Abstract
Importance
Bedside monitor alarms are designed to alert nurses to life-threatening physiologic changes, yet nurses’ response times are slow.
Objective
To identify factors associated with physiologic monitor alarm response time.
Design
Prospective cohort study using 551 hours of video-recorded patient care between July 22, 2014 and November 11, 2015.
Setting
Children’s hospital medical unit.
Participants
100 children, 38 nurses.
Exposure(s)
Patient, nurse, and alarm-level factors hypothesized to predict response time.
Main Outcome and Measure(s)
We used multivariable accelerated failure-time models stratified by nurse and adjusted for clustering within patients to evaluate associations between exposures and response time to alarms that occurred while the nurse was outside the room.
Results
Of 11,745 alarms in 100 children, 50 (0.5%) were actionable. Adjusted median response time was 10.4 minutes (95% CI 5.0–15.8) and varied based on the following variables: patient on complex care service (5.3 minutes (1.4–9.3) versus 11.1 minutes (5.6–16.6) in general pediatrics patients), family absent from bedside (6.3 minutes (2.2–10.4) versus 11.7 minutes (5.9–17.4) when family present), nurse with <1 year experience (4.4 minutes (3.4–5.5) versus 8.8 minutes (7.2–10.5) for nurses with >=1 year experience), 1:1 nursing assignment (3.5 minutes (1.3–5.7) versus 10.6 minutes (5.3–16.0) for nurses caring for 2 or more patients), prior alarm requiring intervention (5.5 minutes (1.5–9.5) versus 10.7 minutes (5.2–16.2) for patients without intervention), and lethal arrhythmia alarm (1.2 minutes (−0.6–2.9) versus 10.4 minutes (5.1–15.8) for alarms for other conditions). Each hour elapsed in a nurse’s shift was associated with a 15% longer response time (6.1 minutes (2.8–9.3) in hour 2 versus 14.1 minutes (6.4–21.7) in hour 8). The number of nonactionable alarms to which the nurse was exposed in the preceding 120 minutes was not associated with response time.
Conclusions and Relevance
Response time was associated with factors that likely represent heuristics nurses use to assess the probability that an alarm represents a life-threatening condition. Nurse:patient ratio and physical/mental fatigue (measured by hours into shift) represent modifiable factors associated with response time. Chronic alarm fatigue resulting from long term exposure to nonactionable alarms may be a more important determinant of response time than short term exposure.
INTRODUCTION
Physiologic monitors are intended to help clinicians detect clinical emergencies by alarming when vital signs exceed pre-set thresholds or cardiac arrhythmias occur. For alarms to be effective, they should activate only for serious physiologic changes and be considered important by staff.1 Unfortunately most current physiologic monitors generate high rates of alarms that are rarely actionable.2,3 In 6 studies of pediatric wards and intensive care settings, monitor alarm rates ranged from 1.5–24 alarms per monitored patient-hour.4–9 In pediatric intensive care settings, 5 studies found that 3–13% of physiologic monitor alarms warranted action4–8; 1 study performed on a pediatric ward found that only 1% warranted action.4 Perhaps as a result, nurses’ response times to alarms are slow.4 A delayed response to a patient whose alarm represents impending cardiac arrest could be catastrophic.10
National surveys of healthcare staff performed in 2005 and 2011 both ranked “frequent false alarms, which lead to reduced attention or response to alarms when they occur” the most important alarm issue to address.11,12 In both surveys, more than 75% of respondents agreed or strongly agreed that nuisance alarms reduce trust in alarms and cause caregivers to inappropriately turn alarms off.11,12 As a result, nurses likely develop the expectation that most physiologic monitor alarms are not important and prioritize other routine care tasks more highly than responding to alarms when they are busy, unless there are specific concerning features of the patient or the alarm.
Video recording provides an opportunity to thoroughly evaluate and improve the quality of care delivered in hospitals.13 In a previous pilot study using video, we identified an association between increased nonactionable physiologic monitor alarm exposure (a proxy for acute alarm fatigue) and delayed nurse response time.4 In this study, we examined a broader set of exposure variables and evaluated the associations of patient, nurse, and alarm-level factors with nurse response time to physiologic monitor alarms.
METHODS
Study definitions are provided in Box 1.
Box 1. Study definitions.
| Out of room alarm: An alarm that (a) occurs while no clinicians are in the patient’s room or are viewing the central monitoring station, and (b) sends an automatic text message to the bedside nurse (alarms for asystole, ventricular tachycardia, ventricular fibrillation, apnea, heart rate (HR), respiratory rate (RR), oxygen saturation (SpO2), SpO2 probe off, and leads fail). The text message includes the following elements: General Electric alarm level (crisis, warning, advisory, or system), alarm type and value that triggered the alarm (such as the HR HI 190), and the date and time. |
| Clinical alarm: An alarm for a cardiac arrhythmia or a physiologic parameter that is outside of the range set for the patient on the bedside monitor. |
| Valid alarm: A clinical alarm that correctly identifies the physiologic status of the patient. Validity was based on waveform quality, signal strength indicators, and artifact conditions, referencing the monitor’s operator’s manual. |
| Actionable alarm: A valid clinical alarm that either (a) leads to an observed clinical intervention (such as initiating supplemental oxygen) or (b) leads to an observed consultation with another clinician (such as discussing the patient’s tachycardia with a resident) at the bedside or (c) warrants intervention or consultation for a clinical condition (such as a prolonged desaturation) but the condition was unwitnessed: occurring while no clinicians are present and resolving before any clinicians entered the room or visualized the central monitoring station. |
| Nonactionable alarm: An alarm that does not meet the actionable definition above, including invalid alarms such as those caused by motion artifact, alarms that are valid but nonactionable, and technical alarms. |
| Technical alarm: An alarm for a problem with the physiologic monitor device or associated sensors. |
We conducted this prospective cohort study on 1 inpatient unit admitting patients to the general pediatrics and medically complex services at The Children’s Hospital of Philadelphia between July 22, 2014 and November 11, 2015. The medically complex service admits patients with a wide range of comorbidities and forms of technology dependence. Examples include patients with intestinal failure, complications of extreme prematurity, genetic syndromes, and other diagnoses that result in a population that is medically fragile and often requires ICU care during admissions. Patients on this unit undergoing continuous cardiorespiratory and/or pulse oximetry monitoring were eligible. In order to gain the analytic benefits of contrasting the effects of different exposures within individual nurses over time,14,15 we invited nurses of eligible patients to participate in multiple sessions.
This study was approved by the Institutional Review Board of The Children’s Hospital of Philadelphia. We obtained written in-person consent from the patient’s parent and nurse. We obtained a Certificate of Confidentiality from the National Institutes of Health to further reassure and protect hospital employees in the unlikely event research video was subpoenaed.16 In that situation we could use the Certificate to legally refuse to disclose video or associated data.
Monitoring equipment and secondary notification
All bed spaces included General Electric Dash 3000 monitors that were used if ordered. Physicians specified alarm parameters using an electronic health record order set with age-based defaults. Changing parameters required new orders. A central monitoring station with a display of all of the unit’s monitored patients’ waveforms was at the nurses’ station but there were no staff assigned to review alarms centrally.17
On the unit we studied, approximately 40% of patients were continuously monitored. In addition to alarming at the bedside, alarms for asystole, ventricular tachycardia, ventricular fibrillation, apnea, HR, RR, SpO2, SpO2 probe off, and leads fail also automatically sent a text message to the bedside nurse (Table 1).
Table 1.
Characteristics of the 11,710 evaluable clinical alarms. All alarms generated immediate audible alerts at the bedside and at the central monitoring station. Only a subset of the alarms generated automatic text messages and sent them to the bedside nurse’s phone, as shown in the Table.
| Alarm label | Alarm condition | Text to nurse phone | Total n | Valid n | Valid % | Actionable n | Actionable % |
|---|---|---|---|---|---|---|---|
| Critical arrhythmias | |||||||
| ASYSTOLE | Asystole | Yes | 44 | 0 | 0.0% | 0 | 0.0% |
| V TACH | 6+ beats of ventricular tachycardia | Yes | 18 | 0 | 0.0% | 0 | 0.0% |
| VFIBVTAC | Ventricular fibrillation | Yes | 2 | 0 | 0.0% | 0 | 0.0% |
| Noncritical arrhythmias | |||||||
| PVC | Premature ventricular contraction | No | 1,220 | 10 | 0.8% | 0 | 0.0% |
| PVC HI | Threshold for number of premature ventricular contractions exceeded | No | 106 | 0 | 0.0% | 0 | 0.0% |
| IRREGULAR | Irregular rhythm | No | 135 | 36 | 26.7% | 0 | 0.0% |
| COUPLET | Couplet | No | 159 | 1 | 0.6% | 0 | 0.0% |
| BIGEMINY | Bigeminy | No | 3 | 0 | 0.0% | 0 | 0.0% |
| TRIGEMINY | Trigeminy | No | 3 | 0 | 0.0% | 0 | 0.0% |
| R ON T | Ventricular contraction near T-wave peak | No | 28 | 0 | 0.0% | 0 | 0.0% |
| PAUSE | R-R interval exceeds set duration | No | 26 | 0 | 0.0% | 0 | 0.0% |
| VT>2 | 3–5 rapid ventricular contractions | No | 30 | 0 | 0.0% | 0 | 0.0% |
| Pulse oximetry | |||||||
| SPO2 LO | Low oxygen saturation | Yes | 3,265 | 608 | 18.6% | 43 | 1.3% |
| Heart ratea | |||||||
| TACHY | Tachycardia using ECG arrhythmia algorithm | No | 1,531 | 1,512 | 98.8% | 0 | 0.0% |
| HR HI | Tachycardia using ECG rate algorithm | Yes | 1,328 | 1,291 | 97.2% | 1 | 0.1% |
| RATE HI | Tachycardia using SpO2 pulse rate algorithm | No | 251 | 245 | 97.6% | 0 | 0.0% |
| BRADY | Bradycardia using ECG arrhythmia algorithm | No | 346 | 221 | 63.9% | 2 | 0.6% |
| HR LO | Bradycardia using ECG rate algorithm | Yes | 95 | 62 | 65.3% | 1 | 1.1% |
| RATE LO | Bradycardia using SpO2 pulse rate algorithm | No | 1 | 1 | 100.0% | 0 | 0.0% |
| Respiratory rate | |||||||
| RSP HI | Respiratory rate high | Yesb | 574 | 431 | 75.1% | 1 | 0.2% |
| RSP LO | Respiratory rate low | Yesb | 312 | 202 | 64.7% | 0 | 0.0% |
| APNEA | No breath detected in 15 sec (infant) or 20 sec (child/adult) | Yes | 13 | 9 | 69.2% | 2 | 15.4% |
| Blood pressure | |||||||
| NBP S HI | Systolic blood pressure high | No | 21 | 12 | 57.1% | 0 | 0.0% |
| NBP M HI | Mean blood pressure high | No | 8 | 8 | 100.0% | 0 | 0.0% |
| NBP D HI | Diastolic blood pressure high | No | 27 | 18 | 66.7% | 0 | 0.0% |
| S-T segment | |||||||
| ST-I HI | S-T segment elevation in lead I | No | 1 | 0 | 0.0% | 0 | 0.0% |
| Overall | 9547 | 4667 | 48.9% | 50 | 0.5% | ||
TACHY and BRADY heart rate alarms are generated using a different algorithm than HR HI and HR LO; all 4 are generated using the ECG leads. RATE LO and RATE HI alarms are generated using the SpO2 probe.
Respiratory rate high and low alarms are sent to the nurse’s phone 15 seconds after they occur at the bedside. If the condition resolves during the 15 seconds, a text message is not sent. All other text messages are sent as soon as alarms occur at the bedside, without a delay.
Data collection
We combined video recordings with time stamped alarm data from BedMasterEx software (Excel Medical Electronics) running on a server connected to the monitor network. Our recording and annotation methods have been described previously,4,18 and the acceptability, feasibility, and costs of performing this study have been reported.19 Briefly, we temporarily mounted up to 6 small video cameras in patients’ rooms and 1 camera on the central monitoring station and recorded for approximately 6 hours per session. The camera on the central monitoring station captured any responses that occurred when nurses visualized monitors remotely.
Video review
We trained a research assistant to review video and determine validity and actionability of clinical alarms. During the training period, the research assistant reviewed 4675 clinical alarms (every clinical alarm that required interpretation from the first 42 patients) with oversight from an expert in physiologic monitoring (Bonafide).
We then determined if the research assistant could accurately review alarms independently. The research assistant and expert separately reviewed every clinical alarm that required interpretation from 10 additional patients (n=883 alarms). The research assistant and expert agreed on the validity determination for 99.3% of the 883 alarms. Using the expert as the gold standard, the research assistant’s sensitivity (assessed alarm as valid when physician also assessed as valid) was 99.4% (95%CI 97.9–99.9%) and specificity (assessed alarm as invalid when physician also assessed as invalid) was 99.3% (95%CI 98.1–99.8%). The research assistant and expert agreed on the actionability determination for 99.7% of the 883 alarms. The research assistant’s sensitivity was 100% (1-sided 97.5% CI 39.8–100%) and specificity was 99.7% (95% CI 99.0–99.9%). Based on these reassuring results, for the remaining 48 patients the expert performed secondary review of only the clinical alarms that the research assistant determined to be valid.
Response time outcome
Our analysis examined response time, measured as the number of seconds elapsed between the start time of each audible alarm on the bedside monitor and the time a clinical staff member either entered the patient’s room or viewed the central monitoring station. Since all patients on the unit have their waveforms displayed on the central monitoring station, we conservatively assumed that any viewing of the central monitoring station included viewing the vital signs of the patient under study and counted as a response. Responses by other clinical staff were censored at the time of their response.
We limited analyses of response time to “out of room” alarms that (a) occurred while no clinicians were in the patient’s room or were viewing the central monitoring station, and (b) sent an automatic text message to the bedside nurse (see Study definitions section). We focused on this group of alarms because we wanted to identify factors influencing the decision nurses must make when they receive an alarm notification: whether to interrupt their current patient care task and respond immediately or delay responding until their current task is complete.
Exposures of interest
In planning the exposure variables to measure and include in the analysis, we developed a theoretical framework linking constructs such as “nurse’s concern that patient is at increased risk of a life-threatening event” with specific variables and response time (eFigure in the Supplement). Since our previous pilot study identified an association between the number of nonactionable alarms to which a nurse was exposed for the same patient in the preceding 120 minutes and response time, we also evaluated nonactionable alarm exposure groups as exposure variables.4 We divided the nonactionable alarm counts into quartiles of increasing alarm frequency exposure in our primary analysis. To explore the stability of our findings, we also examined nonactionable alarm count exposure divided into tertiles and the categories we previously used in our pilot.
Statistical analysis
We first analyzed the data without the nonactionable alarm exposure variables. We visually examined the relationships between each exposure variable and nurse response time with unadjusted Kaplan-Meier failure plots. We then constructed a multivariable accelerated failure-time model based on the Weibull distribution20,21 stratified by nurse with clustering by patient. This stratified model evaluated the within-nurse effects of different exposures. Accelerated failure-time models are comparable to Cox models, but emphasize time to event rather than hazards.20,21 We included all of the variables in the multivariable model, regardless of significance level, in order to describe the adjusted contribution of each. We reported the adjusted median response times and 95% confidence intervals for each variable subgroup as estimated in the model and the P values contrasting pairs of subgroups, adjusted for multiple comparisons using Bonferroni’s method whenever more than 1 comparison was made. Since nurse experience does not vary within nurse, it could not be estimated directly from the stratified model. We obtained this estimate adjusted for all the variables included in the stratified model from a separate model that allowed for estimation of nurse-level factors and accounted for nurse clustering.
We then restricted the dataset to alarms occurring after the first 120 minutes of video recording in order to estimate the association between nonactionable alarm exposure in the preceding rolling 120 minute window and response time. We analyzed the relationship between nonactionable alarm exposure groups and response time, adjusted for all of the other variables in the theoretical framework, in the same multivariable accelerated failure-time model stratified by nurse with clustering by patient.
For further details on model building and evaluation, please refer to the eMethods in the Supplement. We used REDCap22 for data entry and management, and Stata/SE 14.1 for statistical analysis.
RESULTS
We performed 100 video recordings among 100 patients and 38 nurses over 551 hours. Each nurse participated in a median of 3 (range 1–6) video recordings. Nurses were female (100%) and predominantly White (92%). They had a median of 2 years of experience (range 2 months – 20 years). Most (79%) worked 36 or more hours per week, usually in 8–12 hour shifts.
Most patients (82%) were admitted to the general pediatrics service, with 18% on the medically complex service. Consistent with the unit’s population, patients were young, with 75% under 6 months old. Fifty-one percent were male. The race distribution was 45% Black or African American, 33% White, 4% Asian, 18% Other.
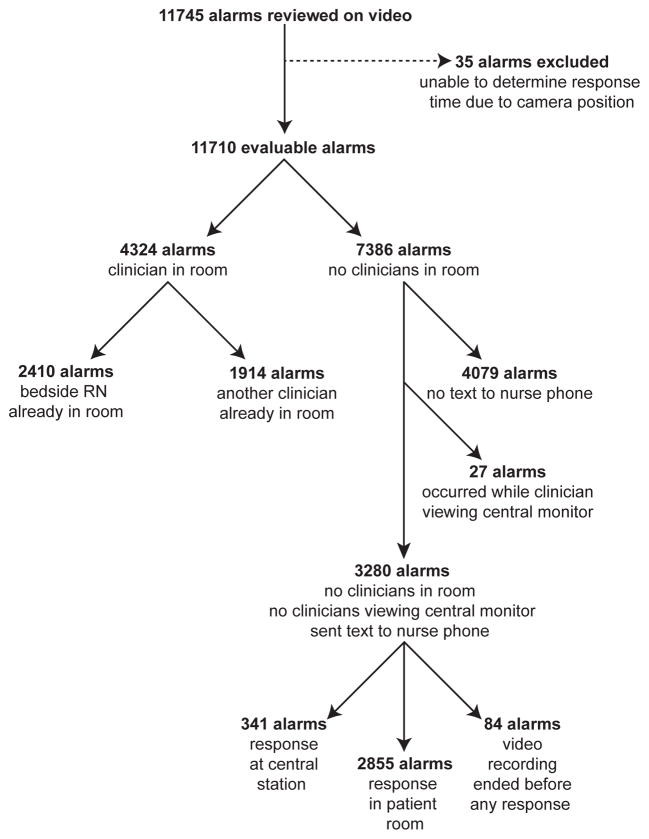
We captured 11,745 alarms on video (9582 clinical and 2163 technical), averaging 21.3 alarms per monitored patient-hour. Alarms per patient ranged from 0 to 484 (median 90, IQR 59–163). We excluded 35 alarms (0.3%) for which response could not be determined due to camera obstruction. Overall, 48.9% of the clinical alarms were valid and 0.5% (50 alarms in 19 patients) were actionable. Further details about actionable alarms are in the eTable in the Supplement. There were no instances of missed alarms that required emergency assistance in the form of a rapid response or code blue team call. Details by alarm type are in Table 1. The data flow diagram is in Figure 1.
Figure 1.
Alarm data flow diagram. The analysis examined response time to the 3280 “out-of-room” alarms that (a) occurred while no clinicians were in the patient’s room or were viewing the central monitoring station, and (b) sent automatic text messages to the bedside nurse (alarms for asystole, ventricular tachycardia, ventricular fibrillation, apnea, HR, RR, SpO2, probe off, and leads fail).
The observed (unadjusted) median response time to the 3280 out-of-room alarms was 7.0 minutes (bootstrapped 95% CI accounting for nurse clustering: 5.2–8.8 minutes). The adjusted median response time was 10.4 minutes (95% CI 5.0–15.8). The multivariable model results are shown in Table 2. We found that with each successive hour that passed in a nurse’s shift, response time was slower. Nurses with less than 1 year of experience and those in a 1:1 nurse:patient assignment responded more quickly than nurses with more experience and those caring for more than 1 patient, respectively. Patients without family present at the bedside, and those on the complex care service had their alarms responded to more quickly than those with parents present, and those on the general pediatrics service, respectively. Patients with prior alarms during the nurse’s shift that required interventions were responded to more quickly than those without prior interventions. Lethal arrhythmia alarms received the fastest responses. Patient age, central venous lines and nasogastric/nasojejunal tubes were not associated with response time.
Table 2.
Results of multivariable model.
| Variable | Adjusted median response time (95%CI), minutes | P value of contrast between groups |
|---|---|---|
| Overall response time | 10.4 (5.0–15.8) | |
| Nurse-level factors | ||
| Nurse experiencea | ||
| <1 year | 4.4 (3.4–5.5) | <.001 |
| 1 or more years | 8.8 (7.2–10.5) | |
| Nurse:patient ratio | ||
| 1:1 | 3.5 (1.3–5.7) | <.001 |
| 1:2 or more | 10.6 (5.3–16.0) | |
| Each successive hour in nurse’s shiftb | ||
| Hour 2 | 6.1 (2.8–9.3) | .03 |
| Hour 8 | 14.1 (6.4–21.7) | |
| Patient-level factors | ||
| Family at bedside | ||
| Present | 11.7 (5.9–17.4) | .01 |
| Absent | 6.3 (2.2–10.4) | |
| Hospital service | ||
| Complex care | 5.3 (1.4–9.3) | .03 |
| General pediatrics | 11.1 (5.6–16.6) | |
| Patient had prior alarm requiring intervention | ||
| Yes | 5.5 (1.5–9.5) | .049 |
| No | 10.7 (5.2–16.2) | |
| Central venous line | ||
| Present | 8.2 (1.0–15.3) | .46 |
| Absent | 10.4 (5.1–15.8) | |
| NG or NJ tube | ||
| Present | 10.3 (3.0–17.6) | .97 |
| Absent | 10.4 (5.0–15.9) | |
| Patient age | ||
| <2 months | 8.8 (4.9–12.7) | >.97 for all comparisonsc |
| 2–<6 months | 13.1 (4.1–22.1) | |
| >=6 months | 12.5 (3.4–21.5) | |
| Alarm-level factors | ||
| Alarm type | ||
| Lethal arrhythmia | 1.2 (−0.6–2.9) | <.001 |
| Other alarm conditions | 10.4 (5.1–15.8) | |
Abbreviations: CI, confidence interval; NG, nasogastric; NJ, nasojejunal.
Since nurse experience does not vary within nurse, its association with response time could not be estimated directly from the stratified model. The adjusted estimate reported here was obtained from a separate model that allowed for estimation of nurse-level factors and accounted for nurse clustering.
Evaluated as a continuous variable in model; hours 2 and 8 presented as representative examples. Time ratio 1.15 (95% CI 1.04–1.28, P=0.008).
Adjusted for 3 comparisons using Bonferroni’s method.
In evaluating the association between nonactionable alarm exposure quartiles and response time, we found that the middle quartiles had significantly slower response times than the lowest quartile, possibly consistent with acute alarm fatigue, but there was no evidence of a dose-response relationship between increasing nonactionable alarm exposure and slowing of response time (Table 3). We examined nonactionable alarm count exposure divided into tertiles and the groupings we previously used in our pilot and the results were similar (data not shown).
Table 3.
Relationship between quartiles of nonactionable alarm exposure in the preceding 120 minutes and response time, adjusted for all variables in Table 2.
| Variable | Adjusted median response time (95% CI), minutes | P valuea |
|---|---|---|
| Quartile 1: 0–17 alarms | 8.3 (3.8–12.8) | |
| Quartile 2: 18–31 alarms | 12.3 (6.5–18.2) | .13 (vs Quartile 1) |
| Quartile 3: 32–52 alarms | 12.3 (3.9–20.6) | .85 (vs Quartile 1) |
| Quartile 4: 53+ alarms | 10.1 (5.5–14.7) | >.99 (vs Quartile 1) |
Abbreviation: CI, confidence interval.
Adjusted for 3 comparisons using Bonferroni’s method.
Note: Model required restricting the dataset to alarms occurring after the first 120 minutes of video recording to accurately assess alarm exposure counts.
DISCUSSION
This study’s main findings were: (1) alarms for patients on the complex care service and patients without family at the bedside received faster responses than alarms for patients without those characteristics, (2) nurses responded faster if they had <1 year experience, if they were in a 1:1 assignment, or if they had previously responded to an actionable alarm for the same patient that required intervention, (3) lethal arrhythmia alarms received the fastest responses among all of the variables we measured, (4) each hour that passed in a nurse's shift was associated with slower response time, and (5) the number of nonactionable alarms to which the nurse was exposed in the preceding 120 minutes was not associated with a dose-dependent slowing of response time.
It is well established that nurses use intuition and heuristics in clinical decision making.23 We theorize that the decision whether or not to respond immediately to an alarm is based on heuristics, to which many of the factors described above contribute. These heuristics are then used to rapidly make intuitive judgments about the probability that the alarm represents a life-threatening condition that warrants an immediate response to prevent patient harm. The finding of faster responses in the least experience nurses could be explained by a lack of chronic alarm fatigue, although alternative explanations (e.g. the least experience nurses were more nervous, or less skilled at triaging important versus unimportant alarms) are also possible. Slower response time with each hour of work likely represents physical and mental fatigue.
In comparison to the pilot study we previously published using the same video recording methods on the same unit,4 in this study response time was faster overall (7.0 vs 9.8 minutes) and we did not find a dose-dependent slowing of response time with increasing nonactionable alarm exposure. There are a number of possible explanations for this. The first is that this is a larger study with over 5-fold more alarms in the same setting, and the association seen in the pilot study could have occurred by chance. Another possibility is that nursing practice surrounding alarm management changed over time. Since completing the pilot study, there have been initiatives including a communication campaign focused on spreading awareness of the 2014 Joint Commission National Patient Safety Goal focused on improving the safety of clinical alarm systems24 and hospital-wide mandatory education focused on hospital alarm policy and practice. These could have blunted some of the acute alarm fatigue effects seen previously. An additional difference is that there was nursing turnover on this unit between studies, with recent nursing school graduates replacing older, more experienced nurses. In the pilot study, nurses from this unit had a median of 9.8 years of experience with a range of 2–28 years. In this study, nurses had a median of 2 years of experience with a range of 2 months – 20 years. Since nurses with less experience respond to alarms more quickly, this might have contributed to the observed differences.
Few other studies have examined factors that impact response time to alarms. Voepel-Lewis and colleagues evaluated response time to pulse oximetry alarms in a higher risk setting, a postoperative orthopedic unit where one-third of alarms were actionable and the median response time was under 1 minute.25 They found a longer response time for patients in the highest quartile of alarms compared to those in lower quartiles, which they attributed to possible alarm fatigue. In an adult intensive care unit study, Deb and Claudio found that alarm responses were driven by nurse personality characteristics (extraversion, agreeableness, neuroticism), mental workload, apathy, noise level, time elapsed since the start of the nurse’s shift, and the nurse:patient ratio.26
This study has several limitations. First, it was performed on just 1 inpatient unit. We did this intentionally to take advantage of the natural experiment that occurs when 1 nurse is observed across multiple patients and many alarms. Despite the statistical advantages offered by the repeated measures within nurses, the generalizability is still limited. Multicenter studies would allow us to determine if these findings are externally valid. Second, while we showed that response time was dependent upon a number of variables, we did not directly measure the effect of an intervention on response time. As we strive to improve response time so that any alarm that is potentially life-threatening and occurs while a nurse is outside the room gets an immediate response, we need to know not only the individual factors that predict response time but also the interventions that are effective in improving it. Next steps in this line of research could include evaluating the effect of interventions such as shorter shifts and higher nurse:patient ratios on response time. In addition, to begin reducing nonactionable alarms, researchers should develop guidelines establishing which children should be monitored (including metrics for over and under-monitoring), what vital signs should be continuously measured, what settings optimize detection of actionable events, and when monitoring should be discontinued.27,28
CONCLUSIONS
We identified a set of patient, nurse, and alarm-level factors that were associated with faster responses to physiologic monitor alarms. We theorize that many of these factors contribute to the heuristics nurses use to rapidly make intuitive judgments about the probability that the alarm represents a life-threatening condition that warrants an immediate response to prevent patient harm. Changing nurses’ baseline assumption that most alarms do not represent life-threatening conditions will likely require hospitals to critically evaluate their alarm management practices and commit to reducing low priority alarms that are unlikely to represent life-threatening conditions. Nurse:patient ratio and physical/mental fatigue (measured by hours into shift) represent additional modifiable factors associated with response time that should be included in intervention studies. Chronic alarm fatigue, the result of long term exposure to nonactionable alarms during a nurse’s career, may be a more important predictor of response time than short term exposure to nonactionable alarms.
Supplementary Material
eFigure. Theoretical framework of the predictor constructs we hypothesized contributed to the 2 components of alarm response time and the variables in our dataset that we mapped to the predictor constructs. Abbreviations: BSI, bloodstream infection; RN, registered nurse.
Acknowledgments
Funding/Support: This study was supported by a Mentored Patient-Oriented Research Career Development Award from the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL116427 (Bonafide, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organization.
Footnotes
Conflict of Interest Disclosures: Dr. Lin reports consulting for General Electric (GE) Medical Systems.
Role of the Funder/Sponsor: The funder had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author Contributions: Dr. Bonafide had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bonafide, Keren, Localio, Nadkarni, Holmes, Roberts, Lin.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Bonafide.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Bonafide, Localio.
Obtained funding: Bonafide.
Administrative, technical, or material support: Stemler, MacMurchy, Zander.
Study supervision: Keren, Localio, Nadkarni, Holmes.
References
- 1.Chopra V, McMahon LF., Jr Redesigning hospital alarms for patient safety: alarmed and potentially dangerous. JAMA. 2014;311(12):1199–1200. doi: 10.1001/jama.2014.710. [DOI] [PubMed] [Google Scholar]
- 2.Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136–144. doi: 10.1002/jhm.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cvach M. Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–277. doi: 10.2345/0899-8205-46.4.268. [DOI] [PubMed] [Google Scholar]
- 4.Bonafide CP, Lin R, Zander M, et al. Association between exposure to nonactionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6):345–351. doi: 10.1002/jhm.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawless ST. Crying wolf: false alarms in a pediatric intensive care unit. Crit Care Med. 1994;22(6):981–985. [PubMed] [Google Scholar]
- 6.Rosman EC, Blaufox AD, Menco A, Trope R, Seiden HS. What are we missing? Arrhythmia detection in the pediatric intensive care unit. J Pediatr. 2013;163(2):511–514. doi: 10.1016/j.jpeds.2013.01.053. [DOI] [PubMed] [Google Scholar]
- 7.Talley LB, Hooper J, Jacobs B, et al. Cardiopulmonary monitors and clinically significant events in critically ill children. Biomed Instrum Technol. 2011;(Suppl):38–45. doi: 10.2345/0899-8205-45.s1.38. [DOI] [PubMed] [Google Scholar]
- 8.Tsien CL, Fackler JC. Poor prognosis for existing monitors in the intensive care unit. Crit Care Med. 1997;25(4):614–619. doi: 10.1097/00003246-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 9.van Pul CVD, Mortel HPMEVD, Bogaart JJL, Mohns T, Andriessen P. Safe patient monitoring is challenging but still feasible in a neonatal intensive care unit with single family rooms. Acta Paediatr. 2015;104(6):e247–254. doi: 10.1111/apa.12907. [DOI] [PubMed] [Google Scholar]
- 10.Andersen LW, Berg KM, Saindon BZ, et al. Time to epinephrine and survival after pediatric in-hospital cardiac arrest. JAMA. 2015;314(8):802–810. doi: 10.1001/jama.2015.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korniewicz DM, Clark T, David Y. A national online survey on the effectiveness of clinical alarms. Am J Crit Care. 2008;17:36–41. [PubMed] [Google Scholar]
- 12.Funk M, Clark JT, Bauld TJ, Ott JC, Coss P. Attitudes and practices related to clinical alarms. Am J Crit Care. 2014;23(3):e9–e18. doi: 10.4037/ajcc2014315. [DOI] [PubMed] [Google Scholar]
- 13.Makary MA. The power of video recording: taking quality to the next level. JAMA. 2013;309:1591–1592. doi: 10.1001/jama.2013.595. [DOI] [PubMed] [Google Scholar]
- 14.Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22:2591–2602. doi: 10.1002/sim.1524. [DOI] [PubMed] [Google Scholar]
- 15.Localio AR, Berlin JA, Ten Have TR, Kimmel SE. Adjustments for center in multicenter studies: an overview. Ann Intern Med. 2001;135(2):112–123. doi: 10.7326/0003-4819-135-2-200107170-00012. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed August 31, 2016];NIH Certificates of Confidentiality Kiosk. http://grants.nih.gov/grants/policy/coc/
- 17.Funk M, Parkosewich JA, Johnson CR, Stukshis I. Effect of dedicated monitor watchers on patients’ outcomes. Am J Crit Care. 1997;6(4):318–323. [PubMed] [Google Scholar]
- 18.Bonafide CP, Zander M, Graham CS, et al. Video methods for evaluating physiologic monitor alarms and alarm responses. Biomed Instrum Technol. 2014;48(3):220–230. doi: 10.2345/0899-8205-48.3.220. [DOI] [PubMed] [Google Scholar]
- 19.MacMurchy M, Stemler S, Zander M, Bonafide CP. Acceptability, feasibility, and cost of using video to evaluate alarm fatigue. Biomed Instrum Technol. 2017 doi: 10.2345/0899-8205-51.1.25. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collett D. Modelling Survival Data in Medical Research. 2. Boca Raton, FL: Chapman & Hall/CRC Press; 2003. Chapter 6: Accelerated Failure Time and other Parametric Models; pp. 197–229. [Google Scholar]
- 21.Cleves M, Gould W, Gutierrez R, Marchenko Y. An Introduction to Survival Analysis Using Stata. 3. College Station, TX: Stata Press; 2010. Chapter 12: Parametric Models; pp. 229–244. [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckingham CD, Adams A. Classifying clinical decision making: interpreting nursing intuition, heuristics and medical diagnosis. J Adv Nurs. 2000;32(4):990–998. [PubMed] [Google Scholar]
- 24.The Joint Commission. The Joint Commission announces 2014 National Patient Safety Goal. Jt Comm Perspect. 2013;33:1–4. [PubMed] [Google Scholar]
- 25.Voepel-Lewis T, Parker ML, Burke CN, et al. Pulse oximetry desaturation alarms on a general postoperative adult unit: a prospective observational study of nurse response time. Int J Nurs Stud. 2013;50(10):1351–1358. doi: 10.1016/j.ijnurstu.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Deb S, Claudio D. Alarm fatigue and its influence on staff performance. IIE Trans Healthc Syst Eng. 2015;5(3):183–196. [Google Scholar]
- 27.Bonafide CP, Roland D, Brady PW. Rapid response systems 20 years later: new approaches, old challenges. JAMA Pediatr. 2016;170(8):729–730. doi: 10.1001/jamapediatrics.2016.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karnik A, Bonafide CP. A framework for reducing alarm fatigue on pediatric inpatient units. Hosp Pediatr. 2015;5(3):160–163. doi: 10.1542/hpeds.2014-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Theoretical framework of the predictor constructs we hypothesized contributed to the 2 components of alarm response time and the variables in our dataset that we mapped to the predictor constructs. Abbreviations: BSI, bloodstream infection; RN, registered nurse.