at rates of 20–40%.17–19 In our study, all 4 cases suffered from a complication of postoperative fracture. Two patients suffered a fracture within the first postoperative year, and 2 patients experienced a fracture at 4 and 8 years postoperatively. The high fracture rate in this population may be due to the relatively reduced weight-bearing potential of the recipient limb after initial transfer. Furthermore, the young age of this patient cohort naturally renders them less compliant to long periods of immobilization and predisposes these patients to activities that may place them at a higher risk for suffering a fracture of their graft.2,17 The authors advocate for the use of compression plate fixation at the osteosynthesis site and careful protection of the extremity to limit the incidence of graft fracture. It should be noted, however, that the fracture rates seen within 1 year postoperatively do not vary significantly from prior reports. Fractures that occur after 1 year may not be directly attributable to the vascularized graft and a direct result of the surgery. Alternative osteosynthesis such as a bridge plate may be considered in the future to reduce the risk of postoperative fracture.17 A larger cohort is needed to make a more conclusive statement on the likelihood of fracture in this population. A primary advantage of the vascularized fibula flap is that bone union rates after fracture have been reported to be significantly higher than avascular constructs, which demonstrate a higher fracture rate and weaker bone formation.15,20 In addition, the ability of vascularized fibula flaps to continue lengthening after a fracture is critically important in the pediatric population. In our study, all 3 grafts that were successfully managed with Sarmiento brace and/or casting progressed to union with no impact on bone growth. This is similar to findings in other studies that found consistent bone lengthening over time after traumatic injury.15,20
A disadvantage of using a vascularized fibula graft is that a healthy fibula is utilized resulting in a potential functional and sensory deficit at the donor site, mainly involving the common peroneal nerve. In addition, because resection of malignant tumors involving the proximal humerus often requires dissections of the deltoid muscle, rotator cuff muscles, and the axillary nerve, patients may lose abduction, flexion, and extension at the shoulder. However, most patients will retain excellent elbow, wrist, and hand function.6,21 In our study, only 1 patient experienced peroneal nerve damage leading to unremitting foot drop and dorsal foot numbness, requiring the use of an AFO to walk. The frequency of this injury is due to the proximity of the peroneal nerve to the anterior tibial vascular supply of the fibula head, making isolation and protection of this nerve difficult.18,22,23 Caution must be taken to isolate and protect this nerve during fibula dissection. Some surgeons advocate for dividing branches of the peroneal nerve at the musculature and performing coaptation after fibula harvest. Similar to previous studies, our findings suggest that the majority of cases presenting with postoperative peroneal nerve damage will resolve over time and that patients will regain the ability to dorsiflex their foot.2,18 With regard to shoulder function, at the most recent patient follow-ups (4–11 years postoperatively), all patients demonstrated very minimal active shoulder abduction and forward flexion. Their active range of abduction ranged from 10° to 30°, whereas their passive range of motion spanned from 45° to 90°. Poor postoperative shoulder function is most likely due to an inability to adequately reattach the rotator cuff to the fibula graft after rotator cuff excision and potential compromise of the axillary nerve during dissection. Similar to previous studies, all patients exhibited normal wrist and hand motion along with a normal arc of elbow flexion and extension.12,18
CONCLUSIONS
In the pediatric patient who requires oncologic resection and reconstruction of an extremity involving the epiphyseal plate, the vascularized fibula epiphyseal transfer offers the ability to preserve longitudinal limb growth throughout adolescence. Furthermore, this study emphasizes the need for future technical advances and larger prospective studies to further minimize postoperative complications.
Footnotes
Institutional review board approval was obtained for this study.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Isakoff MS, Bielack SS, Meltzer P, et al. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33:3029–3035.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zelenski N, Brigman BE, Levin LS, et al. The vascularized fibular graft in the pediatric upper extremity: a durable, biological solution to large oncologic defects. Sarcoma. 2013;2013:321201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldekhayel S, Govshievich A, Neel OF, et al. Vascularized proximal fibula epiphyseal transfer for distal radius reconstruction in children: a systematic review. Microsurgery. 2016;36:705–711.. [DOI] [PubMed] [Google Scholar]
- 4.Bibbo C, Ehrlich DA, Kovach SJ., 3rd Reconstruction of the pediatric lateral malleolus and physis by free microvascular transfer of the proximal fibular physis. J Foot Ankle Surg. 2015;54:994–1000.. [DOI] [PubMed] [Google Scholar]
- 5.Innocenti M, Delcroix L, Romano GF, et al. Vascularized epiphyseal transplant. Orthop Clin North Am. 2007;38:95–101, vii.. [DOI] [PubMed] [Google Scholar]
- 6.Erdmann D, Garcia RM, Blueschke G, et al. Vascularized fibula-based physis transfer for pediatric proximal humerus reconstruction. Plast Reconstr Surg. 2013;132:281e–287e.. [DOI] [PubMed] [Google Scholar]
- 7.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997.. [PubMed] [Google Scholar]
- 8.Team, R. C. R: A Language and Environment for Statistical Computing. 2013. Vienna, Austria: R Foundation for Statistical Computing; ISBN 3-900051-07-0, 2014. [Google Scholar]
- 9.Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:48. [Google Scholar]
- 10.Shea KG, Coleman SS, Coleman DA. Growth of the proximal fibular physis and remodeling of the epiphysis after microvascular transfer. A case report. J Bone Joint Surg Am. 1997;79:583–586.. [PubMed] [Google Scholar]
- 11.Tang CH. Reconstruction of the bones and joints of the upper extremity by vascularized free fibular graft: report of 46 cases. J Reconstr Microsurg. 1992;8:285–292.. [DOI] [PubMed] [Google Scholar]
- 12.Innocenti M, Ceruso M, Manfrini M, et al. Free vascularized growth-plate transfer after bone tumor resection in children. J Reconstr Microsurg. 1998;14:137–143.. [DOI] [PubMed] [Google Scholar]
- 13.Grimer RJ. Surgical options for children with osteosarcoma. Lancet Oncol. 2005;6:85–92.. [DOI] [PubMed] [Google Scholar]
- 14.Nunley JA, Spiegl PV, Goldner RD, et al. Longitudinal epiphyseal growth after replantation and transplantation in children. J Hand Surg Am. 1987;12:274–279.. [DOI] [PubMed] [Google Scholar]
- 15.Innocenti M, Delcroix L, Romano GF. Epiphyseal transplant: harvesting technique of the proximal fibula based on the anterior tibial artery. Microsurgery. 2005;25:284–292.. [DOI] [PubMed] [Google Scholar]
- 16.Crowder C, Austin D. Age ranges of epiphyseal fusion in the distal tibia and fibula of contemporary males and females. J Forensic Sci. 2005;50:1001–1007.. [PubMed] [Google Scholar]
- 17.Gebert C, Hillmann A, Schwappach A, et al. Free vascularized fibular grafting for reconstruction after tumor resection in the upper extremity. J Surg Oncol. 2006;94:114–127.. [DOI] [PubMed] [Google Scholar]
- 18.Innocenti M, Delcroix L, Manfrini M, et al. Vascularized proximal fibular epiphyseal transfer for distal radial reconstruction. J Bone Joint Surg Am. 2004;86-A:1504–1511.. [DOI] [PubMed] [Google Scholar]
- 19.Rose PS, Shin AY, Bishop AT, et al. Vascularized free fibula transfer for oncologic reconstruction of the humerus. Clin Orthop Relat Res. 2005;438:80–84.. [DOI] [PubMed] [Google Scholar]
- 20.Weiland AJ, Moore JR, Daniel RK. Vascularized bone autografts. Experience with 41 cases. Clin Orthop Relat Res. 1983:87–95.. [PubMed] [Google Scholar]
- 21.Bilgin SS. Reconstruction of proximal humeral defects with shoulder arthrodesis using free vascularized fibular graft. J Bone Joint Surg Am. 2012;94:e94. [DOI] [PubMed] [Google Scholar]
- 22.Aldekhayel S, Govshievich A, Neel OF, et al. Vascularized proximal fibula epiphyseal transfer for distal radius reconstruction in children: a systematic review. Microsurgery. 2016;36:705–711.. [DOI] [PubMed] [Google Scholar]
- 23.Ghert M, Colterjohn N, Manfrini M. The use of free vascularized fibular grafts in skeletal reconstruction for bone tumors in children. J Am Acad Orthop Surg. 2007;15:577–587.. [DOI] [PubMed] [Google Scholar]


