Abstract
Objective
To investigate the trends of diagnosed celiac disease (CD), undiagnosed CD, and people without CD avoiding gluten (PWAG) in the civilian non-institutionalized US population from 2009 to 2014.
Patients and Methods
We studied the occurrence of CD and PWAG in the 2009–2014 National Health and Nutrition Examination Surveys (NHANES). The sera of all participants 6 years and older from NHANES 2009–2014 were tested for CD serology at Mayo Clinic. Participants were interviewed about a diagnosis of CD and the use of a gluten-free diet (GFD). The design effects of the survey and sample weights were incorporated in all statistical analyses.
Results
In the US general population, the prevalence of CD did not significantly change from 2009–2010 [0.7%, 95% CI (0.6–0.8)] to 2011–2012 [0.8%, 95% CI (0.4–1.2)] to 2013–2014 [0.7%, 95% CI (0.3–1.0)]. However, the prevalence of undiagnosed CD decreased from 0.6% in 2009–2010 to 0.3% in 2013–2014. In contrast, the prevalence of PWAG significantly increased from 0.5% (95% CI, 0.2–0.9) in 2009–2010 to 1.0% (95% CI, 0.6–1.4) in 2011–2012 to 1.7% (95% CI, 1.1–2.4) in 2013–2014 (P for trend = .005).
Conclusion
While the overall prevalence of CD remained stable between 2009 and 2014, the proportion CD that is hidden significantly decreased. Moreover, the prevalence of individuals without CD but following a GFD markedly increased between 2009 and 2014. Long-term health consequences of GFD warrant further investigation.
Keywords: Celiac disease, gluten-free diet, prevalence, trends
Introduction
Celiac disease (CD) is an autoimmune disease that affects approximately 1% of the U.S. population and amounts to a significant public health burden.1–3 Symptomatic (diagnosed) CD can be a devastating disease, and is associated with increased mortality and substantial accumulated morbidity.2,4 Until recently, most of what we knew about CD was based on studies of diagnosed CD. Although the rise of CD is documented worldwide,2,5–7 we have shown that even with modern clinical laboratory techniques of CD detection widely available, more than 80% of patients with CD remain undetected clinically.3,8
The gluten free diet (GFD) is mainly considered to be treatment for gluten-related conditions including CD and wheat allergy,4 but was not widely used previously for other disorders. However, several studies found that many people without CD are interested in following a GFD.9–11 The benefits of following a GFD in people without CD have not been tested rigorously, and indeed nutritional concerns have been raised about deficient iron, calcium and fiber consumption.12,13 In contrast to public interest in following a GFD, it remains uncertain if there is any benefit of following a GFD for people without gluten-related conditions.14 Nonetheless, recent public attention to the GFD has dramatically increased along with the market shares of nutraceutical products.3,10,11,15–19 However, the real magnitude of this emerging public health issue and population-based prevalence of gluten-related conditions, including CD and people without CD avoiding gluten (PWAG), are largely unknown in the United States. Many health claims have been based on market research directed surveys that may have inherent selection bias, such as polling in shopping malls, or may have covered a short duration.16,17,20 Thus, the purpose of this study was to 1) estimate the prevalence of CD and PWAG, 2) further investigate trends in the prevalence of gluten-related occurrence including CD and PWAG using the data of the 2009–2014 National Health and Nutrition Examination Surveys (NHANES).
METHODS
NHANES Background
The NHANES is designed and conducted to evaluate the health and nutritional status of the United States population.21–23 NHANES accurately represents the U.S. non-institutionalized, civilian population because of its complex, stratified, multi-stage, probability-based sampling design. Approximately 5,000 persons per year were recruited to collect information on health and nutritional status by standardized household interviews, physical examinations, and testing of biologic samples.21–23 More detailed information on the survey design for the NHANES and methods to evaluate CD and PWAG using gluten-related questions and CD laboratory testing- is available in our previous publications.3,8 Since 1999, NHANES has been conducted in 2-year cycles continuously and oversampled non-Hispanic blacks, Hispanics, Asians (2011–2014), low-income whites, and persons aged 80 years or older.
Laboratory tests for CD
Starting with the NHANES 2009–2010, we designed study components to estimate the prevalence of CD and PWAG in the US population. CD serology testing was performed at Mayo Clinic and two structured questions related to diagnosis of CD and the status of GFD were asked. The methods for CD serology testing has been described in detail elsewhere.3,8 Briefly, serum samples from 2009–2014 NHANES participants aged 6 years or older were collected and shipped to the Celiac Disease Research Laboratory at Mayo Clinic, Rochester, MN, USA. Sequential serology testing was used to detect CD, which we have shown to be an effective method to detect undiagnosed cases in the general population.24,25 The human recombinant tTG-based enzyme-linked immunosorbent assay (Inova Diagnostics, San Diego, CA) was used to measure titers of tissue transglutaminase IgA (tTG IgA).3,26 If tTG IgA titers were ≥ 4.0 U/mL, then sequential testing with IgA endomysium antibodies (EMA) was performed by indirect immunofluorescence using the reticulin component of the endomysium of the smooth muscle in monkey esophagus tissue (Inova Diagnostics).26 Serological diagnosis of CD was based on the double positivity (positive to tTG-IgA and subsequent EMA confirmation), which has previously shown high sensitivity and specificity.26–28
Continuous NHANES 2009–2014
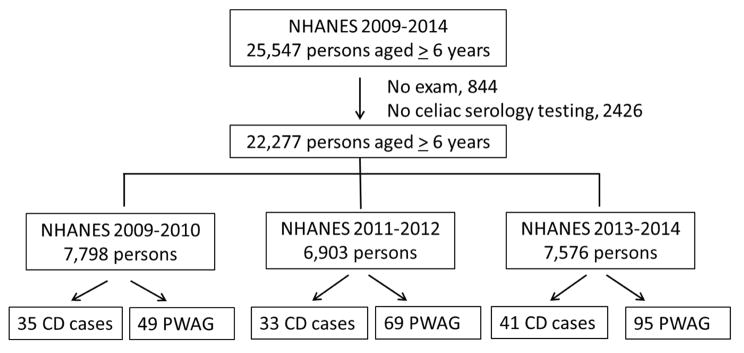
For this analysis, data from NHANES 2009–2010, 2011–2012, and 2013–2014 were used. Among a total of 25,547 participants aged 6 years or older between 2009 and 2014, 844 persons who did not receive a health examination were excluded. Our analysis sample comprised a total of 22,277 participants to estimate the prevalence of CD or PWAG after 2,426 subjects who were not tested for CD serology or did not respond to CD-related questions were also excluded. The flow chart for the analysis sample is shown in Figure 1. The following definitions were adapted from our previous studies3,8; CD was confirmed if individuals had double positivity (tTG IgA and EMA) or a participant-reported clinical diagnosis of CD. The responses to two following CD-related questions were used to define a reported clinical diagnosis of CD and PWAG: 1) has a doctor or other health professional ever told you that you have celiac disease, also called sprue? and 2) are you on a gluten-free diet? While a reported clinical diagnosis of CD was defined by positive responses to these two questions, PWAG was identified if the individual adhered to a GFD without a health professional diagnosis of CD and was negative on serologic testing.
Figure 1.
From an eligible population of 25,547 participants aged 6 years or older in the NHANES 2009–2014, 22,277 had serologic samples tested for CD. Of 7,798 participants in NHANES 2009–2010, 35 had a diagnosis of CD and 49 were PWAG. Similarly, 33 had CD and 69 had PWAG in NHANES 2011–2012 (n=6,903), while 41 had CD and 95 had PWAG out of 7,576 participants in NHANES 2013–2014. CD, celiac disease; PWAG, people without CD avoiding gluten; NHANES, National Health and Nutrition Examination Survey.
Statistical Analyses
All statistical analyses followed the National Center for Health Statistics analytic guidelines, which incorporated the effect of complex study design and used appropriate, published weights. Statistical analyses were performed using SAS version 9.4 software. All estimates of prevalence of CD and PWAG were based on weighted data. In addition, a complex study design of oversampling and nonparticipation in the household interview and physical examination and for the changing sampling strategies or priorities over each biennium was incorporated in all analyses. The prevalence of CD, undiagnosed CD, or PWAG between 2009 and 2014 was estimated in the total population, as well as by race (whites, blacks, and Hispanics), children and adults, and gender, if number of cases was greater than or equal to 20. Trend analyses of the prevalence of CD, undiagnosed CD, or PWAG were performed in the three study periods i.e. 2009–2010, 2011–2012, and 2013–2014, using the χ2 test. P values < 0.05 were considered statistically significant.
RESULTS
Overall prevalence of celiac disease in NHANES 2009–2014
Of 22,277 individuals aged 6 years or older from 2009–2014 NHANES, 39% (n=8,652) were non-Hispanic white, 22 % non-Hispanic black (n=4,825), 27% Hispanic (n=6,098), and 12 % were other race groups including Asians (n=2,702). Based on serological or clinical diagnosis, CD was confirmed in 109 participants. Among participants aged 6 years or older, the prevalence of CD in NHANES 2009–2014 was 0.7% (95% confidence interval [CI], 0.5–0.9) (Table 1), which equates to approximately 2.0 million Americans. Among 8,652 non-Hispanic whites, 75 had CD; the weighted prevalence of CD among non-Hispanic whites was 1.0% (95% CI, 0.7–1.2). Only 34 individuals had CD among 13,626 individuals who were not non-Hispanic white (0.2%, 95% CI: 0.1–0.3); 6 out of 4,826 non-Hispanic blacks, 19 persons of Hispanic origin (n=6,098), and 9 persons of other race (including Asian and other non-Hispanic races, n=2,702). The prevalence of CD was higher among women (0.9 %, 95% CI: 0.6–1.2) than men (0.5%, 95% CI: 0.4–0.7) (P=.007), but was similar between children (aged 6–18 years) and adults (18 years or older) (Table 1). When restricted to undiagnosed CD (positive on sequential CD serology testing without a reported clinical diagnosis), the prevalence in NHANES 2009–2014, was 0.5% (n=81, 95% CI, 0.4–0.7), which equates to approximately 1.4 million Americans. The weighted prevalence of undiagnosed CD among non-Hispanic whites was 0.7 % (95 % CI, 0.5–0.9%). The prevalence of undiagnosed CD did not differ between males (0.5%, 95% CI: 0.3–0.6) and females (0.6, 95% CI: 0.3–0.8) (P=.2), and was similar among children (aged 6–18 years, 0.6%, 95% CI: 0.3–0.9) and adults (18 years or older, 0.5%, 95% CI: 0.4–0.6) (P=.4).
Table 1.
Prevalence of CD and PWAG in the United States from 2009–2014*
| Characteristic | No of cases | Weighted prevalence % (95% CI) |
|---|---|---|
| Serologically diagnosed CD and/or reported clinical diagnosis | ||
| Total population | 109 | 0.72 (0.53–0.90) |
| Non-Hispanic white | 75 | 0.97 (0.71–1.24) |
| Hispanic | 19 | 0.30 (0.14–0.48) |
| Male | 40 | 0.54 (0.40–0.69) |
| Female | 69 | 0.88 (0.56–1.20) |
| Age < 18 years | 26 | 0.76 (0.44–1.08) |
| Age ≥ 18 years | 83 | 0.71 (0.52–0.90) |
| Serologically-diagnosed CD without reported clinical diagnosis | ||
| Total population | 81 | 0.51 (0.37–0.65) |
| Non-Hispanic white | 54 | 0.68 (0.47–0.88) |
| Hispanic | 15 | 0.27 (0.11–0.42) |
| Male | 34 | 0.46 (0.34–0.59) |
| Female | 47 | 0.55 (0.29–0.80) |
| Age < 18 years | 24 | 0.59 (0.31–0.89) |
| Age ≥ 18 years | 57 | 0.49 (0.35–0.63) |
| PWAG | ||
| Total population | 213 | 1.11 (0.82–1.41) |
| Non-Hispanic white | 84 | 1.18 (0.77–1.59) |
| Non-Hispanic black | 53 | 1.13 (0.77–1.50) |
| Hispanic | 43 | 0.80 (0.51–1.08) |
| Other race | 33 | 1.17 (0.64–1.70) |
| Male | 95 | 0.83 (0.60–1.07) |
| Female | 118 | 1.38 (0.94–1.82) |
| Age < 18 years | 27 | 0.58 (0.23–0.93) |
| Age ≥ 18 years | 186 | 1.20 (0.88–1.52) |
CD, celiac disease; PWAG, people without CD avoiding gluten; CI, confidence interval.
Population estimates are not reported for categories with < 20 cases, except for Hispanic ethnicity.
Overall prevalence of PWAG in NHANES 2009–2014
Among 22,277 participants aged 6 years or older in NHANES 2009–2014, 243 responded as being on a GFD. Of 243 participants on a GFD, 30 people who had a reported clinical diagnosis of CD were excluded from the PWAG group. Among participants aged 6 years or older, the prevalence of PWAG in NHANES 2009–2014 was 1.1% (n=213, 95% CI, 0.8–1.4), corresponding to approximately 3.1 million Americans. While the prevalence of PWAG was similar among racial and ethnicity groups, the prevalence of PWAG was significantly higher (P=.006) in females than in males (table 1).
Trends in prevalence of CD and PWAG between NHANES 2009 and 2014
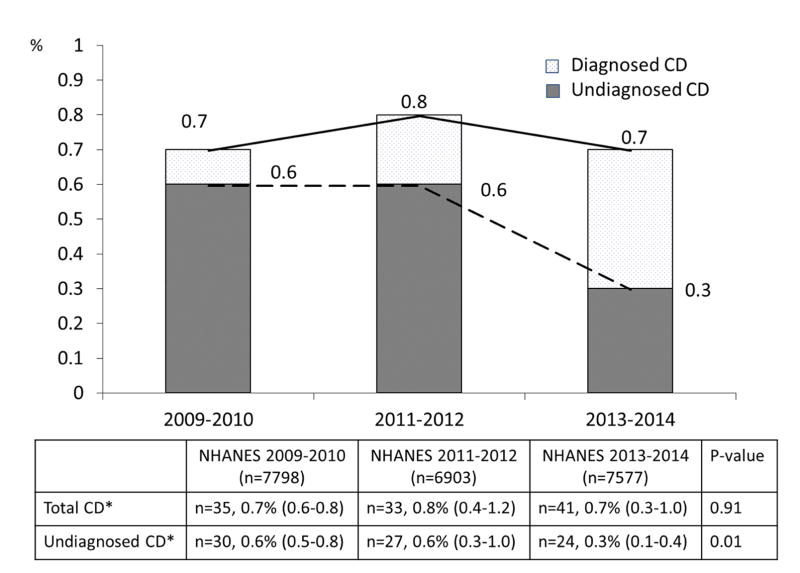
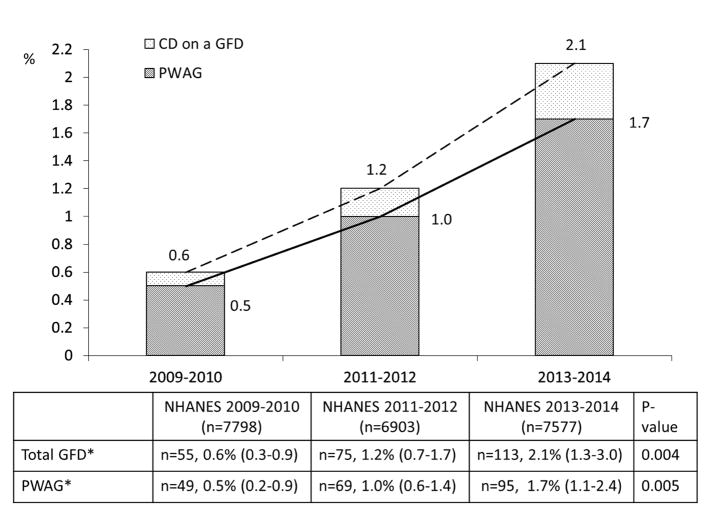
Figure 2 shows the trend in the prevalence of CD and undiagnosed CD in individuals aged 6 years and older in NHANES 2009–2010, 2011–2012, and 2013–2014. Overall, the prevalence of CD was 0.7% (n=35, 95% CI 0.6–0.8) in NHANES 2009–2010, 0.8% (n=33, 95% CI 0.4–1.2) in 2011–2012, and 0.7% (n=41, 95% CI 0.3–1.0) in 2013–2014, indicating that the prevalence of CD was stable over this period. In contrast to overall CD prevalence, the prevalence of undiagnosed CD significantly decreased over time (p for trend=0.01); the prevalence of undiagnosed CD was 0.6 % (n=29, 95% CI, 0.5–0.8) in NHANES 2009–2010, 0.6 % (n=27, 95% CI, 0.3–1.0) in NHANES 2011–2012, and 0.3 % (n=23, 95% CI, 0.1–0.4) in NHANES 2013–2014. The discrepancy between the absolute number of cases and calculated prevalence of undiagnosed CD in NHANES 2013–2014 was caused by the weighted analysis appropriate for NHANES sampling. While there has been an increase in the percent of those with celiac disease diagnosed the numbers are too small to permit truly accurate prevalence numbers over each biennium, however there is clear reduction in the percentage that remain undiagnosed. The decreasing trend in undiagnosed CD prevalence was predominantly found among the non-Hispanic white group; 0.9% (95% CI, 0.7–1.2) in NHANES 2009–2010, 0.8 % (95% CI, 0.3–1.4) in NHANES 2011–2012, and 0.3% (95% CI, 0.07–0.5) in NHANES 2013–2014. In addition, a similar trend pattern in adults aged 18 years or older with undiagnosed CD was observed, decreasing from 0.7% (95% CI, 0.5–0.8) in NHANES 2009–2010, to 0.6% (95% CI, 0.3–1.0) in NHANES 2011–2012, to 0.2% (95% CI, 0.05–0.4) in NHANES 2013–2014. Figure 3 shows the trend in the prevalence of GFD in participants aged 6 years and older in NHANES 2009–2010, 2011–2012, and 2013–2014. The proportion of participants on the GFD continuously increased over the 6-year period from 0.6% (n=55, 95% CI, 0.3–0.9) in NHANES 2009–2010 to 1.2% (n=75, 95% CI, 0.7–1.7) in NHANES 2011–2012, to 2.1% (n=113, 95% CI, 1.3–3.0) in NHANES 2013–2014 (P for trend=0.004). In particular, the prevalence of PWAG increased more than 3-fold in this period (p for trend=.005); from 0.5% (n=49, 95% CI, 0.2–0.9) in NHANES 2009–2010 to 1.0% (n=69, 95% CI, 0.6–1.4) in NHANES 2011–2012 to 1.7% (n=95, 95% CI, 1.1–2.4) in NHANES 2013–2014 (Figure 3). Figure 4 summarizes the trends of gluten-related occurrence (combination of CD and PWAG) between NHANES 2009 and 2014. The overall prevalence of gluten-related occurrence increased over time, but this was largely attributed to the increase in PWAG prevalence. Interestingly, the proportion of undiagnosed CD among total gluten-related occurrence has decreased from 51% in 2009–2010 to 35% in 2011–2012 to 12% in 2013–2014 (Figure 4).
Figure 2.
Trends in total and undiagnosed CD prevalence between 2009 and 2014. While the prevalence of total CD remained stable in this period, the prevalence of undiagnosed CD decreased in the last 2-year cycle. Interestingly, the prevalence of diagnosed CD increased dramatically in the last 2-year cycle. Total CD included participants with either serologically diagnosed CD or a reported clinical diagnosis of CD. CD, celiac disease; NHANES, National Health and Nutrition Examination Survey; *, % (95% confidence interval).
Figure 3.
Trends in GFD and PWAG prevalence between 2009 and 2014. The prevalence of total GFD and PWAG significantly increased between NHANES 2009 and 2014. Total GFD included participants who positively responded to following a GFD regardless of CD status. CD, celiac disease; GFD, gluten-free diet; PWAG, people without CD avoiding gluten; NHANES, National Health and Nutrition Examination Survey; *, % (95% confidence interval)
Figure 4.
Trends and relative proportions of CD and PWAG among gluten-related occurrences (GRO) between NHANES 2009 and 2014. The prevalence of GRO increased from NHANES 2009–2010 to 2013–2014. This increasing trend in GRO prevalence was predominantly due to increases in PWAG. However, the proportion of undiagnosed CD decreased from 51% in NHANES 2009–2010 to 12% in NHANES 2013–2014. CD, celiac disease; PWAG, people without CD avoiding gluten; NHANES, National Health and Nutrition Examination Survey; *, % (95% confidence interval)
DISCUSSION
In recent years, public attention to gluten-related conditions, including CD and the practice of avoiding gluten in the diet, has dramatically increased, reflecting the marked growth of the gluten-free food industry.16,20 Many studies from Western countries showed that the prevalence of CD is increasing recently,3,6,29 reflecting not only advances in serologic testing, but also increased awareness of CD among the general public and physicians. In the present study, we showed that the overall prevalence of CD in the US remained stable between NHANES 2009 and 2014. Interestingly, the prevalence of undiagnosed CD significantly decreased in this period, suggesting a success in detection, increased awareness of CD, or maybe increasing preference for a GFD. During this same period the prevalence of PWAG more than tripled, rising to an estimated 3.1 million persons.
In the past, CD was a rare disorder in the USA, measuring 1 in 652 persons from a military cohort in the 1950s.2 However, the prevalence of CD in the world has dramatically increased.3,6,29,30 In our previous, nationally-representative US study, we showed that the prevalence of CD in adults aged 50 years and older more than doubled between 1988 and 2012.3 Indeed, this trend is similar to that in other Western countries where the overall prevalence of CD is on the rise.6,29,31 Our current study showed stable CD prevalence in the U.S. population over a recent 6 year period. This may represent a true leveling in prevalence or too short of a period to see a change in prevalence. The recent decline in the prevalence of undiagnosed CD suggests an increased awareness of the disease in the general public and health care providers. However, this finding should be cautiously interpreted because the number of undiagnosed CD cases is small and the prevalence decreased only in the last two-year cycle. Another possible explanation for the decreased prevalence of undiagnosed CD is that positivity to serologic CD testing may be influenced by the increased proportion of people on a GFD in the US population. However, further studies of the relationship between CD and the public perception of gluten-free foods as a healthier dietary option are needed. It is also possible that persons who become symptomatic from undiagnosed CD may try a GFD empirically without testing thereby moving into the PWAG group. Future epidemiology studies, including serological surveys, will need to take into account this secular trend toward gluten avoidance.
As expected, racial disparity in the prevalence of CD was observed in the current study; most CD cases (75/109) were among non-Hispanic whites. Only 6 cases met the CD diagnosis out of 4,826 non-Hispanic blacks, which was about 1 in 804 people. Consistent with other studies of gender differences, our study showed that the prevalence of CD is higher in females than in males. Interestingly, the prevalence of undiagnosed CD was similar between females and males. Differences in health risk behavior in men and women may partly impact the gender difference in prevalence of diagnosed and undiagnosed CD. Another interesting finding regarding CD prevalence is the difference between children and adults. In contrast to other studies30,32,33 that have shown a higher CD prevalence in children compared to adults, we found that the prevalence of CD was similar between children age 6–18 and adults. This difference between studies may be due to population selection or small sample size of previous studies, most of which were conducted in selected populations from referral centers.32,33
We found a steady increase in the proportion of people on a GFD between 2009 and 2014. Interestingly, this increasing trend was predominantly in people who did not have a diagnosis of CD, and more than tripled from 0.5% in NHANES 2009–2011 to 1.7% in NHANES 2013–2014. Although the reasons why people choose to follow a GFD are unclear, the trend of more people choosing a GFD is significant in NHANES. Several surveys, including market research, gallop, and consumer reports, showed more than 20% of Americans seek gluten-free products.16,17,20 These polls have several limitations including selection bias, low response rate, and inadequate validity. In contrast, our study using the NHANES, a robust and representative sample of the US general population with direct interview responses to gluten- and CD-related questions, seems to most likely reflect the actual prevalence and trends of the GFD in the US. The health or nutritional benefits of a GFD in people without CD and/or wheat allergy have not been rigorously tested and hence are unproven. Rather, several studies raised nutritional concerns in people following a GFD, such as high fat and sugar consumption, micronutrient deficiency, and induction of the metabolic syndrome.34–37 In contrast to these studies, DiGiacomo et al.19 showed a slightly better cardio-metabolic profile including normal weight and higher HDL-cholesterol in individuals without CD on a GFD. Thus, further studies of PWAG are warranted to evaluate the association with clinical deterioration, similarities to CD, and social and economic burdens.
The current study has several limitations. With regard to CD diagnosis, serology may not detect persons with CD and Ig A deficiency. However, very few people with undiagnosed CD should have been missed in our study due to the low prevalence of selective Ig A deficiency. Although our study did not confirm the diagnosis of CD by intestinal biopsy, double sequential serologic testing to detect undiagnosed CD had been validated in our laboratory and others before the current study using NHANES was designed.24,25,38 Moreover, this study applied a highly sensitive and specific serologic test and interview questions to a representative US population; the NHANES provided us the opportunity for an unbiased study of the epidemiology of gluten-related conditions, including CD and GFD, in the US population. We did not ask participants why they chose a GFD, so it is possible that some persons with undetected CD had started on a GFD and thereby were incorrectly assigned to the PWAG group. One survey in Australia found that 80% of people who reported avoiding wheat had experienced at least one negative reaction to the consumption of wheat-based products.11 In addition, Biesiekierski et al.39 reported that 3 in 4 individuals with self-reported nonceliac gluten sensitivity had symptom improvement from avoiding gluten.
CONCLUSION
Our study, which was based on the US representative population, showed that the prevalence of CD remained stable between 2009 and 2014, but the proportion of undiagnosed CD among total CD significantly decreased in this period. This trend was more prominent among non-Hispanic whites and adults. Remarkably, the proportion of individuals maintaining a GFD in the absence of a diagnosis of CD increased markedly in this period. In light of this increasing trend in the use of a GFD, the clinical consequences of GFD require further investigation.
Acknowledgments
Financial support: This work was partly supported by the Centers for Disease Control and Prevention Contract No. M26561 and the NIDDK
We thank the staff of the celiac research laboratory and Immunodermatology Laboratory at Mayo Clinic for conducting the serologic testing. Also, the authors wish to thank Deborah I. Frank for her assistance in the preparation of the manuscript.
Abbreviations
- CD
celiac disease
- NHANES
National Health and Nutrition Examination Surveys
- GFD
gluten-free diet
- PWAG
people without CD avoiding gluten
- tTGA
tissue transglutaminase antibody
- EMA
endomysial antibody
- GFD
gluten-free diet
- IgA
immunoglobulin A
- OR
odds ratio
- CI
confidence interval
Footnotes
Specific author contributions:
The original study was conceived by James Everhart and Joseph Murray
Study concept and design: Joseph A. Murray and Rok Seon Choung;
Analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content: Rok Seon Choung, Aynur Unalp-Arida, Constance E. Ruhl, and Joseph A. Murray;
Statistical analysis: Rok Seon Choung;
Administrative, technical, or material support: Tricia L. Brantner;
Study supervision: Joseph A. Murray
Potential competing interests: Dr. Murray has received grant funding from Alba Therapeutics and Alvine Pharmaceuticals, Inc.; served on an advisory board for Alvine Pharmaceuticals, Inc; and has served as a consultant for AMAG Pharmaceuticals, BioLineRx, and GlaxoSmithKline.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163(3):286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137(1):88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choung RS, Ditah IC, Nadeau AM, et al. Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: findings from the National Health and Nutrition Examination Surveys from 1988 to 2012. Am J Gastroenterol. 2015;110(3):455–461. doi: 10.1038/ajg.2015.8. [DOI] [PubMed] [Google Scholar]
- 4.Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108(5):656–676. doi: 10.1038/ajg.2013.79. quiz 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JY, Kang AH, Green A, Gwee KA, Ho KY. Systematic review: worldwide variation in the frequency of coeliac disease and changes over time. Aliment Pharmacol Ther. 2013;38(3):226–245. doi: 10.1111/apt.12373. [DOI] [PubMed] [Google Scholar]
- 6.Lohi S, Mustalahti K, Kaukinen K, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26(9):1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 7.Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107(8):1248–1255. doi: 10.1038/ajg.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA, Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107(10):1538–1544. doi: 10.1038/ajg.2012.219. quiz 1537, 1545. [DOI] [PubMed] [Google Scholar]
- 9.Tanpowpong P, Broder-Fingert S, Katz AJ, Camargo CA., Jr Predictors of gluten avoidance and implementation of a gluten-free diet in children and adolescents without confirmed celiac disease. J Pediatr. 2012;161(3):471–475. doi: 10.1016/j.jpeds.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 10.Tanpowpong P, Ingham TR, Lampshire PK, et al. Coeliac disease and gluten avoidance in New Zealand children. Arch Dis Child. 2012;97(1):12–16. doi: 10.1136/archdischild-2011-300248. [DOI] [PubMed] [Google Scholar]
- 11.Golley S, Corsini N, Topping D, Morell M, Mohr P. Motivations for avoiding wheat consumption in Australia: results from a population survey. Public Health Nutr. 2015;18(3):490–499. doi: 10.1017/S1368980014000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson T, Dennis M, Higgins LA, Lee AR, Sharrett MK. Gluten-free diet survey: are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J Hum Nutr Diet. 2005;18(3):163–169. doi: 10.1111/j.1365-277X.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 13.Saturni L, Ferretti G, Bacchetti T. The gluten-free diet: safety and nutritional quality. Nutrients. 2010;2(1):16–34. doi: 10.3390/nu20100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaesser GA, Angadi SS. Gluten-free diet: imprudent dietary advice for the general population? J Acad Nutr Diet. 2012;112(9):1330–1333. doi: 10.1016/j.jand.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Di Sabatino A, Corazza GR. Nonceliac gluten sensitivity: sense or sensibility? Ann Intern Med. 2012;156(4):309–311. doi: 10.7326/0003-4819-156-4-201202210-00010. [DOI] [PubMed] [Google Scholar]
- 16.The Hartman Group, Inc. [Accessed June 14, 2016];The Hartman Group’s Health & Wellness 2015 and Organic & Natural 2014 reports. http://www.hartman-group.com/acumenPdfs/gluten-free-2015-09-03.pdf.
- 17.Gallup. Riffkin R. [Accessed June 14, 2016];One in Five Americans Include Gluten-Free Foods in Diet. 2015 Jul 21; Retrieved from http://www.gallup.com/poll/184307/one-five-americans-include-gluten-free-foods-diet.aspx.
- 18.Tavakkoli A, Lewis SK, Tennyson CA, Lebwohl B, Green PH. Characteristics of patients who avoid wheat and/or gluten in the absence of Celiac disease. Dig Dis Sci. 2014;59(6):1255–1261. doi: 10.1007/s10620-013-2981-6. [DOI] [PubMed] [Google Scholar]
- 19.DiGiacomo DV, Tennyson CA, Green PH, Demmer RT. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand J Gastroenterol. 2013;48(8):921–925. doi: 10.3109/00365521.2013.809598. [DOI] [PubMed] [Google Scholar]
- 20.Reilly NR. The Gluten-Free Diet: Recognizing Fact, Fiction, and Fad. J Pediatr. 2016 doi: 10.1016/j.jpeds.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Ezzati TM, Massey JT, Waksberg J, Chu A, Maurer KR. Sample design: Third National Health and Nutrition Examination Survey. Vital Health Stat. 1992;2(113):1–35. [PubMed] [Google Scholar]
- 22.Curtin LR, Mohadjer LK, Dohrmann SM, et al. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital Health Stat. 2013;2(160):1–23. [PubMed] [Google Scholar]
- 23.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011–2014. Vital Health Stat. 2014;2(162):1–33. [PubMed] [Google Scholar]
- 24.Walker MM, Murray JA, Ronkainen J, et al. Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology. 2010;139(1):112–119. doi: 10.1053/j.gastro.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz KD, Rashtak S, Lahr BD, et al. Screening for celiac disease in a North American population: sequential serology and gastrointestinal symptoms. Am J Gastroenterol. 2011;106(7):1333–1339. doi: 10.1038/ajg.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulkanen S, Halttunen T, Laurila K, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998;115(6):1322–1328. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- 27.Chorzelski TP, Beutner EH, Sulej J, et al. IgA anti-endomysium antibody. A new immunological marker of dermatitis herpetiformis and coeliac disease. Br J Dermatol. 1984;111(4):395–402. doi: 10.1111/j.1365-2133.1984.tb06601.x. [DOI] [PubMed] [Google Scholar]
- 28.Ladinser B, Rossipal E, Pittschieler K. Endomysium antibodies in coeliac disease: an improved method. Gut. 1994;35(6):776–778. doi: 10.1136/gut.35.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lionetti E, Gatti S, Pulvirenti A, Catassi C. Celiac disease from a global perspective. Best Pract Res Clin Gastroenterol. 2015;29(3):365–379. doi: 10.1016/j.bpg.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Volta U, Bellentani S, Bianchi FB, et al. High prevalence of celiac disease in Italian general population. Dig Dis Sci. 2001;46(7):1500–1505. doi: 10.1023/a:1010648122797. [DOI] [PubMed] [Google Scholar]
- 31.West J, Fleming KM, Tata LJ, Card TR, Crooks CJ. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. Am J Gastroenterol. 2014;109(5):757–768. doi: 10.1038/ajg.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marine M, Farre C, Alsina M, et al. The prevalence of coeliac disease is significantly higher in children compared with adults. Alimentary pharmacology & therapeutics. 2011;33(4):477–486. doi: 10.1111/j.1365-2036.2010.04543.x. [DOI] [PubMed] [Google Scholar]
- 33.Pratesi R, Gandolfi L, Garcia SG, et al. Prevalence of coeliac disease: unexplained age-related variation in the same population. Scand J Gastroenterol. 2003;38(7):747–750. doi: 10.1080/00365520310003255. [DOI] [PubMed] [Google Scholar]
- 34.Wu JH, Neal B, Trevena H, et al. Are gluten-free foods healthier than non-gluten-free foods? An evaluation of supermarket products in Australia. Br J Nutr. 2015;114(3):448–454. doi: 10.1017/S0007114515002056. [DOI] [PubMed] [Google Scholar]
- 35.Miranda J, Lasa A, Bustamante MA, Churruca I, Simon E. Nutritional differences between a gluten-free diet and a diet containing equivalent products with gluten. Plant Foods Hum Nutr. 2014;69(2):182–187. doi: 10.1007/s11130-014-0410-4. [DOI] [PubMed] [Google Scholar]
- 36.Tortora R, Capone P, De Stefano G, et al. Metabolic syndrome in patients with coeliac disease on a gluten-free diet. Alimentary pharmacology & therapeutics. 2015;41(4):352–359. doi: 10.1111/apt.13062. [DOI] [PubMed] [Google Scholar]
- 37.Penagini F, Dilillo D, Meneghin F, Mameli C, Fabiano V, Zuccotti GV. Gluten-free diet in children: an approach to a nutritionally adequate and balanced diet. Nutrients. 2013;5(11):4553–4565. doi: 10.3390/nu5114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker MM, Murray JA. An update in the diagnosis of coeliac disease. Histopathology. 2011;59(2):166–179. doi: 10.1111/j.1365-2559.2010.03680.x. [DOI] [PubMed] [Google Scholar]
- 39.Biesiekierski JR, Newnham ED, Shepherd SJ, Muir JG, Gibson PR. Characterization of Adults With a Self-Diagnosis of Nonceliac Gluten Sensitivity. Nutr Clin Pract. 2014;29(4):504–509. doi: 10.1177/0884533614529163. [DOI] [PubMed] [Google Scholar]