Abstract
Background
Several highly effective but costly therapies for hepatitis C virus (HCV) are available. As a consequence of their high price, thirty-five state Medicaid programs limited treatment coverage to patients with more advanced HCV stages. States have only limited information available to predict the long-term impact of these decisions.
Methods
We adapted a validated hepatitis C microsimulation model to the Pennsylvania Medicaid population to estimate the existing HCV prevalence in Pennsylvania Medicaid and estimate the impact of various HCV drug coverage policies on disease outcomes and costs. Outcome measures included rates of advanced-stage HCV outcomes and treatment and disease costs in both Medicaid and Medicare.
Results
We estimated that 46,700 individuals in Pennsylvania Medicaid were infected with HCV in 2015, 33% of whom were still undiagnosed. By expanding treatment to include mild fibrosis stage (Metavir F2), Pennsylvania Medicaid will spend an additional $274 million on medications in the next decade with no substantial reduction in the incidence of liver cancer or liver-related death. Medicaid patients who are not eligible for treatment under restricted policies would get treatment once they transition to the Medicare program, which would experience 10% reduction in disease-related costs due to early treatment in Medicaid. Further expanding treatment to patients with early fibrosis stages (F0 or F1) would cost Medicaid an additional $693 million during the next decade but would reduce the number of individuals in need of treatment in Medicare by 46% and decrease Medicare treatment costs by 23%. In some scenarios, outcomes could worsen with eligibility expansion if there is inadequate capacity to treat all patients.
Conclusions and Relevance
Expansion of HCV treatment coverage to less severe stages of liver disease may not substantially improve liver related outcomes for patients in Pennsylvania Medicaid in scenarios in which coverage through Medicare is widely available.
Keywords: Hepatitis C, Pennsylvania Medicaid, microsimulation, direct-acting antivirals, claims analysis, hepatocellular carcinoma, liver transplant
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is a major, and costly, health problem in the United States, affecting 2.7–3.2 million people (1) with the majority unaware of their disease (2). Beginning in 2014, interferon-free HCV therapies, such as sofosbuvir, simeprevir, ledipasvir (3), were introduced, leading to substantially improved sustained virologic response (SVR) rates – a surrogate for cure – as high as 98% (4), with shorter treatment duration and few adverse effects. However, their high prices ($40,000– $94,500 for 12-week therapy) in combination with a large number of treatment candidates translates into substantial budgetary impact for health-care payers.
The prevalence of HCV is higher among low-income populations, who are often enrolled in Medicaid (5). Although state Medicaid programs are eligible to receive at least a 23.1% rebate off average manufacturer prices, they spent $1.1 billion on treating HCV-infected individuals in 2014 (6–8). Pennsylvania Medicaid, which is the 5th largest Medicaid program by health expenditures and the 6th largest by enrollment in the United States (9, 10), spent about 4% of its 2014 prescription drug expenditures on sofosbuvir alone (11).
Facing high costs of treatment and operating within budgetary constraints, 36 state Medicaid programs have developed treatment authorization guidelines (12) to prioritize HCV treatment to patients with more advanced disease. These decisions have been criticized by patient advocacy groups and the Centers for Medicare and Medicaid Services (4, 13). Nevertheless, only seven out of these 36 states had expanded treatment to patients with mild fibrosis scores as of February 2015 (14). Pennsylvania expanded treatment to patients with F2 fibrosis score in July of 2015 (15) and is currently considering further expansions.
State Medicaid coverage decisions are complicated by the absence of reasonable estimates of HCV prevalence. Such estimates are difficult to generate given that roughly half of patients are unaware of infection (16). Medicaid programs also lack fibrosis scores and genotype information in their administrative data, which are required for treatment planning (12). Additionally, the impact of Medicaid treatment strategies on long-term disease and cost outcomes is difficult to measure. Since chronic HCV is a slowly progressive disease, Medicaid’s decisions could impact downstream HCV spending in Medicare once individuals reach age 65 or become dually enrolled due to disability.
Many of these challenges can be addressed with the use of simulation modeling. The objective of our study was twofold: (I) To use a well-validated national HCV simulation model to estimate the number of people currently infected with HCV in Pennsylvania Medicaid along with their disease characteristics; and (II) to use the model to project the economic and disease impact of different prior authorization criteria for treatment in Pennsylvania Medicaid.
METHODS
We used a three-step approach to address the above objectives. First, we estimated the observed HCV burden in Pennsylvania Medicaid using claims data from 2007–2012. Second, we adapted our previously developed and validated HCV disease burden model (HEP-SIM) (17, 18) to Pennsylvania Medicaid using claims data and other published studies. Finally, we used HEP-SIM to estimate the disease burden (both observed and unobserved) of HCV and evaluated the long-term disease and economic impact of different prior authorization guidelines for treatment in Pennsylvania Medicaid.
Analysis of Pennsylvania Medicaid Claims Data
We obtained data from the Pennsylvania Medicaid program for paid claims and encounters covering services rendered in 2007–2012 for enrollees both in fee-for-service Medicaid and in Medicaid managed-care-organizations. We identified individuals diagnosed with HCV for the purposes of model validation, defined by the presence of at least one paid inpatient, outpatient or professional claim with an ICD-9 diagnosis code for HCV (eTable 1). Among HCV-diagnosed individuals, we identified those with potential treatment contraindications, HCV-related complications, liver transplants and rates of HCV treatment, for use as inputs in the microsimulation model (Supplement A).
Microsimulation Model for Pennsylvania Medicaid
HEP-SIM has been extensively validated with the National Health and Nutrition Examination Surveys and several published data sources (1, 17, 19, 20). The natural history of HCV in the model was defined using the Metavir scoring system for fibrosis stages: F0 for no fibrosis, F1 for portal fibrosis without septa, F2 for portal fibrosis with few septa, F3 for numerous septa without cirrhosis, and F4 for compensated cirrhosis (eFigure 1 and eTable 2 of Supplement B). Patients in the F4 stage could further progress to decompensated cirrhosis or hepatocellular carcinoma, receive a liver transplant, or die from liver-related complications.
We incorporated Pennsylvania Medicaid’s population characteristics into the HEP-SIM model, including demographics, HCV incidence, new enrollments in Medicaid, HCV screening (both risk-based and birth-cohort) rate, and historic HCV treatment rate. Supplement B and eTables 3–5 provide detailed descriptions of model parameters and how the model was adapted to fit Pennsylvania, using a combination of prior literature, publically available data sources, and the Medicaid claims data.
Coverage Scenarios
We simulated three coverage scenarios according to different treatment authorization guidelines starting in 2014: (I) Our base-case scenario, in which HCV treatment is available to patients with a fibrosis score of F2–F4, consistent with the recent Pennsylvania Medicaid HCV treatment authorization criteria(15); (II) the scenario to expand treatment to all diagnosed HCV patients; and (III) the scenario to limit treatment to F3–F4 patients only, consistent with the treatment authorization criteria in Pennsylvania Medicaid prior to July 2015, and in several other states.
In each scenario, we assumed that 40% of diagnosed HCV-infected individuals who are treatment candidates received treatment each year after 2014 - defined in our model as ‘treatment penetration rate’ - in order to account for limitations in provider availability and patient’s preference (eTable 5). Using a 40% treatment penetration rate across scenarios, we assumed that a larger number of individuals could be treated annually under F0–F4 coverage (8,200 patients) than with F3–F4 (2,500 patients). We address this assumption in more detail in the sensitivity analyses. Note that a treatment penetration rate of 40% is greater than the actual treatment rate in Pennsylvania Medicaid in 2014.
Cost
We set the weekly costs of older HCV therapies, peginterferon, ribavirin, boceprevir, and telaprevir, at $587, $309, $1100, and $4100, respectively (21). We set the weekly costs of sofosbuvir at $7000, ledipasvir/sofosbuvir at $7875, and paritaprevir, ritonavir, ombitasvir, and dasabuvir at $6,943 (21, 22). We applied 23% and 46% discounts to the available average wholesale drug costs in 2014 and in 2015 and beyond, respectively, according to the average reported discounts and rebates provided to health-care payers (23) (eTable 6). We also included the cost of managing early and advanced stages of HCV including hepatocellular carcinoma and liver transplantation, which were obtained from prior literature (eTable 7) (24, 25).
Model Outputs
We projected the temporal trends in the prevalence of HCV, number of people aware and unaware of their infection, and distribution of fibrosis scores. Since HCV is a slow-progressive disease and the benefits of HCV treatment will accrue years later, we simulated our model for a long time horizon, from 2015 to 2050. Under each coverage scenario described above, we projected the incidence of advanced liver disease, number of liver transplants, and liver-related deaths in 2015–2050. We also estimated the long-term cost of chronic HCV management until 2050. Because of variable HCV treatment costs in the future, we also estimated the short-term budget impact on Medicaid from 2015–2025.
Medicare Outputs for Transitions between Medicaid and Medicare
Since several benefits of HCV treatment will accrue after some patients have transitioned from Medicaid-only coverage to Medicare-only or dual coverage, we estimated the impact of Medicaid’s coverage decisions on the disease and cost outcomes in Medicare. In all scenarios, we assumed that patients who did not receive or failed to respond to HCV treatment in Medicaid would transition to Medicare at the age of 61, a transition age calculated according to our claims-based analyses and a published study (26). We assumed that all patients who transitioned to Medicare, who were aware of their infection, and eligible for treatment, would receive treatment irrespective of their fibrosis score once in Medicare.
Sensitivity analyses
Using one-way deterministic sensitivity analysis, we analyzed the effect of model parameters on the incidence of advanced-stage liver diseases and budget needed for disease management and treatment costs (Supplement C). We examined the impact of expanded treatment coverage scenarios on model outcomes assuming there is a fixed maximum number of patients who can be treated in a given year (because of the number of liver specialists, availability of appointments, etc.), instead of a variable treatment penetration rate. We assessed the effect of alternative treatment penetration rates on model outcomes in the base case (F2–F4 treatment), and also added scenarios in which the expansion of treatment to F2 patients might be delayed until 2017 or 2020, instead of 2015 in the base case.
RESULTS
Diagnosed HCV Population in Claims Data
The number of enrollees who had a claim with one or more HCV diagnosis codes increased steadily from 18,955 (882 per 100,000) in 2007 to 26,432 (1,023 per 100,000) in 2012 (Table 1 and Supplement D). The number of enrollees who initiated medication therapy increased from 797 in 2007 to 1,025 in 2012; however, the proportion of individuals who initiated treatment during this period remained nearly constant (4%). Pennsylvania Medicaid covered twelve liver transplants performed on enrollees with HCV on average each year in 2007–2012.
Table 1.
HCV-diagnosed population in the Pennsylvania Medicaid claims, 2007–2012, excluding Medicare dual eligibles.
| Parameter | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|---|---|
|
Number of hepatitis C-infected individuals |
18,955 | 20,242 | 23,234 | 24,352 | 26,061 | 26,432 |
| Mean age | 44.7 | 44.9 | 45.0 | 45.4 | 45.8 | 46.3 |
| Sex (%) | ||||||
| Female | 46.7 | 46.5 | 46.2 | 46.0 | 45.8 | 46.1 |
| Male | 53.3 | 53.5 | 53.8 | 54.0 | 54.2 | 53.9 |
| Age distribution (%) | ||||||
| <18 | 1.0 | 1.1 | 1.1 | 1.1 | 1.0 | 0.9 |
| 18–29 | 14.6 | 14.8 | 15.0 | 14.6 | 13.4 | 12.4 |
| 30–39 | 13.1 | 13.4 | 13.8 | 14.1 | 15.5 | 17.0 |
| 40–49 | 30.4 | 28.3 | 26.0 | 23.9 | 21.8 | 19.7 |
| 50–60 | 35.3 | 36.0 | 37.1 | 38.5 | 39.2 | 39.7 |
| 61–64 | 4.2 | 4.9 | 5.5 | 6.2 | 7.0 | 8.0 |
| 65+ | 1.5 | 1.5 | 1.5 | 1.7 | 2.1 | 2.4 |
| Number of months enrolled in Medicaid (%)* | ||||||
| <2 | 3.3 | 2.9 | 2.7 | 2.2 | 2.7 | 2.6 |
| 2–6 | 11.5 | 10.2 | 10.2 | 9.4 | 9.0 | 8.6 |
| 6+ | 85.2 | 86.9 | 87.1 | 88.5 | 88.3 | 88.7 |
| Eligibility type (%) | ||||||
| General assistance | 42.5 | 41.7 | 42.0 | 40.9 | 40.0 | 36.8 |
| Supplemental Security Income | 43.3 | 44.3 | 44.4 | 45.2 | 47.0 | 49.7 |
| Temporary assistance for needy families |
14.2 | 14.0 | 13.6 | 13.9 | 13.1 | 13.4 |
| Waiver | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 |
|
Number (%) of individuals with any interferon contraindication (substance abuse/depression) |
7,302 (39%) |
7,999 (40%) |
9,956 (43%) |
10,693 (44%) |
11,771 (45%) |
12,337 (47%) |
|
Number (%) of people who initiated treatment |
797 (4.2%) |
863 (4.3%) |
977 (4.2%) |
855 (3.5%) |
807 (3.1%) |
1,025 (3.9%) |
Source: Authors’ analysis of Pennsylvania Medicaid claims data of 2007–2012.
Enrolled during the calendar year, not the total number of months ever enrolled.
Model Validation
The model-based estimates of the number of patients who were aware of their HCV infection in 2007–2012 matched closely the number of HCV-diagnosed enrollees in claims data (Table 2). The model predicted in 2012 a total of 49,500 patients with HCV (including those unaware/undiagnosed), with 14 liver transplants. The projected trend in the number of liver transplants from the model was comparable to the trend observed in claims data (eFigure 2 in Supplement E). In addition, the projected percentage of individuals with cirrhosis who were aware of their disease during 2007– 2012 was within 5% of the number of enrollees diagnosed with cirrhosis in the analyses of claims data. These findings indicate that the model was appropriately calibrated to approximate the characteristics of the Pennsylvania Medicaid population.
Table 2.
Estimated prevalence of HCV infected patients in Pennsylvania Medicaid in 2007–2015 and the number of new episodes of decompensated cirrhosis, hepatocellular carcinoma, and liver transplant.
| Year | HCV cases in Claims data* |
Model-based HCV-infected population in Medicaid† | ||||
|---|---|---|---|---|---|---|
| Total with diagnoses |
Total aware |
Total | DC incidence |
HCC incidence |
Liver transplants |
|
| 2001 | - | 19,700 | 50,000 | 50 | 57 | 12 |
| 2002 | - | 19,600 | 50,000 | 40 | 43 | 14 |
| 2003 | - | 19,600 | 50,000 | 56 | 51 | 11 |
| 2004 | - | 19,500 | 49,800 | 70 | 59 | 9 |
| 2005 | - | 19,700 | 50,200 | 81 | 49 | 10 |
| 2006 | - | 19,900 | 50,000 | 94 | 57 | 11 |
| 2007 | 18,955 | 20,400 | 49,500 | 101 | 60 | 12 |
| 2008 | 20,242 | 21,800 | 50,800 | 111 | 65 | 11 |
| 2009 | 23,234 | 22,800 | 50,400 | 120 | 66 | 12 |
| 2010 | 24,352 | 24,000 | 50,200 | 129 | 70 | 13 |
| 2011 | 26,061 | 25,400 | 49,900 | 141 | 79 | 14 |
| 2012 | 26,432 | 26,700 | 49,500 | 149 | 83 | 14 |
| 2013 | - | 28,500 | 48,400 | 143 | 84 | 15 |
| 2014 | - | 30,100 | 47,700 | 139 | 86 | 15 |
| 2015 | 31,200 | 46,700 | 108 | 78 | 16 | |
Source: Simulation model results
The numbers of HCV cases identified from claims data are included for comparison in the highlighted column.
The model-based results for each year indicate values at the end of the calendar year.
Abbreviations: HCV = hepatitis C virus; DC = decompensated cirrhosis; HCC = hepatocellular carcinoma.
HCV Burden in Pennsylvania Medicaid - Model Predictions
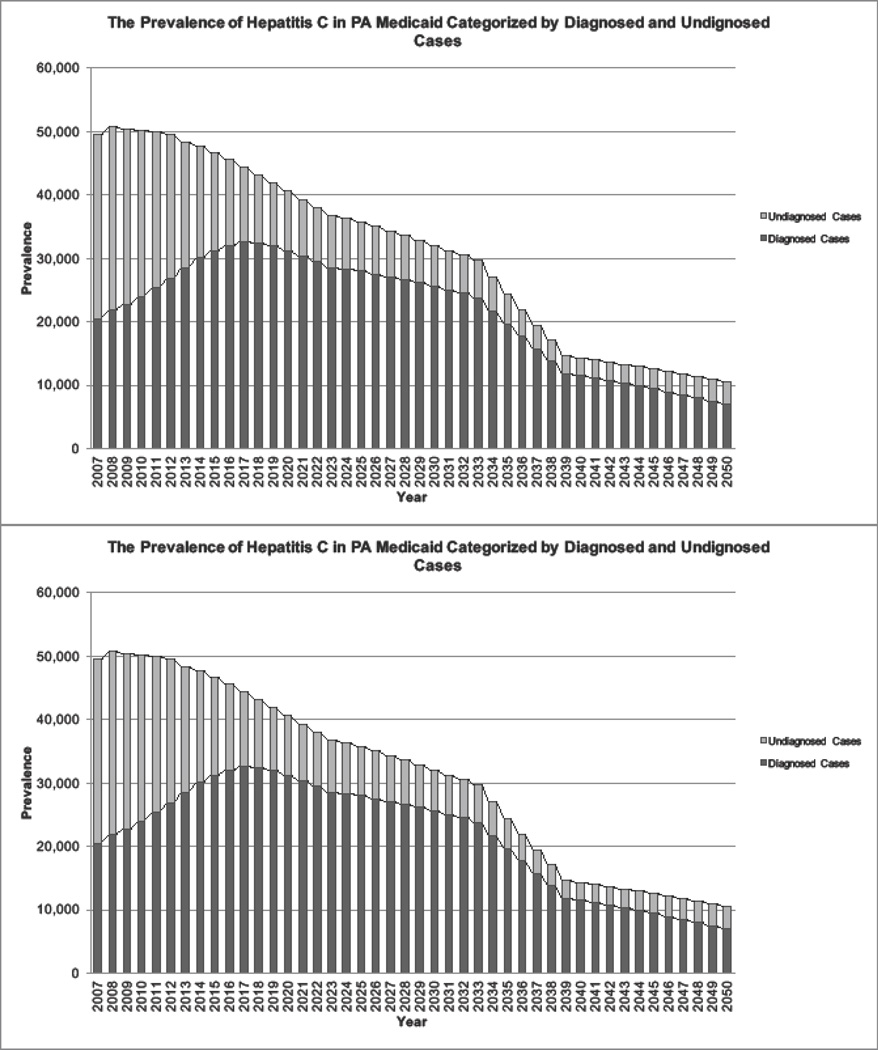
The model projected the HCV-infected population at the end of 2015 at 46,700, with 31,200 (67%) aware of their diagnosis (Table 2). In the base case (treatment for F2–F4), the overall burden of HCV in Pennsylvania Medicaid and the prevalence of undiagnosed cases are projected to decrease by 23% and 50% from 2015 to 2025, respectively (Figure 1).
Figure 1.
The projected prevalence of hepatitis C in Pennsylvania Medicaid categorized by diagnosed and undiagnosed cases in 2007–2050.
Note: After 2014, the projection of HCV prevalence was calculated under the coverage scenario of treating patients with F2–F4 fibrosis levels.
Table 3 shows the projected cumulative incidence of advance liver diseases, liver-related mortality, chronic disease cost in 2015–2050, and cumulative antiviral treatment cost in 2015–2025 under each scenario. With a base-case treatment penetration rate of 40%, up to 4,300 HCV-infected individuals were treated annually in 2015 and beyond (eTable 8). Compared to the base case, limiting treatment coverage to F3–F4 with a 40% treatment penetration rate (treating up to 2,500 HCV-infected individuals annually) would reduce cumulative treatment cost from $955 million to $682 million ($274 million reduction) during the next decade (Table 3, Panel A), incur 15% ($60 million) increase in downstream cumulative chronic disease cost from 2015–2050, but minimally affect the cumulative incidence of liver complications and liver-related mortality in Pennsylvania Medicaid through 2050. Compared to the base-case coverage scenario (F2–F4 treatment), the further coverage expansion to F0 and F1 fibrosis scores (treating up to 8,200 HCV-infected individuals annually) would increase the cumulative cost of treatment by an additional $693 million by 2025, reduce the long-term cost incurred by chronic HCV cases by 35% ($116 million), but not substantially decrease the overall burden of liver complications in Medicaid through 2050. The majority of the 10-year cumulative cost of treatment among these coverage scenarios occurred in the first 5 years, a period when the majority of HCV patients received treatment (eFigure 3, Panel A).
Table 3.
Cumulative incidence of HCV outcomes and costs in 2015–2050 under each coverage scenario (Panel A) and with different treatment penetration rates under base-case coverage (Panel B).
| Cumulative results in Pennsylvania Medicaid | Cumulative results incurred to Medicare | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence 2015–2050 | Cost ($million) | Incidence 2015–2050 | Cost ($million) | |||||||||
| DC | HCC | LT | LRD | Chronic disease 2015–2050* |
Treatment 2015–2025† |
DC | HCC | LT | LRD | Chronic disease 2015–2050* |
Treatment 2015–2025† |
|
|
Panel A. Coverage Scenarios | ||||||||||||
| F3–F4 | 708 | 645 | 138 | 1,360 | 392 | 682 | 840 | 721 | 106 | 1,440 | 175 | 702 |
|
F2–F4 (base case) |
696 | 636 | 136 | 1,351 | 331 | 955 | 830 | 714 | 104 | 1,482 | 173 | 619 |
| F0–F4 | 688 | 633 | 136 | 1,340 | 215 | 1,648 | 823 | 703 | 102 | 1,452 | 170 | 475 |
|
Panel B. Treatment uptake in PA Medicaid‡ | ||||||||||||
| 20% | 950 | 783 | 161 | 1,595 | 387 | 838 | 1,025 | 859 | 123 | 1,796 | 219 | 613 |
| 40% | 696 | 636 | 136 | 1,351 | 331 | 955 | 830 | 714 | 104 | 1,482 | 173 | 619 |
| 60% | 625 | 588 | 126 | 1,261 | 311 | 975 | 786 | 681 | 98 | 1,505 | 163 | 616 |
| 80% | 595 | 576 | 125 | 1,241 | 305 | 986 | 779 | 681 | 98 | 1,449 | 162 | 610 |
| 100% | 570 | 560 | 121 | 1,212 | 299 | 995 | 775 | 673 | 95 | 1,432 | 161 | 605 |
Chronic disease cost is the cost incurred by chronic stages of hepatitis C virus and the cost of managing associated liver complications.
Cost of HCV treatment with new oral antiviral therapies.
Panel B presents the results of different annual penetration rates, assuming treatment of F2-F4 after 2015. Treatment penetration rate is the annual percentage of treatment-eligible Medicaid enrollees who receive treatment. This parameter could be affected by the number of physicians to provide HCV treatment, and individuals’ care-seeking behavior.
Abbreviations: F0 = Metavir stage for no liver fibrosis; F1 = Metavir stage for portal fibrosis without septa; F2 = Metavir stage for portal fibrosis with few septa; F3 = Metavir stage for numerous septa without cirrhosis; F4 = Metavir stage for cirrhosis; DC = decompensated cirrhosis; HCC = hepatocellular carcinoma; LT = liver transplant; LRD = liver-related death.
HCV Burden in Transitions from Medicaid to Medicare
Under Medicaid’s F2–F4 treatment coverage and 40% treatment penetration rate (base-case), HCV-infected individuals who failed treatment in Medicaid or transitioned to Medicare at 61 years old without receiving treatment would incur an economic disease burden of $173 million in 2015–2050 and treatment cost burden of $619 million in 2015–2025 (Table 3, Panel A). Expanding treatment to include F0 and F1 fibrosis scores in Pennsylvania Medicaid reduced the costs for treatment in Medicare by 23%, or $144 million (from $619 million to $475 million) through 2025 and reduced the number of individuals receiving treatment in Medicare from 2015–2050 by 46%, from 6,600 to 3,500. Changes in treatment coverage in Medicaid, however, did not substantially impact the burden of new cases of decompensated cirrhosis, hepatocellular carcinoma or liver transplant in Medicare.
Sensitivity Analyses
Variations in treatment penetration rate in Pennsylvania Medicaid would have a substantial impact on the annual HCV treatment costs (eFigure 3, Panel B) and the incidence of advanced liver disease (Table 3, Panel B, and eFigure 4). For example, if all treatment-eligible patients (100%) were to receive treatment under F2–F4 coverage, costs of therapy would increase by $40 million (4%) in the next decade when compared to a 40% treatment penetration rate (i.e. 955 million to 995 million) (Table 3, Panel B). However, the incidence of liver transplant would drop by 11% (15 fewer liver transplants) through 2050 and liver-related death decrease by 10% (139 deaths).
Setting a maximum numbers of individuals who could be treated annually in 2015 and beyond (instead of setting a treatment penetration rate) substantially altered model outputs in different coverage scenarios (Table 4). Compared to F2–F4 coverage, expanding treatment to F0 and F1 fibrosis when only 2,200 patients can be treated annually increased the cumulative incidence of advanced liver diseases and liver-related deaths by 30%. It was only in a scenario of unlimited treatment capacity that expansion to F0–F4 did not increase the incidence of liver complications and death.
Table 4.
Cumulative incidence of HCV outcomes and costs in 2015–2050 under various coverage scenarios, altering the maximum number of individuals treated annually in 2015 and beyond.
| Maximum individuals treated annually in 2015 and beyond |
Coverage Scenario |
Cumulative results in Pennsylvania Medicaid |
Cumulative results incurred to Medicare | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence 2015–2050 | Cost ($million) | Incidence 2015–2050 | Cost ($million) | ||||||||||
| DC | HCC | LT | LRD | Chronic disease 2015–2050* |
Treatment 2015–2025** |
DC | HCC | LT | LRD | Chronic disease 2015–2050* |
Treatment 2015–2025** |
||
| 2,200 |
Panel A. |
||||||||||||
| F3–F4 | 744 | 659 | 140 | 1,3 82 | 396 | 660 | 873 | 750 | 108 | 1,6 31 | 184 | 696 | |
|
F2–F4 (base case) |
957 | 784 | 163 | 1,600 | 386 | 838 | 1,020 | 855 | 125 | 1,839 | 219 | 611 | |
| F0–F4 | 1,424 | 1,050 | 212 | 2,061 | 399 | 1,004 | 1,409 | 1,117 | 159 | 2,443 | 296 | 425 | |
| 4,300 |
Panel B. |
||||||||||||
| F3–F4 | 614 | 585 | 125 | 1,257 | 369 | 702 | 782 | 688 | 98 | 1,470 | 163 | 704 | |
|
F2–F4 (base case) |
696 | 636 | 136 | 1,351 | 331 | 955 | 830 | 714 | 104 | 1,482 | 173 | 619 | |
| F0–F4 | 896 | 742 | 155 | 1,531 | 269 | 1,489 | 972 | 818 | 119 | 1,795 | 207 | 503 | |
| 6,400 |
Panel C. |
||||||||||||
| F3–F4 | 572 | 563 | 121 | 1,214 | 361 | 712 | 782 | 682 | 97 | 1,487 | 161 | 699 | |
|
F2–F4 (base case) |
623 | 587 | 129 | 1,259 | 311 | 975 | 784 | 687 | 98 | 1,487 | 164 | 616 | |
| F0–F4 | 742 | 660 | 141 | 1,381 | 229 | 1,592 | 859 | 741 | 108 | 1,603 | 181 | 486 | |
| 8,500 |
Panel D. |
||||||||||||
| F3–F4 | 576 | 560 | 120 | 1,214 | 361 | 712 | 774 | 677 | 97 | 1,415 | 162 | 699 | |
|
F2–F4 (base case) |
595 | 575 | 124 | 1,240 | 305 | 985 | 782 | 675 | 99 | 1,456 | 162 | 610 | |
| F0–F4 | 672 | 621 | 132 | 1,310 | 208 | 1,642 | 816 | 704 | 102 | 1,519 | 170 | 475 | |
| Unlimited |
Panel E. |
||||||||||||
| F3–F4 | 584 | 567 | 121 | 1,224 | 362 | 712 | 781 | 677 | 97 | 1,398 | 162 | 698 | |
|
F2–F4 (base case) |
575 | 559 | 121 | 1,214 | 300 | 996 | 771 | 673 | 97 | 1,403 | 161 | 604 | |
| F0–F4 | 574 | 556 | 122 | 1,210 | 180 | 1,718 | 775 | 673 | 96 | 1,465 | 160 | 447 | |
Chronic disease cost is the cost incurred by chronic stages of hepatitis C virus and the cost of managing associated liver complications.
Cost of HCV treatment with new antiviral therapies.
Abbreviations: F0 = Metavir stage for no liver fibrosis; F1 = Metavir stage for portal fibrosis without septa; F2 = Metavir stage for portal fibrosis with few septa; F3 = Metavir stage for numerous septa without cirrhosis; F4 = Metavir stage for cirrhosis; DC = decompensated cirrhosis; HCC = hepatocellular carcinoma; LT = liver transplant; LRD = liver-related death.
The impact of delaying the inclusion of F2 fibrosis levels in treatment coverage depended on the treatment penetration rate. Waiting until 2017 or 2020 to expand treatment to F2 (compared to expanding in 2015) would have beneficial effects on liver-related outcomes if treatment penetration is limited, while it would have a modest negative impact if treatment penetration is 100% (eTable 9).
Overall, model projections were robust to changes in other model parameters (eTable 10).
DISCUSSION
Our study applied microsimulation modeling to estimate the prevalence of HCV in Pennsylvania Medicaid and analyze the cost and disease burden impact of broadening treatment coverage. We projected that including F2 fibrosis patients in treatment coverage - something only seven states had done as of February 2016 - compared to limiting treatment to F3–F4 patients only, would increase the cumulative treatment cost by $274 million in 2015–2025, decrease long-term chronic HCV cost by $60 million in 2015–2050, but would not substantially decrease the incidence of advanced liver diseases or liver-related death in the Medicaid population in Pennsylvania. Expanding treatment in Medicaid would decrease treatment costs in Medicare – an impact that is not fully considered in policy discussions or prior literature (27). Furthermore, our findings highlight the critical importance of treatment penetration rate in estimating the impact of coverage scenarios; in settings of limited treatment penetration or capacity, expansion of eligibility could potentially worsen liver related outcomes.
Our study uses a novel approach of combining claims-based analyses and validated microsimulation modeling to estimate the impact of treatment coverage scenarios on HCV disease and cost burden in the future. Importantly, our analyses do not measure cost-effectiveness, as in prior studies (27,28), but focus on treatment costs and liver-related outcomes for one payer (Pennsylvania Medicaid), uniquely accounting for treatment capacity and for the transition in insurance between Medicaid and Medicare.
Treatment penetration rate is an especially important variable in Pennsylvania, where Medicaid guidelines stipulate that HCV therapies should be prescribed by physicians specialized in infectious disease, gastroenterology, hepatology, or transplantation (15). The limited availability of these specialists in some areas could limit the number of enrollees who are able to pursue treatment and result in low treatment penetration rate (29), although opportunities exist to expand access to specialists through telemedicine. Our findings suggest that within the base-case scenario (treatment of F2–F4), expanding the treatment penetration rate improved liver-related outcomes while increasing cost. However, with a fixed treatment rate and limits on the maximum number of annually treated individuals, expanding treatment to lower fibrosis levels may potentially lead to F0–F2 patients being treated before F3–F4 patients and worse outcomes. In fact, with a low treatment penetration rate among enrollees, the state could potentially benefit by delaying the expansion of treatment, thus ensuring that more severe cases are treated before less severe ones. The expanded treatment coverage in 2015 would be beneficial only if the treatment penetration rate were 80% or higher – a rate that may potentially exceed provider capacity - highlighting the policy significance of ensuring adequate system capacity for treating all HCV patients before eligibility criteria are expanded.
Our results show that expanded HCV treatment policies in Medicaid may not substantially decrease the incidence of liver complications and death in this population. Patients may be successfully treated as they progress to more advanced fibrosis levels while in Medicaid, and others still in early fibrosis stages (F0 or F1) may transition out of Medicaid into Medicare, which offers treatment to all eligible patients in our model regardless of fibrosis levels. Our analysis highlights the potential tradeoffs between Medicaid and Medicare - expanded treatment coverage and the rates of treatment penetration in Medicaid would impact the future disease burden and costs incurred to Medicare when patients transition in coverage. While important, these results can be considered estimates only, given the limitations of the model in precisely defining the moment of transition from Medicaid to Medicare. Nonetheless, our analyses document the importance of expanding the discussion about costs and impacts of treatment beyond Medicaid only for conditions with slow rates of progression like HCV.
One final consideration in evaluating the potential impact of HCV treatment coverage decisions is the expected future drop in drug prices (30). For example, the Department of Veterans Affairs was able to end treatment prioritization and expand HCV treatment to all Veterans regardless of disease severity in February 2016 (31), due to their ability to lower prices and due to an infusion of funds from Congress. Our model is based on current pricing data and will overestimate costs if HCV drug prices for Medicaid fall substantially in the future. Costs, however, will not change the impact of a given coverage decision for HCV on future liver-related health outcomes.
Our study has several limitations. First, our model cannot fully account for transitions from Medicaid coverage only to dual eligibility for Medicare, a transition potentially related to the onset of advanced liver disease. However, our claims-based analyses and a published study (26) suggests that most individuals with HCV transition to Medicare by age 61; thus we assumed Medicare became the primary payer after that age. We also assumed that all patients are treated in Medicare once leaving Medicaid regardless of fibrosis level. Second, we did not analyze the potential effect of treatment on the transmission of HCV in the Medicaid population. However, since the magnitude of HCV incidence did not affect the projected prevalence of HCV according to our sensitivity analyses, we do not expect this omission to substantially change the findings. Third, the costs of chronic disease management were not drawn from Medicaid or Medicare data due to limited data availability. As a result, we mainly focused on the relative (rather than absolute) differences in projected disease management costs between different coverage scenarios. Fourth, our analysis did not incorporate potential benefits of treatment on improved quality of life and increased economic productivity (4). Finally, we did not consider the impact of Medicaid expansion, which was implemented in Pennsylvania in 2015, for which there was no available information on changes in population clinical characteristics at the time of our study.
In conclusion, the expansion of treatment prior authorization criteria would significantly increase the economic burden of HCV treatment and somewhat reduce the cost of chronic HCV in Pennsylvania, but would not substantially decrease HCV-related complications among infected Medicaid enrollees. Concurrent with patient prioritization policies, the issue of treatment accessibility and treatment penetration rate among eligible patients should also be a focus of policy efforts. Expanding eligibility for hepatitis C treatment could potentially be counterproductive if patients with less severe liver disease are treated before those whose disease is more advanced.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported in part by an intergovernmental agreement (IGA) between the Pennsylvania Department of Human Services and the University of Pittsburgh, and by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR000146.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Jagpreet Chhatwal has received a research grant from Gilead Sciences, and consulting fees from Merck & Co. and Gilead Sciences outside the submitted work. No additional financial disclosures.
Disclaimer: The opinions expressed are those of the authors alone and do not necessarily represent the opinions of the United States Government or the State of Pennsylvania.
REFERENCES
- 1.Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic Hepatitis C Virus Infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Annals of Internal Medicine. 2014;160(5):293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razavi H, El Khoury A, Elbasha E, Estes C, Pasini K, Poynard T, et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57(6):2164–2170. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. Hepatitis B and C Treatments. Accessed at http://www.fda.gov/forpatients/illness/hepatitisbc/ucm408658.htm on 10 January 2016.
- 4.Recommendations for Testing, Managing, and Treating Hepatitis C. American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Accessed at http://hcvguidelines.org/sites/default/files/HCV-Guidance_April_2016_d1.pdf on April 9, 2016.
- 5.Epidemiology data; Milliman, Inc., NY. Health Care Reform and Hepatitis C: A Convergence of Risk and Opportunity. Accessed at http://us.milliman.com/uploadedFiles/insight/2013/convergence-of-risk-and-opportunity.pdf on December 25, 2015.
- 6.Costly Hepatitis C Drugs for Everyone? Accessed at http://www.nytimes.com/2015/09/02/opinion/costly-hepatitis-c-drugs-for-everyone.html?_r=0 on December 3, 2015.
- 7.New hepatitis C drugs are costing Medicare billions. Accessed at https://www.washingtonpost.com/national/health-science/medicare-spent-45-billion-on-new-hepatitis-c-drugs-last-year-data-shows/2015/03/29/66952dde-d32a-11e4-a62f–ee745911a4ff_story.html on December 3, 2015.
- 8.State Spending on Hepatitis C Drugs Soared in 2014. Accessed at http://www.hepmag.com/articles/state_hcv_spending_2014_2831_27140.shtml on December 3, 2015.
- 9.Foundation. KF. Total Medicaid Spending. Accessed at http://kff.org/medicaid/state-indicator/total-medicaid-spending/ on December 3, 2015.
- 10.Foundation KF. Health Expenditures by State of Provider. Accessed at http://kff.org/other/state-indicator/total-health-spending/ on December 3, 2015.
- 11.WideVariance in Medicaid Use of CostlyHepatitis C Drug. Accessed at http://articles.philly.com/2015-10-01/business/67015535_1_sofosbuvir-sovaldi-hepatitis-c on December 25, 2015.
- 12.Japsen B. As Pricey Hepatitis Pill Harvoni Joins Sovaldi, States Erect Medicaid Hurdles. Forbes. Accessed on June 30, 2015 at http://www.forbes.com/sites/brucejapsen/2014/10/10/as-hepatitis-pill-harvoni-joins-sovaldi-states-erect-medicaid-hurdles/
- 13.Department of Health and Human Services, Centers for Medicare & Medicaid Services. Assuring Medicaid Beneficieries Access to hepatitis C Drugs. Accessed at https://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Benefits/Prescription-Drugs/Downloads/Rx-Releases/State-Releases/state-rel-172.pdf on December 15, 2015.
- 14.Center for Evidence-based Policy, Oregon Health & Science University. State Medicaid Coverage Policies for Harvoni and Viekira Pak Treatment of Hepatitis C. 2015 [Google Scholar]
- 15.Pennsylvania Department of Human Services. Requirements for Prior Authorization of Hepatitis C Agents. Medical Assistance Handbook, Prior Authorization of Pharmaceutical Services. 2015 Accessed on 22 July 2015 at http://www.dpw.state.pa.us/cs/groups/webcontent/documents/bulletin_admin/c_084858.pdf.
- 16.Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S.primary care settings. Annals of internal medicine. 2012;156(4):263–270. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Annals of internal medicine. 2014;161(3):170–180. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chhatwal JWX, Ayer T, Kabiri M, Chung RT, Hur C, Donohue JM, Roberts MS, Kanwal F. Hepatitis C disease burden in the United States in the era of oral direct-acting antivirals. Hepatology. 2016 doi: 10.1002/hep.28571. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour J-F, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of Internal Medicine. 2006;144(10):705. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 21.Databank F. Drug Pricing Policy. South San Francisco, CA: First Databank; 2014. Accessed on 22 July 2015 at http://www.firstdatabank.com/Support/drug-pricing-policy.aspx. [Google Scholar]
- 22.AbbVie’s Viekira Pak: What You Need to Know about the Newest Hepatitis C Treatment. Accessed on 22 Jult 2015 at http://blogs.hepmag.com/lucindakporter/2014/12/abbvies_viekira_pak.html.
- 23.Gilead reveals deeper discounts in US for Sovaldi, Harvoni, above expectations. Accessed on July 22, 2015 at http://www.firstwordpharma.com/node/1262258-axzz3gdrsH1W3.
- 24.Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. Journal of Clinical Gastroenterology. 2011;45(2):e17. doi: 10.1097/MCG.0b013e3181e12c09. [DOI] [PubMed] [Google Scholar]
- 25.McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-Cause and Incremental Per Patient Per Year Cost Associated with Chronic Hepatitis C Virus and Associated Liver Complications in the United States: A Managed Care Perspective. J Manag Care Pharm. 2011;17(7):531–546. doi: 10.18553/jmcp.2011.17.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scaife J, Kuti E, Acampa L, Million R, Miyasato G, Wang Z, et al. Prevalence Of Chronic Hepatitis C Virus And Commonly-Associated Comorbidities Within A Large Us Commercially-Insured Population. Value in Health. 2013;16(3):A82–A83. [Google Scholar]
- 27.Chidi AP, Bryce CL, Donohue JM, Fine MJ, Landsittel DP, Myaskovsky L, et al. Economic and Public Health Impacts of Policies Restricting Access to Hepatitis C Treatment for Medicaid Patients. Value in Health. 2016 doi: 10.1016/j.jval.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chahal HS, Marseille EA, Tice JA, Pearson SD, Ollendorf DA, Fox RK, et al. Cost-effectiveness of early treatment of hepatitis C virus genotype 1 by stage of liver fibrosis in a US treatment-naive population. JAMA internal medicine. 2016;176(1):65–73. doi: 10.1001/jamainternmed.2015.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayasekera CR, Arora S, Ahmed A. Hepatitis c treatment delivery mandates optimizing available health care human resources: A case for task shifting. JAMA. 2016;315(18):1947–1948. doi: 10.1001/jama.2016.1993. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Medicare and Medicaid Services, Assuring Meicaid Beneficiaries Access to Hepatitis C Drugs. 2015 Nov 5; Accssed at https://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Benefits/Prescription-Drugs/Downloads/Rx-Releases/State-Releases/state-rel-172.pdf on February 11, 2016.
- 31.The United States Department of Veterans Affairs. Hepatitis C Virus Funding and Prioritization Status Update. 2016 Feb; Accessed at http://www.hepatitis.va.gov/provider/policy/choice-program-index.asp on March 22, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.