Abstract
Introduction
Genomic applications raise multiple challenges including the optimization of genomic counseling (GC) services as part of the results delivery process. More information on patients’ motivations, preferences, and informational needs are essential to guide the development of new, more efficient practice delivery models that capitalize on the existing strengths of a limited genetic counseling workforce.
Methods
Semi-structured telephone interviews were conducted with a subset of counselees from the Coriell Personalized Medicine Collaborative following online receipt of multiple personalized genomic test reports. Participants previously had either in-person GC (chronic disease cohort, n=20; mean age 60 years) or telephone GC (community cohort, n=31; mean age 46.8 years). Transcripts were analyzed using a Grounded Theory framework.
Results
Major themes that emerged from the interviews include 1) primary reasons for seeking GC were to clarify results, put results into perspective relative to other health-related concerns, and to receive personalized recommendations; 2) there is need for a more participant driven approach in terms of mode of GC communication (in-person, phone, video), and refining the counseling agenda pre-session; and 3) there was strong interest in the option of follow up GC.
Conclusion
By clarifying counselees’ expectations, views and desired outcomes, we have uncovered a need for a more participant-driven GC model when potentially actionable genomic results are received online.
INTRODUCTION
Genetic counselors assess disease risks based on personal and family medical histories, non-genetic influences, and genetic and genomic information, in order to assist patients in medical and other health-related decisions. Genetic counselors also attend to the emotional ramifications of this information in a client-centered and psychotherapeutic manner (Austin, 2015; Kessler, 1981; Veach, Bartels, & Leroy, 2007). Traditional genetic counseling focuses on a tailored discussion of one or a few disease or risk factors of concern for the patient and their family. Often, this counseling is done in-person, or more recently, by telephone (Cohen et al., 2013; Jacobs et al., 2016; Trepanier & Allain, 2014). With the emergence of technology that facilitates testing an individual for hundreds or thousands of genomic risk variants at a single time point, the traditional model for genetic counseling must naturally evolve toward a more scaleable approach (Bernhardt, 2014; O'Daniel, 2010; Ormond, 2013; Roche, 2012).
Genomic counseling (GC) has been proposed to meet that need. Genomic counseling is a health service designed to provide comprehensive application of genetic and genomic information to individuals and healthcare teams for prevention, improved care, lifestyle changes, and treatment/preventative options (Mills & Haga, 2014; O'Daniel, 2010; Ormond, 2013; Shelton & Whitcomb, 2015). However, more information on patients’ motivations, preferences, and informational needs are essential to guide the development of more efficient practice delivery models that capitalize on the existing strengths of a limited genetic counseling workforce. This transition does not replace the vital work on risk assessment and counseling for single gene (Mendelian) diseases, but builds upon it through careful integration of polygenic and environmental risk information for multiple disease risk indications. The complexity of genomic information lends itself to innovative approaches that have the potential to make GC more accessible and efficient, such as online web portals providing high-quality education and support in a more participatory and less healthcare provider time-intensive fashion (Haga et al., 2014; Mills, Powell, Barry, & Haga, 2014; Ormond, 2013; Trepanier & Allain, 2014). This more participatory approach has several benefits including meeting patients desire to be engaged in their health care, and leading to improvements in health outcomes (Simmons, Wolever, Bechard, & Snyderman, 2014). Though several research studies have been done around delivery of genomic information (Gordon et al., 2012; Haga et al., 2014; Mills & Haga, 2014; Wright et al., 2014), the field of genetic counseling has not yet articulated a model for GC that capatilizes on these technologies and actively involves patients, family members and physicians throughout all phases of the process.
As formative research for development of this model, we conducted qualitative interviews with participants who previously received GC following online receipt of multiple potentially actionable complex disease risk reports (e.g. age-related macular degeneration) and drug-response reports (e.g. CYP2C19 and Clopidogrel). The overarching goals of the interviews were to: a) clarify patient expectations and impressions of GC, b) determine the most and least valuable components of a traditional, in person, genomic counselor driven model and a telephone-based genomic counselor model for which the participant helps determine, pre-session which genomic test results to discuss, and c) identify preferences for follow-up counseling. In this paper we present major themes from patient interviews and begin to outline an expanded model of GC practice delivery based on study findings.
METHODS
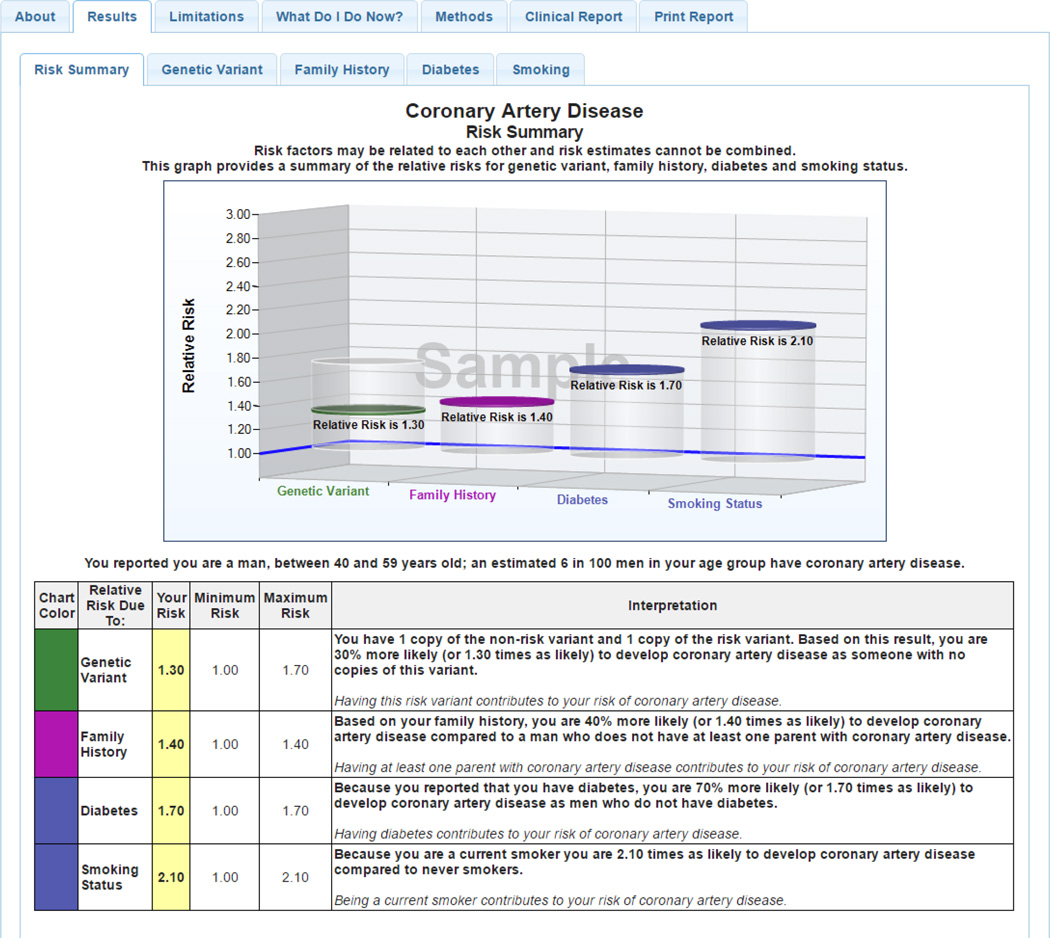
Participants of the Coriell Personalized Medicine Collaborative (CPMC) received potentially actionable complex disease and pharmacogenomic risk result reports through a secure web portal (Keller M, 2010). All participants provided saliva samples for genotyping and completed several online surveys to produce personalized risk reports (Figure 1) that are based on genetic risk factors, family history, and non-genetic factors (e.g. body mass index). Participants in this study received results for up to 19 complex diseases (e.g. age related macular degeneration, Table 1a) and up to 7 drug response reports (ex: CYP2C19 and Clopidogrel), Table 1b). Although most of the complex disease reports provide genetic variant risk based on a single SNP, the relative risk spans a wide range (0.08 – >6.0), and one report is polygenic (e.g. exocrine pancreatic cancer, 3 SNPs). Participants could access risk reports via a secure web portal, which also provides educational tools enabling participants to learn more about their risks and what they can do to positively influence their health. The number of reports received by study participants prior to receipt of GC, and prior to the qualitative interviews, varied based on their route of accrual into the CPMC.
Figure 1. Sample CPMC® Coronary Artery Disease Report.
Solid discs represent the participant’s relative risk, and vertical cylinders depict the range of RR values possible for the risk variable. On-line risk reports are organized using a tabbed approach, with separate tabs for disease condition information, risk results, limitations, methods, and links to request genetic counseling, or review educational material. To ensure readability, the CPMC report design was informed by multiple rounds of pilot testing conducted by allowing individuals with no scientific background to review report drafts and provide feedback. The CPMC chose to report relative risks to study participants because this approach allows for the reporting of all disease risk factors (genetic, family history, and lifestyle) using the same metric and does not require population estimates of disease incidence. The 8 CPMC health condition risk reports included in this study present genetic variant risk based on a single SNP because of the lack of validated multigenic models with robust prediction. Risk estimates provided for non-genetic risk factors (family history and lifestyle or environmental factors) were derived or reported from valid and representative peer-reviewed publications. Non-genetic risk factors were included if they meet two criteria: the risk factor must be collected by the baseline required lifestyle questionnaire and the risk factor must be an established disease risk, included in multiple disease review articles and consistently associated with disease.
Table 1.
a: 19 Coriell Personalized Medicine Collaborative (CPMC)-Approved Complex Disease Reports. b: 7 Coriell Personalized Medicine Collaborative (CPMC) Approved Drug/Drug Class-Gene Pairs
| Complex Disease | Reported SNPs |
|---|---|
| Age-Related Macular Degeneration | rs10490924 |
| Bladder Cancer | rs9642880 |
| Breast Cancer | rs2981582 |
| Colorectal Cancer | rs6983267 |
| Coronary Artery Disease | rs1333049 |
| Crohn's Disease | rs11209026 |
| Exocrine Pancreatic Cancer - polygenic | rs3790844, rs401681, rs4885093 |
| Hemochromatosis | rs1800562 |
| Melanoma | rs910873 |
| Obesity | rs9939609 |
| Osteoarthritis | rs3815148 |
| Periodontitis | rs1143634 |
| Prostate Cancer | rs16901979 |
| Rheumatoid Arthritis | rs6920220 |
| Systemic Lupus Erythematosus | rs3821236 |
| Testicular Cancer | rs995030 |
| Type 1 Diabetes | rs9272346 |
| Type 2 Diabetes | rs7754840 |
| Ulcerative Colitis | rs11209026 |
| Drug/Drug Class | Gene(s) | Reported SNPs |
|---|---|---|
| Celecoxib | CYP2C9 | rs72558189, rs1799853, rs9332131, rs28371685, rs1057910, rs2837168 |
| Clopidogrel | CYP2C19 | rs12248560, rs28399504, rs41291556, rs72558184, rs4986893, rs4244285, rs72558186, rs56337013 |
| Metformin | ATM | rs11212617 |
| Proton Pump Inhibitors |
CYP2C19 | rs4244285, rs4986893, rs28399504, rs56337013, rs72558184, rs72558186, rs41291556, rs17884712, rs6413438, rs12248560 |
| Simvastatin | SLCO1B1 | rs2306283, rs56101265, rs56061388, rs72559745, rs4149056,rs55901008, rs2306283, rs72559746 |
| Thiopurines | TPMT | rs1142345, rs1800584, rs1800460, rs1800462 |
| Warfarin | CYP2C9/VKORC1/CYP4F2 | rs1799853, rs1057910, rs28371686, rs9332131, rs28371685, rs72558189, rs9923231, rs2108622 |
Results from primary outcomes of various trials related to the CPMC have been previously reported (Gordon et al., 2012; Schmidlen T, 2016; Schmidlen et al., 2014; Sweet K, 2016). For this qualitative interview study, participants were either from the Ohio State University chronic disease cohort (OSU-CPMC) comprised of individuals diagnosed with either hypertension or congestive heart failure (n=199) or the CPMC cohort. The CPMC cohort included members of the general public (no selection criteria related to health status; n=4158), employees of the United States Air Force Medical Service, including medical professionals and administrative staff, (n=1290), and individuals with either breast or prostate cancer enrolled through Fox Chase Cancer Center (n=86). Participants who had received GC in either cohort were notified of the opportunity to participate in the qualitative interviews via an email sent through the CPMC study web portal. Two hundred and seven participants subsequently contacted a study research assistant via email, and were provided study details and a link to complete an eligibility survey. Fifty-three participants subsequently completed an online consent form and an interview by phone. One individual was removed for participating twice and providing discrepant stories in her two interviews. Thus, we had 51 telephone interviewees (20, OSU-CPMC; 31, CPMC). This study was approved by Institutional Review Board at each institution.
OSU-CPMC participants
All 199 OSU-CPMC participants received an initial batch of results pertaining to 8 health conditions (coronary artery disease, type 1 diabetes, type 2 diabetes, hemochromatosis, melanoma, age-related macular degeneration, prostate cancer, and lupus) and 1 drug response report (CYP2C19 and Clopidogrel). They were then randomized to and received in-person GC from licensed board certified Ohio State genomic counselors in a hospital based setting. In-person GC consultations followed the format of a traditional genetic counseling appointment, lasted between 60–90 minutes, and provided individualized risk assessment for the nine initial personalized test risk reports. Given that participants had the potential for multiple “increased” risk variables (genetic variant, family history and health behaviors, Table 2), “decreased” risk variant(s) for DM1, and differing ranges of relative risk for each disease, a tabular one-page visual display that summarized each of the risk factors into a quick reference summary (QRS) was provided to OSU-CPMC participants (Sweet et al., 2014). All individual increased risk variables were highlighted, and risk was compared to the general population risk for each disease. The genomic counselors also reviewed, expanded and assessed the patient’s family history to obtain at least a 3-generation pedigree, reviewed the patient’s medical and social histories, environmental risk factor information, and current health promotion and screening practices. Specific actions to prevent and/or lower disease risk were also provided. A detailed GC summary letter (Figure 2) and PDF copies of the nine personalized test reports were also generated for all OSU-CPMC participants and their healthcare team, and was made available within the EPIC electronic medical record. Once OSU-CPMC participants completed all required study activities, they received additional risk reports on a monthly basis until all 26 reports were delivered online.
Table 2.
Reportable disease risk values for a Caucasian OSU-CPMC Participant
| Disease | Genetic Variant RR |
Family History RR* |
BMI RR | Smoking RR |
Diabetes*** RR |
|---|---|---|---|---|---|
| AMD | 2.4, 6.0 | 4.0 | NA | 1.4a, 2.0b | NA |
| CAD | 1.3, 1.7 | 1.2F, 1.4M | NA | 2.1M,2.7F | 1.7M, 2.4F |
| DM1 | 0.08, 0.3 | 2.3; 6.6 | NA | NA | NA |
| DM2 | 1.2, 1.3 | 1.9 | 2.3c, 5.9d |
NA | NA |
| HH | 1.0M, 27.0M** |
NA | NA | NA | NA |
| LUP | 1.4, 2.0 | 4.1, 11.3 | NA | 1.0a, 1.5b | NA |
| MEL | 1.7, 3.0 | 2.2 | NA | NA | NA |
| PRO | 1.5M, 1.5M | 1.9M | NA | NA | NA |
AMD: Age Related Macular Degeneration
CAD: Coronary Artery Disease
DM1: Type 1 Diabetes
DM2: Type 2 Diabetes
HH: Hemochromatosis
LUP: Systemic Lupus Erythematosus
MEL: Melanoma
PRO: Prostate cancer
RR: Relative Risk
NA: not applicable risk factor
M: Male
F: Female
former smoker
current smoker
BMI=25.0 – 29.9 kg/m2
BMI=≥ 30 kg/m2
- AMD: one or more first-degree relatives with age-related macular degeneration
- CAD: one or both parents diagnosed with coronary artery disease
- DM1: one (RR 2.3) or more (RR 6.6) first-degree relatives diagnosed with type 1 or type 2 diabetes
- DM2: one or both parents with type 2 diabetes
- LUP: one (RR 4.1) or two or more (RR 11.3) first-degree relatives with a history of any of the following autoimmune diseases: Systemic lupus erythematosus (SLE/lupus), Sjogren's syndrome, rheumatoid arthritis, vitiligo, multiple sclerosis, celiac disease, type 1 diabetes (IDDM), autoimmune hyperthyroidism-Grave's disease, autoimmune hypothyroidism, Crohns disease, ulcerative colitis and psoriasis
- MEL: one or more first-degree relatives with melanoma
- PRO: father and/or any brothers diagnosed with prostate cancer
RR only provided to males. Male heterozygotes and homozygote wild type received an RR of 1.0; females got absolute risk: homozygotes received 16% lifetime risk, heterozygotes and wild type homozygotes received a lifetime risk of 1%.
Both Type 1 and Type 2 diabetes
Figure 2. Sample GC Summary Letter.
CPMC participants
From study inception in December 2007 through January 2012, study participants received all available CPMC reports when genotyping was complete and then additional reports on an ongoing basis as they were created for inclusion in the study. For participants enrolled starting in January 2012, CPMC participants received results on an ongoing basis following study enrollment, no more frequently than 1 per month until all 26 study reports were released. Telephone-based GC was available to all CPMC participants, free of charge, but was not mandatory. Alternatively, questions could be submitted to a genomic counselor via email. Study participants may request GC at any time (pre- or post-result receipt) however; all requests to date have come after results were issued. All telephone GC is provided by a licensed board certified genomic counselor employed by the Coriell Institute for Medical Research. CPMC participants can submit requests for a GC session as often as needed via email, by phone, or through the secure CPMC web portal. Telephone-based GC sessions are participant-driven, meaning that the content of the counseling interaction is primarily dictated by the specific questions and concerns of the participant, with the genomic counselor providing appropriate contextual information in “teachable moments” that arise throughout the natural course of the conversation. Genomic counselors only provided counseling for the (n=26) test reports that study participants received, and made referrals to other healthcare specialists (e.g. ophthalmologist) or to a clinical genetic counselor when warranted (e.g. participant family history suggestive of a hereditary cancer syndrome). These telephone GC sessions on average lasted approximately 27 minutes (range 10–65 minutes).
Phone Interviews
Phone interviews were conducted by LW, who was trained to conduct the interviews by EG and TS who are experienced interviewers and qualitative researchers (Gollust et al., 2012; Gordon et al., 2012; Schmidlen et al., 2014). The interviews used a semi-structured guide (Supplemental Table 1) which contained questions designed to determine participants’ expectations and experiences with GC, the characteristics of their GC session and nature of their results, as well as their expectations and preferences regarding GC communication and topics. We also assessed the extent to which participants shared results with their family members and/or health care providers. The interview guide was pre-tested with 5 study participants who had received GC to make additional edits for clarity, determine the length of the interview, and to ensure that the interviewer (LW) had received adequate training. Interviews lasted 45 – 90 minutes and participants received a $50 amazon.com e- gift card for participating.
Data analysis
Phone interviews were recorded and transcribed verbatim. Transcripts were analyzed using NVivo 9 using a deductive coding method. Transcribed interviews were first read as a whole by the research team (KS, TS, AS, LW, KW) and marginal notes were made of major concepts and themes that emerged in grounded theory format. An initial codebook was developed (KS, TS, ACS, KW, BAB, ESG) including major codes and sub-codes, which captured key thoughts or concepts from the interviews. KW, SH and KS, working together, discussed the category definitions, individually coded a portion of the interview transcripts, and subsequently reviewed this work to test reliability. Category definitions were then revised after this pre-testing to harmonize the approach to coding. They assigned codes and revised the codebook as necessary using NVivo. Results of descriptive coding were then organized into major and minor themes to better understand participant expectations and impressions of the GC process, parts of the separate service delivery model(s) that worked well and those that need modification, and preferences for follow up GC.
RESULTS
Participant Characteristics
Participant demographics are presented in Table 4. The majority of participants were white and highly educated; most had incomes greater than $75,000 per year. Of the 51 participants, there were five who worked in health care related occupations (3 physicians, 2 nurses).
Table 4.
Demographics
| OSU-CPMC | CPMC | P-value | Test | ||
|---|---|---|---|---|---|
| N | 20 | 31 | |||
| Mean Age (SE) | 60 (2.03) | 45.68 (2.51) | 0.00005 | Welch's T-test | |
| Education | High school graduate | 0 | 1 | 0.2572 | Fisher's exact test |
| Some college | 3 | 2 | 0.0738 | Ordinal Logistic Regression | |
| Associate degree | 4 | 2 | |||
| Bachelors degree | 7 | 9 | |||
| Graduate degree | 6 | 17 | |||
| Annual Household Income | Less than 25,000 | 2 | 1 | 0.03345 | Fisher's exact test |
| 25,000 to 49,999 | 4 | 2 | 0.774 | Ordinal Logistic Regression | |
| 50,000 to 74,999 | 1 | 11 | |||
| 75,000 to 99,999 | 3 | 4 | |||
| More than 100,000 | 8 | 13 | |||
| NA | 2 | 0 | |||
| Race | Asian | 0 | 1 | 1 | Fisher's exact test |
| Black or African-American | 1 | 1 | |||
| White (Caucasian) | 19 | 29 | |||
| Gender | Female | 10 | 24 | 0.06765 | Fisher's exact test |
| Male | 10 | 7 | |||
| Healthcare Practitioners | Licensed Practical Nurse | 0 | 1 | ||
| Registered Nurse or BSN | 0 | 1 | |||
| Physician | 0 | 3 | |||
| Non-Healthcare Occupations | Other | 20 | 26 |
Fisher’s exact test
Ordinal Logistic Regression
OSU-CPMC: Chronic Disease cohort receiving in-person GC
CPMC: CPMC cohort receiving telephone GC
In total, 91 (45.7%) OSU-CPMC participants received in-person GC between August 2011–May 2014 as part of the randomized trial. For participants in this qualitative interview study (n=20), the average number of disease reports viewed at the time of in-person GC was 6.9 (range: 1–10) and the number of PGx reports was 1.7 (range: 0–3). By the time of the interviews (May–July 2014), an average of 8.6 complex disease reports (range: 4–18) and 2.7 PGx reports (range: 0–5) had been viewed. The mean number of days between counseling and the phone interviews was 427 (range: 23–966). The mean age of OSU-CPMC study participants was 60 years (versus 46.8 years in the CPMC cohort).
Overall, 191 of 5534 (3.5%) CPMC participants opted to have telephone GC. For CPMC interviewees in this qualitative study (n=31), an average of 10.9 (range: 2–19) disease reports and 3.1 PGx (range: 0–5) reports had been viewed upon receipt of telephone GC. By the time of the qualitative interviews (May–July 2014), 14.6 disease reports (range: 5–19) and 4.2 PGx reports (range: 0–7) had been viewed (Table 3). The mean number of days between counseling and the phone interviews was 320 (range 7–1189).
Table 3.
Number of complex disease and pharmacogenomics reports viewed
| OSU-CPMC | Mean # Complex Disease Reports |
Mean # Pharmacogenomic Reports |
|---|---|---|
| By the time of in-person GC | 6.9 (range: 1–10) | 1.7 (range: 0–3) |
| By the time of phone interview |
8.6 (range: 4–18) | 2.7 (range: 0–5) |
| CPMC | ||
| By the time of telephone GC | 10.9 (range: 2–19) | 3.1 (range: 0–5) |
| By the time of phone interview |
14.6 (range: 5–19) | 4.3 (range: 0–7) |
OSU-CPMC: Chronic Disease cohort receiving in-person GC
CPMC: CPMC cohort receiving telephone GC
Major Themes
There were a number of major themes that emerged from the interviews, including participants’: (1) diverse reasons for participating in GC; (2) positive impressions of GC, independent of the communication channel (in-person versus phone), (3) desire for a more participant-driven model for GC and (4) stated need for follow-up GC as new results become available. We present these major findings in greater detail below.
Diverse reasons for participating in GC
When asked about their reasons for seeking GC, the most common reason stated amongst participants in both groups (in-person and phone counseling) was to clarify testing results in more detail (n=28 (54.9%) participants: 15 (75%) in-person, 13 (41.2%) phone) While only 13 participants [7 (35%) in-person, 6 (16.1%) phone] felt the complex disease test reports were too technical, about two-thirds [n=33 (64.7%) participants: 11 (55%) in-person, 22 (71%) phone] said there was at least one result that was difficult for them to understand. One participant noted that she desired counseling, “to go through the results in detail and help me understand how these results affected my everyday life” (OSU-CPMC, female, age 61). Another participant noted that the counselor was able to give her, “… a little bit more explanation or to make sure that what I explained to her or how I thought I was reading it that it was correct or not” (CPMC, female, 33).
Participants also sought GC to gain a better understanding of the interaction of multiple risk factors (genetic variants, family history, non-genetic risk factors) on their reported disease risk. One participant said she joined the study because of the option to see a genetic counselor and noted it was important to “have somebody with expertise to really give me the insight on what it all means and how it all fits together was what I was looking for” (OSU-CPMC, female, age 54). Another said that her counselor “gave me a tremendous amount of interpretation. It was just very helpful to me. He was able to say well look, this is what this means” (OSU-CPMC, female, age 68). Close to 70% of participants in both groups explicitly remembered the counselor talking about gene/environment interactions, and how multiple factors influence risk for a given disease [n=35: 14 (70%) in-person, 21 (67.7%) phone]. As one participant noted, “there’s so many factors involved with diabetes, and weight, and age, and you know, gender, and history, family history, genetic variants, multiple genetic variants. I found that one to be a little bit more confusing. I do have a family history of that, so I just wanted to make more clear perhaps my likelihood based on the genetic results of me getting it with my family history” (CPMC, male, age 39).
Participants often asked [16 (80%) in-person; 17 (54.8%) phone] for concrete, specific ideas and recommendations to assist them in taking appropriate action in response to the test results. For example, “which are the things I need to be most concerned about, or most aware of, or most make sure I discuss with my physician, whether that means I am at more of a genetic risk in the future or there is something I can do environmentally, you know, to change it. To me, what are the action items?” (OSU-CPMC, male, age 46). Another counselee said, “But it would have been more reassuring if there was some way that they could say well; I’m telling you how to be proactive, other than to talk to your doctor” (CPMC, female, age 68). Almost two-thirds of participants [n=33: 16 (80%) in-person, 17 (54.8%) phone] said the counselor made specific behavioral recommendations (e.g. exercise or lose weight) in response to the risk information; however, some thought they would receive more of an action plan for behavorial recommendations. For example, one participant said that she “thought that I would receive more of a game plan than I got” (CPMC, female, age 68).
Positive Impressions of Counseling
In general, participants had positive impressions of GC, independent of the means of service delivery (telephone versus in-person). The majority of participants said they were satisfied with their GC experience and that it exceeded their expectations [n=48; 19 (95%) in-person, 29 (93.5%) phone]. One-third of respondents [n=17: 9 (45%) in-person, 8 (25.8%) phone] mentioned that the GC helped put their results into perspective (e.g., what their risk was compared to the general population; how family history vs. genetic variant vs. environmental risk factors like body mass index play a role and to what extent; and why they have certain conditions in their family, or not). Many, especially those receiving participant-driven phone counseling, raised unsolicited positive comments regarding GC, stating that their counselor took the time to listen to them and answer questions in a spontaneous fashion [n=35: 11 (55%) in-person, 24 (77.4%) phone]. However, slightly more individuals receiving in-person counseling (12; 60%) than phone counseling (n=10; 32.3%), thought their counseling was thorough and provided complete information; this finding is likely attributable to the more intensive GC-led in-person session for the chronic disease cohort. As one counselee noted, “I just came away with a much better understanding, not only about genetic counseling, but also about myself and my risk to stuff and my health and how to better communicate with my doctors at appointments.” The participant also noted that her doctors “don’t have time in a 20 or 30 minute appointment to talk about our family history, and go over whatever it is that we came to see, and add that all into the bigger picture. It just is something that gets lost sometimes” (CPMC, female, age 32).
Desire for a more participant-driven approach in terms of mode of GC communication, and agenda
In asking participants about preferences regarding means of service delivery (e.g. in-person, telephone), we found that participants often liked the format of GC they had received. For example, 74% (n=23) of those who said they preferred telephone GC received phone counseling. One participant said, “Over the phone was fine and probably preferable just based on scheduling convenience. I don’t think there’s any benefit in doing it face-to-face. I think maybe in-person, you’d have less compliance, because people don’t want to have to take time out of their day to drive somewhere, and then wait, and then go in.” Some did prefer a modality other than the one they received, however. Six participants who received phone counseling stated a preference for in-person, and two were fine with either format. As one of these counselees noted, “Just like talking to another human being and just being there. It can be like comforting. Like you know, the counselor is there to sort of, you know, help read off of you and help you”(CPMC, female, age 57). Participants were also asked whether online counseling (via Skype or other video chat provider) would be a viable option and about half [9 (45%) in-person, 17 (54.8%) phone] agreed that it would, stating that “video chats give you that face to face option and phones don’t” (OSU-CPMC, male, age 52). Phone counselees, in particular, were more open to mediated communication (phone or online) than those in the in-person cohort. Some felt [n=8 (30.8%): 2 in-person, 6 phone] having a combination of methods (e.g., view results online while talking to the counselor) would be helpful. However, delivery method may ultimately depend on the severity and risk level of the information being communicated. As one participant explained, “if the genetic counseling comes up, say Mr. ___ has no risk for diabetes type 1 or 2 let patients access it right online like they do now. But if Mr. ___ has a three time risk for diabetes I think that deserves counseling, regardless. So I don’t know that I would give the option of allowing somebody who has a significant medically actionable disease the option of looking at it themselves” (CPMC, female 54).
We identified a need for more flexibility in determining the structure and direction of the GC session, and of results disclosure. Most participants [n=48 (17 (85%) in-person, 31 (100%) phone)] thought the GC session was “just right” in terms of length. The OSU-CPMC chronic disease participants often said the in-person sessions were led by the genomic counselor (n=11; 55%), while many in the phone counseling group felt the session was led by the participant (n=12; 38.7%) or was jointly led by the participant and the counselor (n=8; 25.8%). As one telephone participant noted, “I didn’t notice that there was a very structured format to it but maybe there was. But whatever it was, it worked. She talked for as long as necessary to answer my questions and have a good discussion about the topic.” (CPMC, female, age 41). Some participants also said they would like to help choose, pre-session, the areas of focus for the GC session (e.g. discussion of specific test results). For example, “I communicated by email, you know, to set it up, and told her the specific areas so I wouldn’t have expected her to go beyond that. And that was my, you know…, she met my expectations” (CPMC, male, age 25). In-person counselees (n=15 (75%) had greater preference for going over all available test reports. As one participant noted, “I think it would be helpful to go over all of them, because… to kind of say that, look, alright, you can’t ignore these other ones either. I mean they’re helpful, because you know, you might want to keep tabs on these to kind of remember…” (OSU-CPMC, male, age 35). However, this finding might be related to underlying health concerns, or the flow of test reports release through the web portal for each cohort as the converse was true for telephone counselees [16 (51.2%)] who often only wanted to discuss increased risk results. Telephone counselees had on average received more reports at the time of GC (CPMC, 10.9 disease reports (range: 2–19) and 3.1 PGx reports (range: 0–5); OSU-CPMC, 6.9 disease reports (range: 1–10), 1.7 PGx reports (range: 0–3). As one CPMC participant (female, age 58) noted, “Somebody else might not mind, but personally, I don’t want all of the results, but if there was something that is flagged in his or her mind, I wouldn’t mind them reviewing it with me.”
Web portal and tools for GC sessions
Generally, people liked the CPMC test report content and the web portal where they accessed the information, as well as risk communication tools such as visual aids. “I’m going to say that the causes were helpful, the how common graph. I like the risk summary (Figure 1). So what I like about the risk summary is it kind of separates out by risk. And I can clearly see on that graph where each of those, the family history and other risk factors such as for the one I’m looking at is smoking. The genetic variant, it’s just at a glance I can tell how much genetics plays into the role versus maybe some other risk factors” (OSU-CPMC, female, age 54). Most OSU-CPMC counselees (14; 70%) recalled the in-person GC summary letter (Figure 2). For example, “Besides just again reinforcing everything, having it really kind of summarized in just a few page document, the history, the risk assessment, the… kind of the increased risk, tying in the family history as well as the genetic testing into one nice tidy little summary here, and the recommendations, was just nice to have it all in one small package” (OSU-CPMC, male, age 56). Also, many liked the idea of having additional "tools/resources" available for use pre- and post-session including a concise “Summary Report” that highlights what the participant is at increased (or decreased) risk for, and what the risk estimate is based on. Participants in both groups, liked being able to see their results while talking to the counselor: “Let’s say an interactive with the counselor present and maybe a visual of some kind on the computer. Or an explanation with the sense the person’s here, also watching this with me. And I can question him or her as to exactly what is needed, etc” (OSU-CPMC, male, age 67).
Interest for Follow-Up
It was notable that 90% of interviewees in both groups were interested in having the option of follow up with the genomic counselor as more results became available. As one counselee noted, “as much as you try to bone up on a subject and make sure that you understand everything there’s always that little pang of doubt that you’re misreading something. Especially if the results are a little different than what you would have thought or if your interpretation is boy, the last results were great, but this one I’m not so sure about. Just having the option to reach out to somebody, to go back a little bit, maybe that’s a time where sending an email would be good enough. Saying hey, I’m reading this result and I’m not sure I’m reading it right. Can you maybe clarify?” (OSU-CPMC, male, age 52). Preferences for mode of follow-up were primarily telephone or in-person in both groups. There were also some participants who felt performing GC in one format, and then having the option of choosing a different counseling modality as new results became available was appealing. As one in-person counselee suggested, “an initial meeting with a genetic counselor to explain what your risk factors are, what the markers mean, and then anything after that when they add something to your list, then maybe phone, maybe internet.” In contrast, a phone counselee suggested that “questions could be answered by email first and then, if the results were complicated, the participant could request a phone counseling or face-to-face interview” (CPMC, female, age 29).
DISCUSSION
Clinical application of genomic technologies raises multiple challenges including communicating large amounts of actionable genetic variant information in the context of additional non-genetic risk information to patients in a way they can readily understand, apply currently for health promotion and possibly treatment, and utilize over the lifespan (Ashley, 2015; Collins & Varmus, 2015). More accurate disease risks estimates, for both highly penetrant Mendelian conditions and common, complex diseases are becoming more readily available (Collins & Varmus, 2015; Khoury & Evans, 2015). Accordingly, further availability of genetic and genomic counseling services within the results delivery process to patients will be essential and directly applicable for new large-scale genomic sequencing efforts (Collins & Varmus, 2015; Kaufman, Bollinger, Dvoskin, & Scott, 2012; Select Committee on Science and Technology, 2009). Currently very little is known about how participants offered either in-person or telephone genomic counseling for multiple common diseases with actionable components perceive its potential benefits. This is important to understand since most previous studies show around 10% or less uptake of counseling for genomic based results received through online delivery (Bloss, Wineinger, Darst, Schork, & Topol, 2013; Kaufman et al., 2012; Schmidlen et al., 2014). Results from this study provide a number of key into insights regarding participant expectations and desired effects from genomic counseling and illustrate the need for a more participant-driven model of GC. These results provide an important vantage point to further develop models of counseling service delivery for actionable genomic based on results received online (Kaphingst et al., 2012; Mills et al., 2014; Simmons et al., 2014).
The essential themes which emerged from the interviewees were the desire through genomic counseling to clarify test results, put results into perspective relative to other health-related and environmental risk factors, and to receive personalized recommendations. Participants also were interested in greater explanation regarding the interaction of multiple influences (genetic variants, family history, lifestyle/environment) on common disease risk. The frequency with which interviewees recalled the counselor discussing the influence of multiple risk factors on the development of common diseases suggests that discussing the genetic and environmental contributions together, in a holistic fashion, may provide a unique opportunity to provide education regarding healthy behaviors. Our approach to counseling has been to focus on the influence of both genes and environment on disease development, especially given the context of multiple test results that provide a wide range of disease risk and actionable components. However, from the interviews it was not clear (in either group) whether people thought about multiple diseases together and the relationships between them (e.g. diabetes as a risk factor for development of cardiovascular disease), or focused on one disease at a time. Focusing the counseling on the natural history of a given disease, especially in light of modifiable lifestyle changes, may assist with development of a personalized action plan (Mills & Haga, 2014; Shelton & Whitcomb, 2015). Although respondents often recall the counselor making specific behavioral recommendations (e.g. exercise or lose weight) in response to the risk information, this portion of the practice model requires more attention. Although most previous studies document that knowledge of genetic risk for common disease does not lead to behavior change (Hollands et al., 2016), none of these prior studies attempted to increase participant understanding of risk or help facilitate behavior modification (i.e., providing referrals) via genomic counseling for a range of actionable multifactorial disease risks as did the current study. Providing insight on the varied effect of genomic risk variants through counseling, to include the limited predictive contribution of many of these variants, and the polygenic and multifactorial nature of common disease risk, may allow individuals to develop more accurate perceptions of and appropriate responses to genetic risk for common diseases. Further supplementing our counseling approach with effective health behavior recommendations and interventions (Austin, 2015), perhaps with use of more directive and motivational counseling, may lead to adoption of health behaviors leading to risk reduction (Mills & Haga, 2014; Shelton & Whitcomb, 2015). The inclusion of health coaches and/or nurses for additional patient support and chronic disease intervention may also be beneficial (Bennett, Coleman, Parry, Bodenheimer, & Chen, 2010; Shelton & Whitcomb, 2015), though further research is needed.
Although participants in both groups tended to feel satisfied with the type of counseling they received, the approaches to GC used in this study need to be developed further. Our results suggest there is a need for more flexibility in determining the format of results disclosure and GC delivery, and for follow up counseling. For example, many of the chronic disease participants receiving in-person counseling had preference for discussion of all available test results, while the converse was true for the community participants, who, for the most part only wanted to discuss increased risk results. This difference may be due to the the structure of the in-person GC sessions, which focused on all nine initial test reports, whereas telephone participants had more control over which reports (up to 26) to view and discuss. It might also be attributable to the fact that those receiving in-person counseling in a medical care setting were already dealing with a chronic disease(s). It is notable that for both groups, telephone counseling would be acceptable when presenting personalized genomic results. Both groups also liked being able to visualize their results through the web portal while talking to the counselor, and felt positively when they had time to ask questions and when the counselor took time to listen to them. This suggests that formats whereby the participant may help direct where the conversation might go during the genomic counseling session is important, and that promotion of technology mediated channels of communication (e.g. texting; email; video conferencing; social networking) might be beneficial (Cohen et al., 2013). Assessing participant preferences prior to the counseling appointment, perhaps through technology mediated communication, may assist in refining the counseling agenda, especially in light of the online return of multiple test results, and to allow for follow up counseling for new results or questions.
Taken together, our results suggest that what patients desire is the ability to provide counseling preferences prior to the actual delivery of genomic counseling. Based on our results and to also help increase efficiency and potential effectiveness of genetic/genomic counseling, we propose assessing patient preferences for communication modality (telephone, telegenetic or in-person) prior to counseling. Utilization of technology beyond the standard “in-person” mode of counseling may help facilitate patient access to services that are limited due to geographical or financial barriers, or when in-person counseling is not feasible (Trepanier & Allain, 2014), and these alternative service delivery models have been well-accepted by patients (Buchanan, Rahm, & Williams, 2016). Furthermore, these alternate forms of communication increase patient/client convenience, and expand the scope of practice to include the ability to counsel multiple family members simultaneously who are not all in the same geographic location (Cohen, Huziak, Gustafson, & Grubs, 2016; Trepanier & Allain, 2014).
Second, given patients’ desire for flexibility in GC versus a standard scripted approach for all patients, we propose that an assessment of patient areas of concern, and/or points of discussion for the counseling session can be performed with the use of question prompts that could be emailed or texted to the patient pre-session. Having the counselee provide these preferences pre-session allows for even more pointed contracting and more specific case preparation. This may increase genomic counseling efficiency, and facilitate targeted psychotherapeutic interventions. This is especially relevant when an individual is provided results for multiple disease risks as well as pharmacogenomics results. Moreover, because counseling for common risk variants may not always require advanced or specialized counseling from a genetic counselor, health care professionals, with supplemental training in genetics/genomics (e.g. nurses) could use similar approaches to personalized and targeted genomic counseling (Mills & Haga, 2014; O'Daniel, 2010; Ormond, 2013; Shelton & Whitcomb, 2015). The goal of GC should be to make sure patients understand the information that is relevant to their health. As such, genomic counselors should recognize that the degree of GC intervention needed will vary per patient, and per indication, such that some participants may need counseling for multiple risks, while others may understand that concepts required for the interpretation of results for one condition are also relevant to the interpretation of other results.
Although the receipt of multiple personalized genomic results through a web portal in the context of genomic counseling was well received, our results suggest that there remains opportunity for improvement in terms of the management of the different types of test reports received, and improvement in test report communication. Refinement of the test report makeup and web materials with inclusion of graphs and visuals (e.g., pictographs) with less focus on actual numbers could drive home concepts of risk and encourage more viewing of test reports (Lautenbach, Christensen, Sparks, & Green, 2013). Further modification of summary reports, and summary letters, their format and content, and the way in which this is delivered to the participant and their healthcare team would be beneficial (Sweet et al., 2016). It would be ideal to develop a summary report with explicit breakdown of the areas of risk (e.g. non-genetic versus genetic) for a particular disease to allow for the viewing and discussion of management of multiple types of risk information at once, including hyperlinks for greater detail or to external resources to make the process more dynamic (Vassy et al., 2015), and to allow for more active routing and delivery. The growing availability and accessibility of information technologies (e.g. hand held devices), as well as innovative health communication approaches (e.g. increased access to information and support on demand to include enhanced opportunity to interact with health professionals, or identify support through the use of networking technology; tailoring information to the specific needs or characteristics of an individuals or groups of users) (Hovick, Wilkinson, Ashida, de Heer, & Koehly, 2014; Robinson, Patrick, Eng, & Gustafson, 1998), could be used to accentuate the current CPMC web portal route of genomic results delivery. It could also provide opportunity for education and support in a more participatory and less healthcare provider time-intensive fashion (Chow et al., 2015; Mishra, Neupane, Briffa, & Kallestrup, 2016; Robinson et al., 1998).
There are several limitations associated with this study. This study reports on a small set of qualitative interviews conducted with a self-selected predominantly Caucasian, generally well-educated population recruited to a parent study either by being a 1) patient with chronic heart disease at a large academic medical center, or 2) community, cancer, or military medical service employee participants who sought genomic testing on their own. Those who received telephone counseling (CPMC participants) did so at their own request, thus they may have a different motivation level or interest in genomic counseling than the OSU-CPMC patient participants who were assigned to receive in-person genomic counseling as part of a randomized controlled trial. In addition there are several differences between these groups that may have contributed to the trends that were observed. The OSU cohort was older (mean 60 yrs vs 45 in the CPMC cohort). This age difference could have contributed to comfort with technology, availability for in person appointments, etc. In addition, the length of the appointment was significantly different with the phone counseling averaging 27 minutes and the in person counseling sessions which were at least 60 minutes. It is not known how participants in either group would have responded to the mode of counseling had the approach been consistent. Furthermore, as interviews were only conducted with counselees, comparisons were not made to individuals who did not receive GC, and who may have different views.
Although our findings provide insight into needs for the genomic counseling process among multiple users, more work needs to be done. The fact that our current GC practice model focuses on multiple actionable disease reports delivered through online format, with each disease risk based on several influences (e.g. genetic risk variant(s), family history, environment/lifestyle), and promotes personal health behavior modification on three essential socio-ecological levels (individual, receiving online actionable genomic results; interpersonal, interaction with a GC; and organizational, interaction with health care systems (Division of Cancer Prevention and Control, 2015; Golden, McLeroy, Green, Earp, & Lieberman, 2015), makes this an applicable model moving forward. Incorporation of more comprehensive genomic risk scores, based on multiple risk variants for both common and rare (Mendelian) disease, will allow for a GC model that is adaptable and scalable for application in diverse clinical and research settings. The findings presented here provide a basis for expansion of this approach to genomic counseling which is participant driven in mode of communication (phone, video, in person) and agenda. Given the breadth of genomic information likely to be included in genomic testing reports as the use of WES/WGS increase, this non-traditional approach to genetic counseling (genomic counseling) will be necessary to construct a practice model that will help respond to increasing demands, worldwide, of the genetic counseling and next generation sequencing fields (Bernhardt, 2014; Khoury, Janssens, & Ransohoff, 2013; Manolio et al., 2015; O'Daniel, 2010; Ormond, 2013).
Supplementary Material
Acknowledgments
We thank Hannah Helbert for her assistance with this study.
Funding: Research reported in this publication was supported by the National Human Genome Research Institute of the National Institutes of Health under Award Number R21HG006575. This work was also supported by the Ohio State University Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Coriell Personalized Medicine Collaborative was funded by the William G. Rohrer Foundation, the RNR Foundation and a grant from the endowment of the Coriell Institute for Medical Research.
EG is currently a paid employee of 23andMe. She worked for the Coriell Institute for Medical Research at the time that this study was developed and the majority of the data collection period.
Footnotes
Conflict of Interest: The authors have no additional conflicts of interest to disclose.
Informed Consent: All procedures followed were in accordance with the ethical standards of the local medical ethical boards of the Ohio State University Wexner Medical Center and the Coriell Institute for Medical Research and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Compliance with Ethical Standards
Animal Studies: This article does not contain any studies with animals performed by any of the authors.
REFERENCES
- Ashley EA. The precision medicine initiative: a new national effort. JAMA. 2015;313(21):2119–2120. doi: 10.1001/jama.2015.3595. [DOI] [PubMed] [Google Scholar]
- Austin J. The effect of genetic test-based risk information on behavioral outcomes: A critical examination of failed trials and a call to action. Am J Med Genet A. 2015;167(12):2913–2915. doi: 10.1002/ajmg.a.37289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett HD, Coleman EA, Parry C, Bodenheimer T, Chen EH. Health coaching for patients with chronic illness. Fam Pract Manag. 2010;17(5):24–29. [PubMed] [Google Scholar]
- Bernhardt B. Genetic counselors and the future of clinical genomics. Genome Med. 2014;6(7):49. doi: 10.1186/gm565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss CS, Wineinger NE, Darst BF, Schork NJ, Topol EJ. Impact of direct-to-consumer genomic testing at long term follow-up. J Med Genet. 2013;50(6):393–400. doi: 10.1136/jmedgenet-2012-101207. [DOI] [PubMed] [Google Scholar]
- Buchanan AH, Rahm AK, Williams JL. Alternate Service Delivery Models in Cancer Genetic Counseling: A Mini-Review. Frontiers in Oncology. 2016;6(120) doi: 10.3389/fonc.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, Thiagalingam A. Effect of Lifestyle-Focused Text Messaging on Risk Factor Modification in Patients With Coronary Heart Disease: A Randomized Clinical Trial. JAMA. 2015;314(12):1255–1263. doi: 10.1001/jama.2015.10945. [DOI] [PubMed] [Google Scholar]
- Cohen SA, Huziak RC, Gustafson S, Grubs RE. Analysis of Advantages, Limitations, and Barriers of Genetic Counseling Service Delivery Models. J Genet Couns. 2016 doi: 10.1007/s10897-016-9932-2. [DOI] [PubMed] [Google Scholar]
- Cohen SA, Marvin ML, Riley BD, Vig HS, Rousseau JA, Gustafson SL. Identification of genetic counseling service delivery models in practice: a report from the NSGC Service Delivery Model Task Force. J Genet Couns. 2013;22(4):411–421. doi: 10.1007/s10897-013-9588-0. [DOI] [PubMed] [Google Scholar]
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Division of Cancer Prevention and Control, C. f. D. C. a. P. Social Ecological Model. 2015 Retrieved from http://www.cdc.gov/cancer/crccp/sem.htm.
- Golden SD, McLeroy KR, Green LW, Earp JA, Lieberman LD. Upending the social ecological model to guide health promotion efforts toward policy and environmental change. Health Educ Behav. 2015;42(1 Suppl):8S–14S. doi: 10.1177/1090198115575098. [DOI] [PubMed] [Google Scholar]
- Gollust SE, Gordon ES, Zayac C, Griffin G, Christman MF, Pyeritz RE, Bernhardt BA. Motivations and perceptions of early adopters of personalized genomics: perspectives from research participants. Public Health Genomics. 2012;15(1):22–30. doi: 10.1159/000327296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon ES, Griffin G, Wawak L, Pang H, Gollust SE, Bernhardt BA. "It's not like judgment day": public understanding of and reactions to personalized genomic risk information. J Genet Couns. 2012;21(3):423–432. doi: 10.1007/s10897-011-9476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga SB, Barry WT, Mills R, Svetkey L, Suchindran S, Willard HF, Ginsburg GS. Impact of delivery models on understanding genomic risk for type 2 diabetes. Public Health Genomics. 2014;17(2):95–104. doi: 10.1159/000358413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollands GJ, French DP, Griffin SJ, Prevost AT, Sutton S, King S, Marteau TM. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ. 2016;352:i1102. doi: 10.1136/bmj.i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovick SR, Wilkinson AV, Ashida S, de Heer HD, Koehly LM. The impact of personalized risk feedback on Mexican Americans' perceived risk for heart disease and diabetes. Health Educ Res. 2014;29(2):222–234. doi: 10.1093/her/cyt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AS, Schwartz MD, Valdimarsdottir H, Nusbaum RH, Hooker GW, DeMarco TA, Peshkin BN. Patient and genetic counselor perceptions of in-person versus telephone genetic counseling for hereditary breast/ovarian cancer. Fam Cancer. 2016 doi: 10.1007/s10689-016-9900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphingst KA, McBride CM, Wade C, Alford SH, Reid R, Larson E, Brody LC. Patients' understanding of and responses to multiplex genetic susceptibility test results. Genet Med. 2012;14(7):681–687. doi: 10.1038/gim.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman DJ, Bollinger JM, Dvoskin RL, Scott JA. Risky business: risk perception and the use of medical services among customers of DTC personal genetic testing. J Genet Couns. 2012;21(3):413–422. doi: 10.1007/s10897-012-9483-0. [DOI] [PubMed] [Google Scholar]
- Keller MGE, Stack C, Gharani N, Sill C, Schmidlen T, Mintzer J, Pallies J, Gerry NP, Christman M. The Coriell Personalized Medicine Collaborative: a prospective study of the utility of personalized medicine. Per Med. 2010;7:301–307. doi: 10.2217/pme.10.13. [DOI] [PubMed] [Google Scholar]
- Kessler S. Psychological aspects of genetic counseling: analysis of a transcript. Am J Med Genet. 1981;8(2):137–153. doi: 10.1002/ajmg.1320080204. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Evans JP. A public health perspective on a national precision medicine cohort: balancing long-term knowledge generation with early health benefit. JAMA. 2015;313(21):2117–2118. doi: 10.1001/jama.2015.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury MJ, Janssens AC, Ransohoff DF. How can polygenic inheritance be used in population screening for common diseases? Genet Med. 2013;15(6):437–443. doi: 10.1038/gim.2012.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenbach DM, Christensen KD, Sparks JA, Green RC. Communicating genetic risk information for common disorders in the era of genomic medicine. Annu Rev Genomics Hum Genet. 2013;14:491–513. doi: 10.1146/annurev-genom-092010-110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Abramowicz M, Al-Mulla F, Anderson W, Balling R, Berger AC, Ginsburg GS. Global implementation of genomic medicine: We are not alone. Sci Transl Med. 2015;7(290):290ps213. doi: 10.1126/scitranslmed.aab0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills R, Haga SB. Genomic counseling: next generation counseling. J Genet Couns. 2014;23(4):689–692. doi: 10.1007/s10897-013-9641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills R, Powell J, Barry W, Haga SB. Information-Seeking and Sharing Behavior Following Genomic Testing for Diabetes Risk. J Genet Couns. 2014 doi: 10.1007/s10897-014-9736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SR, Neupane D, Briffa TG, Kallestrup P. mHealth plus community health worker interventions: the future research agenda. Lancet Diabetes Endocrinol. 2016;4(5):387–388. doi: 10.1016/S2213-8587(16)30001-8. [DOI] [PubMed] [Google Scholar]
- O'Daniel JM. The prospect of genome-guided preventive medicine: a need and opportunity for genetic counselors. J Genet Couns. 2010;19(4):315–327. doi: 10.1007/s10897-010-9302-4. [DOI] [PubMed] [Google Scholar]
- Ormond KE. From genetic counseling to "genomic counseling". Mol Genet Genomic Med. 2013;1(4):189–193. doi: 10.1002/mgg3.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TN, Patrick K, Eng TR, Gustafson D. An evidence-based approach to interactive health communication: a challenge to medicine in the information age. Science Panel on Interactive Communication and Health. JAMA. 1998;280(14):1264–1269. doi: 10.1001/jama.280.14.1264. [DOI] [PubMed] [Google Scholar]
- Roche MI. Moving toward NextGenetic counseling. Genet Med. 2012;14(9):777–778. doi: 10.1038/gim.2012.84. [DOI] [PubMed] [Google Scholar]
- Schmidlen TSL, Zhaoyang R, Kasper R, Sweet K, Gordon ES, Keller M, Stack C, Gharani N, Daly MB, Jarvis J, Christman M. Genetic Knowledge Among Participants in the Coriell Personalized Medicine Collaborative. J Genet Couns. 2016;2:385–394. doi: 10.1007/s10897-015-9883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidlen T, Wawak L, Kasper R, Garcia-Espana JF, Christman MF, Gordon ES. Personalized genomic results: analysis of informational needs. J Genet Couns. 2014;23(4):578–587. doi: 10.1007/s10897-014-9693-8. [DOI] [PubMed] [Google Scholar]
- Select Committee on Science and Technology, H. o. L., Second Report. Genomic Medicine. 2009 Retrieved from. [Google Scholar]
- Shelton CA, Whitcomb DC. Evolving Roles for Physicians and Genetic Counselors in Managing Complex Genetic Disorders. Clin Transl Gastroenterol. 2015;6:e124. doi: 10.1038/ctg.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons LA, Wolever RQ, Bechard EM, Snyderman R. Patient engagement as a risk factor in personalized health care: a systematic review of the literature on chronic disease. Genome Med. 2014;6(2):16. doi: 10.1186/gm533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet K, Gordon ES, Sturm AC, Schmidlen TJ, Manickam K, Toland AE, Marsh C. Design and implementation of a randomized controlled trial of genomic counseling for patients with chronic disease. J Pers Med. 2014;4(1):1–19. doi: 10.3390/jpm4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet KSA, Schmidlen T, McElroy J, Scheinfeldt L, Manickam K, Gordon E, Hovick H, Roberts JS, Toland AE, Christman M. Outcomes of a randomized controlled trial of genomic counselling for patients receiving personalized and actionable complex disease reports. 2016 doi: 10.1007/s10897-017-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet K, Sturm AC, Schmidlen T, Hovick S, Peng J, Manickam K, Christman M. EMR Documentation of Physician-Patient Communication Following Genomic Counseling for Actionable Complex Disease and Pharmacogenomic Results. Clin Genet. 2016 doi: 10.1111/cge.12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepanier AM, Allain DC. Models of service delivery for cancer genetic risk assessment and counseling. J Genet Couns. 2014;23(2):239–253. doi: 10.1007/s10897-013-9655-6. [DOI] [PubMed] [Google Scholar]
- Vassy JL, McLaughlin HL, MacRae CA, Seidman CE, Lautenbach D, Krier JB, Green RC. A one-page summary report of genome sequencing for the healthy adult. Public Health Genomics. 2015;18(2):123–129. doi: 10.1159/000370102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veach PM, Bartels DM, Leroy BS. Coming full circle: a reciprocal-engagement model of genetic counseling practice. J Genet Couns. 2007;16(6):713–728. doi: 10.1007/s10897-007-9113-4. [DOI] [PubMed] [Google Scholar]
- Wright MF, Lewis KL, Fisher TC, Hooker GW, Emanuel TE, Biesecker LG, Biesecker BB. Preferences for results delivery from exome sequencing/genome sequencing. Genet Med. 2014;16(6):442–447. doi: 10.1038/gim.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.