Abstract
Aims
Young women with acute myocardial infarction (AMI) have a higher risk of adverse outcomes than men. However, it is unclear how young women with AMI are different from young men across a spectrum of characteristics. We sought to compare young women and men at the time of AMI on 6 domains of demographic and clinical factors to determine whether they have distinct profiles.
Methods and Results
Using data from VIRGO, a prospective cohort study of women and men aged ≤55 years hospitalized for AMI(N=3,501) in the US and Spain, we evaluated sex differences in demographics, healthcare access, cardiovascular risk and psychosocial factors, symptoms and pre-hospital delay, clinical presentation, and hospital management for AMI.
The study sample included 2,349(67%) women and 1,152(33%) men with mean age 47 years. Young women with AMI had higher rates of cardiovascular risk factors and comorbidities than men, including diabetes, congestive heart failure, chronic obstructive pulmonary disease, renal failure, and morbid obesity. They also exhibited higher levels of depression and stress, poorer physical and mental health status, and lower quality of life at baseline. Women had more delays in presentation and presented with higher clinical risk scores, on average, than men; however, men presented with higher levels of cardiac biomarkers and more classic electrocardiogram findings. Women were less likely to undergo revascularization procedures during hospitalization, and women with STEMI were less likely to receive timely primary reperfusion.
Conclusions
Young women with AMI represent a distinct, higher-risk population that is different from young men.
Keywords: myocardial infarction, sex, risk factors, epidemiology, prognosis
Young and middle-aged women are at high risk of adverse outcomes after acute myocardial infarction (AMI). Studies indicate that women younger than 55 years of age experience two- to three-fold higher hospital mortality after AMI and a 50% higher risk of death over two years compared with similarly aged men.1–3 Additionally, young women are more likely to report higher rates of angina, lower health-related quality of life, and reduced physical and mental functioning after discharge than men.4–6 Yet little is known about whether young and middle-aged women with AMI have a profile that is different from men at the time that they present to the hospital.
We do have some information on sex differences in young AMI patients, but it is incomplete. Data from national registries and administrative claims suggest that young women with AMI may be sicker on admission and receive less effective care during hospitalization.1,3,7–12 Yet, these studies have not been designed to study young women specifically and, thus, findings are based on small numbers of young women and have been limited to common cardiovascular risk factors and complications in older populations. Prospective cohort studies of young and middle-aged patients with AMI, such as GENESIS-PRAXY and Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO), have revealed some sex differences in demographic, cardiovascular risk factors, symptoms, and treatment;13–16 however, these studies have focused on a limited number of variables from specific risk factor domains. To date, no study has comprehensively assessed sex differences across a breadth of sociodemographic characteristics, comorbidities, presentation, treatment, or complications to determine whether young and middle-aged women with AMI have an overall profile that is different from men. In addition, prior studies have omitted many potentially important variables and risk factor domains such as socioeconomic status, health insurance, healthcare access and utilization, non-cardiovascular comorbidities, laboratory and electrocardiogram findings, admission and discharge medications, and in-hospital complications. Because AMI occurs in the context of an individual and multiple risk factors may contribute to prognosis independently or in combination, a comprehensive comparison of young women and men with AMI across multiple domains is imperative for understanding sex differences in the pathophysiology and prognosis of AMI in young patients.
The VIRGO study is designed to characterize young and middle-aged women with AMI.17 With detailed clinical information from patient interviews and chart abstractions, the VIRGO study offers the opportunity to comprehensively evaluate sex differences in clinical presentation and hospital course in order to determine to what extent young women and men with AMI have similar or distinct profiles. VIRGO included a diversity of patients with AMI recruited from over 100 centers in the United States. The aims of this study were to compare young and middle-aged women and men hospitalized for AMI on six domains: 1) demographics and socioeconomic status, 2) healthcare access and use prior to admission, 3) cardiovascular risk factors, comorbidities, and psychosocial factors, 4) symptoms and pre-hospital delay, 5) clinical presentation on admission, and 6) hospital management and in-hospital complications. We hypothesized that young women with AMI would differ from young men on several domains making them a distinct population.
METHODS
The VIRGO Study
The VIRGO study is the largest prospective observational study to date of young and middle-aged women and men with AMI and was designed to examine sex differences in the presentation, treatment, and outcomes of young and middle-aged patients with AMI. Details on the study design and methodology have been previously reported.17 In brief, young and middle-aged patients with AMI were enrolled from 103 hospitals in the U.S. and 24 hospitals in Spain between August 2008 and January 2012 using a 2:1 female-to-male enrollment ratio. Eligible patients were between 18–55 years old, met AMI criteria, and presented or transferred to an enrolling institution within the first 24 hours of hospital presentation. AMI criteria included 1) an increase in cardiac biomarkers (troponin I or T or creatine kinase-MB) with at least one value >99th percentile of the upper reference limit within 24 hours of admission and 2) supporting evidence of myocardial ischemia, including symptoms of ischemia, electrocardiogram (ECG) changes indicative of new ischemia (ST-segment changes, left bundle branch block (LBBB), or the development of pathological Q waves), or other evidence of myocardial necrosis on imaging.18 Patients who developed elevated cardiac markers as a complication of elective coronary revascularization were not eligible for VIRGO. Additional exclusion criteria included the inability to speak English or Spanish, to provide informed consent, or to be contacted for follow-up. Of the 5,585 patients who met eligibility criteria, 3,572 patients were enrolled in VIRGO. Of these, we included 3,501 patients (2,349 women and 1,152 men) in our analyses from the US and Spain. The most common cause for exclusion was refusing informed consent. Enrolled and non-enrolled patients had similar demographic characteristics.
Information on baseline patient characteristics and clinical course was obtained by medical chart abstraction and standardized in-person interviews performed by trained personnel during the index admission. Institutional review board approval was obtained at each participating center, and all patients provided written informed consent to participate.
Variable Definitions
Our primary variable of interest was patient sex (women versus men). Information on patient demographics, socioeconomic status, healthcare access, psychosocial risk factors, and symptoms was self-reported by the patient. Psychosocial factors were assessed using previously validated scales: the Patient Health Questionnaire-9 for depressive symptomatology,19 the ENRICHD Social Support Inventory for social support,20 the Perceived Stress Scale for perceived stress,21 the Short Form-12 physical and mental component scales for general health status,22 the Seattle Angina Questionnaire for disease-specific functional status,23 and the Euro-Quality of Life for health-related quality of life.24 Data on medical history, comorbidities, time to presentation, and clinical presentation were largely derived from the medical chart; however, in some cases, information from both the medical chart and patient interviews was combined to ensure variable completeness. Clinical severity was assessed using Killip class, which classifies patients according to signs of heart failure, and the Global Registry of Acute Coronary Events (GRACE) score, which predicts in-hospital and 6-month mortality risk).25 An expert team of reviewers affiliated with the Yale Coordinating Center independently adjudicated electrocardiogram findings. In-hospital course including therapies received, admission and discharge medications, in-hospital complications, length of stay, and disposition were obtained from chart abstraction. Details on the variable definitions are provided in Supplemental Table S1.
Statistical Analyses
We compared baseline variables between women and men using chi-square or Fisher’s exact test for categorical variables and student’s t-tests or Mann Whitney U tests for continuous variables. Categorical variables are presented as number (%) and continuous variables are presented as mean (standard deviation (SD)) or median (interquartile range). All analyses were performed in SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
This VIRGO study included 2,349 women and 1,152 men in the US and Spain aged 18–55 years old with AMI. The average age of both women and men in the sample was 47 (SD 6) years. As compared with men, women were more likely to self-report as black, unemployed, and divorced, separated, or widowed (all p<0.01) (Table 1). Women generally reported lower total household incomes than men and experienced more difficulty making ends meet financially (p<0.01).
Table 1.
Sex differences in patient demographics and socioeconomic status in young patients with acute myocardial infarction*
| Women(n=2349) N(%) |
Men(n=1152) N(%) |
|
|---|---|---|
| Demographics | ||
| Age, mean (SE) | 47.0(6.3) | 46.9(6.0) |
| Race | ||
| White | 1781(76.0) | 961(83.6) |
| Black | 437(18.6) | 113(9.8) |
| Other | 127(5.4) | 76(6.6) |
| Hispanic | 177(7.6) | 92(8.0) |
| Marital status | ||
| Married | 1132(48.7) | 663(58.2) |
| Living with Partner | 162(7.0) | 72(6.3) |
| Divorced/Separated/Widowed | 717(30.9) | 238(20.9) |
| Single | 312(13.4) | 167(14.7) |
| Education | ||
| Less than high school | 138(6.0) | 47(4.2) |
| Some high school | 943(40.8) | 474(42.1) |
| High school graduate | 1228(53.2) | 604(53.7) |
| Employment | ||
| Full-time | 1019(43.6) | 768(67.3) |
| Part-time | 300(12.8) | 70(6.1) |
| Unemployed | 1020(43.6) | 304(26.6) |
| Live alone | 275(11.8) | 166(14.5) |
| Living arrangement | ||
| Own home | 1218(52.8) | 706(62.5) |
| Rent apartment | 859(37.2) | 319(28.2) |
| Friend/relative home | 219(9.5) | 99(8.8) |
| Other | 11(0.5) | 6(0.5) |
| Socioeconomic Status | ||
| Finances at end of month | ||
| Some money left over | 597(25.8) | 427(37.6) |
| Just enough to make ends meet | 870(37.6) | 426(37.5) |
| Not enough to make ends meet | 848(36.6) | 283(24.9) |
| Total household income | ||
| <10,000 | 464(21.4) | 137(13.0) |
| 10–50,000 | 1063(49.1) | 453(43.0) |
| 50–100,000 | 459(21.2) | 293(27.8) |
| >100,000 | 181(8.4) | 171(16.2) |
Men and women were compared on demographic and socioeconomic characteristics. Values given are N(%) unless otherwise specified. See supplemental table for a complete description of variables.
Fewer women were uninsured, but significantly more had government insurance (Medicare, Medicaid, or Veterans Affairs) (Table 2). More women than men reported having a primary care provider (90% versus 82%, p<0.01), but the percentage of men and women seeing general practitioners versus specialists for primary care was similar. Despite higher rates of insurance and primary care, women still reported more difficulty receiving medical care before hospitalization for AMI (p=0.01).
Table 2.
Sex differences in healthcare access and use in young patients with acute myocardial infarction*
| Women(n=2349) N(%) |
Men(n=1152) N(%) |
|
|---|---|---|
| Health insurance | ||
| None | 438(19.2) | 244(21.9) |
| Commerical/PPO | 722(31.6) | 423(38.0) |
| HMO | 278(12.2) | 148(13.3) |
| Government(VA, Medicare/Medicaid) | 403(17.7) | 110(9.9) |
| Other | 441(19.3) | 187(16.8) |
| Usual source of care | ||
| Private doctor’s office | 1253(53.9) | 568(50.3) |
| HMO/Prepaid health plan | 109(4.7) | 50(4.4) |
| Neighborhood clinic | 423(18.2) | 182(16.1) |
| Hospital outpatient | 325(14.0) | 130(11.5) |
| Hospital Emergency Department | 133(5.7) | 97(8.6) |
| Other | 153(6.6) | 105(9.3) |
| No particular place | 98(4.2) | 78(6.9) |
| Primary care provider | ||
| General practitioner | 1600(68.6) | 787(69.5) |
| Obstetrician/Gynecologist | 162(7.0) | 0(0) |
| Specialist | 336(14.4) | 141(12.5) |
| No primary care physician | 233(10.0) | 204(18.0) |
| Prior visit with cardiologist | 759(32.7) | 366(32.3) |
| Difficulty receiving care | ||
| Difficult | 391(16.7) | 167(14.7) |
| Somewhat difficult | 249(10.7) | 92(8.1) |
| Not difficult | 1696(72.6) | 881(77.3) |
| Previously avoided health care due to cost? | 728(31.4) | 328(29.0) |
Abbreviations: HMO, health maintenance organization; PPO, preferred provider organization; VA, Veteran’s Affairs.
Men and women were compared on healthcare access and use. Values given are N(%) unless otherwise specified. See supplemental table for a complete description of variables.
At the time of AMI, women were significantly more likely to have a history of diabetes, congestive heart failure (CHF), stroke, chronic obstructive pulmonary disease (COPD), chronic renal failure, and thyroid disorders than men and were more likely to have higher BMI and to report insufficient physical activity (all p<0.01) (Table 3). Rates of undiagnosed diabetes were similar between women and men. Overall, women had a higher risk factor burden than men with significantly more women having >3 cardiovascular risk factors (diabetes, hypertension, hypercholesterolemia, smoking, and obesity) (p<0.01). Women were also more likely to have a history of cancer, autoimmune disorders, and psychiatric disorders than men (all p<0.01), although the prevalence of these conditions was low in the overall population. In contrast, young men with AMI were more likely to have a history of hypercholesterolemia and alcohol abuse (both p<0.01). There were no differences in the prevalence of hypertension, prior coronary artery disease, or cocaine use between sexes (all p>0.1).
Table 3.
Sex differences in traditional cardiovascular and psychosocial risk factors in young patients with acute myocardial infarction*
| Women(n=2349) N(%) |
Men(n=1152) N(%) |
|
|---|---|---|
| Cardiovascular Risk Factors | ||
| Diabetes | ||
| None | 1438(61.2) | 844(73.3) |
| Undiagnosed | 151(6.4) | 80(6.9) |
| Diagnosed | 760(32.4) | 228(19.8) |
| Hypertension | 1499(63.8) | 718(62.3) |
| Hypercholesterolemia | 1941(82.6) | 1061(92.1) |
| Prior MI, PCI, CABG | 436(18.6) | 236(20.5) |
| Smoking | ||
| Never | 660(28.1) | 305(26.5) |
| Past | 382(16.3) | 227(19.7) |
| Current | 1307(55.6) | 618(53.7) |
| Body mass index(kg/m2) | ||
| Underweight(<18.5) | 32(1.4) | 9(0.8) |
| Normal weight(18.5–24.9) | 497(21.2) | 172(14.9) |
| Overweight(25–29.9) | 624(26.6) | 458(39.8) |
| Obese(30–34.9) | 530(22.6) | 295(25.6) |
| Morbidly obese(>35) | 666(28.4) | 218(18.9) |
| Physical activity† | ||
| Active | 773(33.2) | 473(41.6) |
| Insufficient activity | 657(28.2) | 286(25.2) |
| Inactive | 897(38.6) | 378(33.3) |
| Number of Cardiovascular Risk Factors‡ | ||
| 0 | 117(5.0) | 45(3.9) |
| 1 | 355(15.1) | 204(17.8) |
| 2 | 515(21.9) | 276(24.0) |
| 3+ | 1362(58.0) | 624(54.3) |
| Comorbidities | ||
| Congestive heart failure | 117(5.0) | 24(2.1) |
| Cardiac arrhythmias | 87(3.7) | 39(3.4) |
| Prior transient ischemic attack or stroke | 120(5.1) | 27(2.4) |
| Peripheral artery disease | 56(2.4) | 23(2.0) |
| Hypercoagulability syndrome | 45(1.9) | 7(0.6) |
| Sleep apnea | 102(4.4) | 59(5.1) |
| Alcohol abuse | 105(4.5) | 126(11.0) |
| History of cocaine use | 102(4.4) | 60(5.2) |
| Chronic obstructive pulmonary disease | 296(12.6) | 63(5.5) |
| Chronic renal failure | 272(11.6) | 90(7.9) |
| Cancer | 96(4.1) | 22(1.9) |
| Autoimmune | 93(4.0) | 15(1.3) |
| Thyroid disorder | 223(9.5) | 21(1.8) |
| Psychiatric disorder | 120(5.1) | 25(2.2) |
| Family History§ | ||
| Family history of coronary artery disease | 1696(76.0) | 809(73.6) |
| Family history of diabetes | 1212(52.5) | 481(42.6) |
| Psychosocial Factors | ||
| Diagnosed depression | 1123(47.8) | 275(23.9) |
| Depressive symptomatology(PHQ-9 ≥10) | 874(38.9) | 240(21.6) |
| Social support(ESSI), mean(SD) | 25.6(5.5) | 26.1(5.5) |
| Perceived stress(PSS), mean(SD) | 27.0(9.9) | 23.4(9.0) |
| General health status(SF-12), mean(SD) | ||
| Physical component score | 42.8(12.3) | 46.2(11.4) |
| Mental component score | 43.9(12.8) | 48.4(11.5) |
| Disease-specific functional status(SAQ), mean(SD) | ||
| Angina frequency | 82.7(21.5) | 86.5(18.0) |
| Angina-related physical limitation | 78.5(27.0) | 86.9(20.7) |
| Angina-related quality of life | 54.7(24.7) | 60.4(22.1) |
| Health-related Quality of Life(EQ-5D), mean(SD) | ||
| Utility index score | 0.73(0.23) | 0.81(0.20) |
| Visual analog scale | 63.0(22.0) | 66.7(20.1) |
Abbreviations: CABG, coronary artery bypass grafting; MI, myocardial infarction; EQ-5D, EuroQoL 5D; ESSI, ENRICHD Social Support Inventory; PCI, percutaneous coronary interventions; PHQ-9, Patient Health Questionnaire; PSS, Perceived Stress Scale; SAQ, Seattle Angina Questionnaire; SD, standard deviation; SF-12, Short Form-12.
Men and women were compared on cardiovascular and psychosocial characteristics. Values given are N(%) unless otherwise specified. See supplemental table for a complete description of variables.
Physical activity categories are based on Physical Activity Guidelines for Americans.
Number of cardiovascular risk factors was calculated as the sum of 6 cardiovascular risk factors(diabetes mellitus(diagnosed or undiagnosed), hypertension, hypercholesterolemia, smoking, obesity, and inactivity).
Family history refers to immediate family including parents or siblings.
There were large differences in psychosocial risk factors between young women and men with AMI (Table 3). Women were significantly more likely to have been diagnosed previously with depression and to meet the criteria for moderate depression on the Patient Health Questionnaire-9 scale (p<0.01). They also reported higher levels of perceived stress and poorer physical and mental health status, on average, than men (all p<0.01) but comparable levels of social support (Figure 1). On disease-specific measures, women reported more angina-related limitations and lower health-related quality of life on both the Seattle Angina Questionnaire and the Euro-Quality of Life (all p<0.01).
Figure 1. Baseline differences in psychosocial measurements between young women and men with AMI.
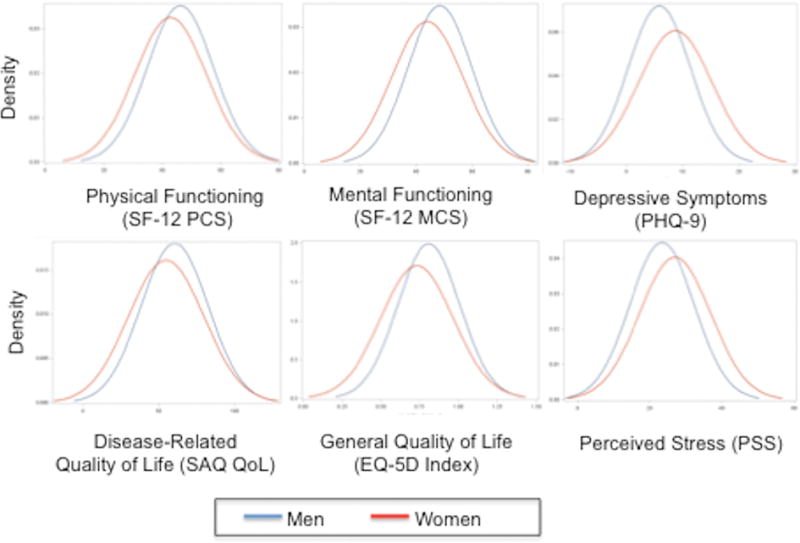
Abbreviations: EQ-5D, Euro-QoL-5D; PHQ-9, Patient Health Questionnaire-9; PSS, Perceived Stress Scale; SAQ QoL, Seattle Angina Questionnaire quality of life; SF-12 PCS, Short Form-12 physical component score; SF-12 MCS, Short Form-12 mental component score/ Women(red) self-reported lower physical and mental functioning and quality of life than men(blue) but higher levels of depressive symptomatology and perceived stress at the time of AMI(all p<0.05).
The majority of women (77%) and men (83%) reported chest pain typical of AMI (Table 4). The second most common symptom in women was nausea (45%), followed by shortness of breath (44%). The reverse was true in men (44% had shortness of breath and 35% reported nausea). Approximately half of young women and men thought that something was wrong with their heart when they first experienced symptoms; however, more men than women reported that providers were able to correctly identify their heart problem at the point of care (87% versus 76%, p<0.01). Women were slightly less likely than men to consider themselves at risk for heart disease or to have been told that they were at risk for heart disease prior to AMI. Additionally, women had significantly longer delays from symptom onset to presentation (>6 hours) (p<0.01).
Table 4.
Sex differences in symptom presentation and pre-hospital delay in young patients with acute myocardial infarction*
| Women(n=2349) N(%) |
Men(n=1152) N(%) |
|
|---|---|---|
| Symptom Presentation | ||
| Presented with typical chest pain | 1813(77.2) | 960(83.3) |
| Patient thought something was wrong with heart | 967(43.0) | 514(47.0) |
| Provider thought something was wrong with heart | 1760(75.5) | 991(86.7) |
| Patient Assessment of Pre-Hospital Risk and Time to Presentation | ||
| Patient considered him/herself at risk for heart disease | 1221(52.2) | 642(55.8) |
| Provider told patient he/she at risk for heart disease | 1039(45.1) | 554(49.2) |
| Time to presentation | ||
| ≤6 hours | 1290(55.1) | 732(63.8) |
| >6 hours | 1050(44.9) | 416(36.2) |
Men and women were compared on symptom presentation and pre-hospital delays. Values given are N(%) unless otherwise specified. See supplemental table for a complete description of variables.
At the time of hospital presentation, men had higher systolic and diastolic blood pressures and higher median levels of peak cardiac markers (troponin and creatine kinase-MB) elevations than women (all p<0.01) (Table 5). There were no sex differences in the percentage of patients presenting with low blood pressure (systolic <90mmHg or diastolic <50mmHg). Men were more likely to have ST-elevation AMIs and new pathological Q-waves on electrocardiogram (both p<0.01); however, women had slightly more severe AMIs as assessed by Killip class and GRACE scores (both p<0.05) (Figure 2).
Table 5.
Sex differences in clinical presentation in young patients with acute myocardial infarction*
| Women(n=2349) N(%) |
Men(n=1152) N(%) |
|
|---|---|---|
| Vitals and Laboratory Studies | ||
| Systolic blood pressure on admission | ||
| <90mmHg | 73(3.1) | 27(2.4) |
| 90–140mmHg | 1055(45.1) | 457(39.7) |
| >140mmHg | 1213(51.8) | 666(57.9) |
| Diastolic blood pressure on admission | ||
| <50mmHg | 64(2.7) | 12(1.1) |
| 50–90mmHg | 1333(57.0) | 546(47.6) |
| >90mmHg | 943(40.3) | 589(51.4) |
| Heart rate(bpm) on admission, mean(SD) | 83.8(20.4) | 81.6(20.1) |
| Peak troponin(ng/mL), median(IQR) | 6.0(1.5, 24.0) | 10.3(2.2, 37.9) |
| Peak CK-MB(IU/L), median(IQR) | 41.4(11.1, 122.6) | 65.1(17.6, 162) |
| Peak creatinine(mg/dL), median(IQR) | 0.82(0.70, 1.00) | 1.03(0.90, 1.20) |
| Electrocardiogram Findings | ||
| Rhythm on qualifying electrocardiogram | ||
| Sinus | 2250(96.9) | 1098(96.7) |
| Atrial fibrillation/flutter | 22(1.0) | 17(1.5) |
| Ventricular tachycardia | 3(0.1) | 3(0.3) |
| Other | 46(2.0) | 18(1.6) |
| ST-elevation or LBBB | 1126(47.9) | 685(59.5) |
| Q-wave | 376(16.0) | 251(21.8) |
| Infarct location† | ||
| Anterior | 737(31.4) | 363(31.5) |
| Inferior | 816(34.7) | 471(40.9) |
| Lateral | 376(16.0) | 169(14.7) |
| Posterior | 132(5.6) | 93(8.1) |
| Right ventricle | 28(1.2) | 12(1.0) |
| Other | 137(5.8) | 36(3.1) |
| AMI Clinical Severity | ||
| Killip class on admission | ||
| I | 2115(94.7) | 1064(97.0) |
| II | 81(3.6) | 24(2.2) |
| III | 22(1.0) | 4(0.4) |
| IV | 16(0.7) | 5(0.5) |
| GRACE score, mean(SD) | 75.4(74.7) | 73.2(72.1) |
| Hemodynamic instability‡ | 210(8.9) | 95(8.3) |
| Left ventricular ejection fraction <40% | 242(10.8) | 126(11.3) |
Abbreviations: IQR, interquartile range; LBBB, left bundle branch block; SD, standard deviation.
Men and women were compared on clinical presentation. Values given are N(%) unless otherwise specified. See supplemental table for a complete description of variables.
Percentage values may not sum to 100% because infarct could have occurred in multiple in locations.
Defined as cardiac arrest prior at presentation or first systolic blood pressure <90mmHg.
Figure 2. Differences in clinical risk scores and length of stay between young women and men with AMI.
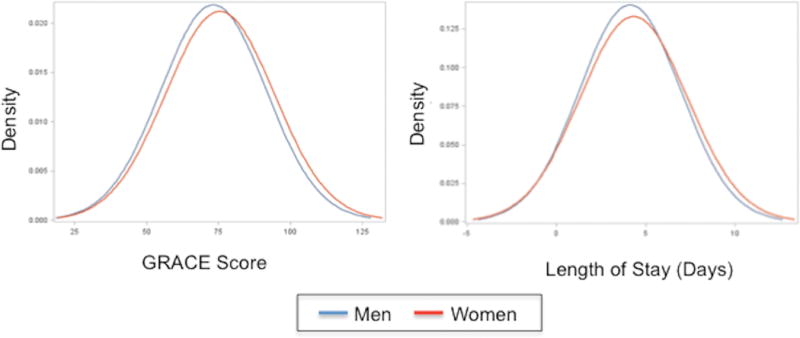
Abbreviations: GRACE, Global Registry of Acute Coronary Events. Women(red) had higher GRACE risk scores(p=0.001) at the time of presentation for AMI and slightly longer hospital lengths of stay(p=0.01) than men.
Given the higher rates of STEMI in men, a greater proportion of men was eligible for primary reperfusion therapy (Table 6). Of those eligible, fewer women received acute reperfusion therapy, and more women received these therapies outside of the recommended time frames (door-to-needle time >30 minutes: 55.1% versus 40.9%; door-to-balloon time >90 minutes: 40.6% versus 29.2%). Cardiac catheterization was performed in >98% of men and women in VIRGO; however, significantly fewer women underwent percutaneous coronary intervention or coronary artery bypass grafting procedures than men (p<0.01). Over 28% of women did not receive any revascularization procedures compared with only 13% of men.
Table 6.
Sex differences in-hospital management, procedures, and in-hospital complications in young patients with acute myocardial infarction*
| Women(n=2349) N(%) |
Men(n=1152) N(%) |
|
|---|---|---|
| Primary Reperfusion Therapy(Ideal Candidates) | ||
| Ideal candidate for primary reperfusion therapy† | 953(40.6) | 581(50.4) |
| Acute reperfusion therapy(among ideal candidates) | ||
| None | 76(8.0) | 25(4.3) |
| Fibrinolytic therapy | 108(11.3) | 80(13.8) |
| Primary angioplasty | 769(80.7) | 476(81.9) |
| Door to needle time >30min | 49(55.1) | 27(40.9) |
| Door to balloon time >90min | 294(40.6) | 133(29.2) |
| Revascularization Procedures(All Patients) | ||
| Cardiac catheterization performed | 2309(98.3) | 1139(98.9) |
| Cardiac catheterization status‡ | ||
| Elective | 523(23.3) | 225(20.2) |
| Urgent | 788(35.0) | 367(32.9) |
| Emergent | 936(41.6) | 522(46.8) |
| Salvage | 2(0.1) | 1(0.1) |
| Percutaneous coronary intervention‡ | 1471(64.1) | 882(77.8) |
| Bare-metal stent | 558(38.1) | 349(39.7) |
| Drug-eluting stent | 938(63.9) | 553(63.1) |
| Coronary artery bypass grafting‡ | 178(7.8) | 115(10.2) |
| No revascularization procedure‡,§ | 647(28.3) | 145(12.8) |
| Other Procedures | ||
| Pacemaker placement | 24(1.0) | 11(1.0) |
| Implantable cardioverter defibrillator placement | 17(0.7) | 6(0.5) |
| Admission Medications|| | ||
| Aspirin on admission | 2235(97.2) | 1122(98.3) |
| Beta blocker on admission | 1872(87.1) | 950(88.6) |
| ACE/ARB inhibitor on admission | 1201(57.5) | 610(57.8) |
| Other antiplatelet agent on admission | 1898(92.4) | 1007(94.9) |
| Glyocprotein IIb/IIIa inhibitor on admission | 1037(62.1) | 608(67.8) |
| Anticoagulant on admission | 2053(93.8) | 1021(94.0) |
| Anti-thrombin agent on admission | 427(33.3) | 228(32.5) |
| Discharge Medications|| | ||
| Aspirin at discharge | 2248(97.8) | 1126(98.4) |
| Beta-blocker at discharge | 2075(95.2) | 1068(97.1) |
| ACEI/ARB at discharge | 1434(67.7) | 797(75.3) |
| Statin at discharge | 2122(92.8) | 1090(96.7) |
| In-hospital Complications | ||
| Reinfarction | 34(1.5) | 9(0.8) |
| Heart failure | 181(7.8) | 61(5.4) |
| Cardiac arrhythmia# | 163(7.0) | 85(7.4) |
| Renal failure | 50(2.1) | 17(1.5) |
| Length of stay, median(IQR) | 3(2, 5) | 3(2, 5) |
| Disposition | ||
| Home/self care | 2195(97.2) | 1083(97.7) |
| Transferred to another institution | 39(1.7) | 8(0.7) |
| Signed out of hospital against medical advice | 22(1.0) | 12(1.1) |
| In-hospital death | 2(0.1) | 5(0.5) |
Abbreviations: ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; interquartile range.
Men and women were compared on in-hospital management, procedures, and in-hospital complications. Values given are N(%) unless otherwise specified. See supplemental table for a complete description of variables.
Patients were considered ideal candidates for primary reperfusion therapy if they presented within 12 hours of symptom onset and demonstrated ST-elevations in ≥2 consecutive leads or new left bundle branch block on ECG.
Percentages calculated among patients undergoing cardiac catheterization.
Includes patients for whom PCI was not indicated or was attempted but unsuccessful and who did not subsequently undergo CABG.
Percentages calculated among patients without documented contraindications only.
Cardiac arrhythmia includes atrial fibrillation/flutter, atrioventricular block, supraventricular tachycardia, or ventricular tachycardia requiring cardioversion or intravenous antiarrhythmics.
Among patients without contraindications, there were no differences in receipt of medications on admission; however, men were more likely to be prescribed statins and angiotensin converting enzyme inhibitors or angiotensin II receptor blockers at discharge (both p<0.010). Women had slightly longer median lengths of stay than men (p=0.01). No differences in other procedures, or in-hospital complications including reinfarction, cardiac arrhythmias, or renal failure were observed between women and men (all p>0.1).
DISCUSSION
In our study we found that compared with men, young and middle-aged women hospitalized with AMI, have distinct risk factor profiles and clinical presentations. We found that women with AMI had a higher burden of cardiovascular risk factors and significantly more comorbidities than men. However, we also identified additional sex differences, extending beyond traditional clinical characteristics. First, women with AMI were more likely to have lower socioeconomic status as assessed by employment, income, and financial strain. Second, they reported higher levels psychosocial risk factors including depression and stress, poorer physical and mental health status, and lower quality of life at the time of AMI. And third, women had more delays in symptom onset to presentation, presented with higher GRACE and Killip scores, and were less likely to receive timely reperfusion therapy. Taken together, these findings suggest that young and middle-aged women with AMI represent a fundamentally distinct, higher-risk population than men, which may contribute to their poorer prognosis over the long-term.
This study is the first comprehensive evaluation of sex differences in baseline risk factors, clinical presentation, and in-hospital course of young and middle-aged women and men admitted for AMI. Like previous studies, we found that women had more traditional cardiovascular risk factors,1,3,7–10,13 were less likely to present with typical chest pain or diagnostic findings on electrocardiogram,1,3,11 and were less likely to receive timely reperfusion.1,8,13 Our findings add to previous reports by identifying sex differences in several additional clinical risk factors and comorbidities. Compared with men, women had significantly higher rates of diabetes, CHF, prior stroke, COPD, chronic renal failure, cancer, autoimmune disease, thyroid disorders, and psychiatric disorders. In addition, comorbidities and risk factors that are typically higher in older male AMI populations (e.g. prior coronary artery disease (CAD) and smoking) were comparable in men and women. These observations suggest that young and middle-aged women with AMI represent a sicker population than men of the same age.
In addition to clinical factors, we identified sex differences in several demographic and psychosocial risk factors. Women in our study were significantly more likely to be divorced, separated, or widowed than men. In addition, they were significantly more likely to be unemployed, to have lower household incomes, and to report financial stress. Studies in older AMI populations have similarly reported higher rates of unmarried and unemployed in women.5,26 Perhaps due to these socioeconomic strains or other life events, women in our study also reported higher levels of perceived stress and depression than men on average. Indeed, other analyses from VIRGO have shown that men and women with PHQ-9 scores ≥9 were less likely to be married, to work full or part time, and to have health insurance.27 Nearly half of women and a quarter of men reported a previous diagnosis of depression or were taking anti-depressive medications on arrival. These rates are significantly higher than those reported in studies of older AMI populations, which have ranged from 7–33%.28 Prior studies have hypothesized that depression may increase a woman’s risk of cardiovascular disease by elevating atherosclerotic and inflammatory biomarkers, reducing pulse rate variability, and enhancing platelet activation.29 Interestingly, we did not observe differences in social support between women and men in VIRGO. However, we did find that women reported poorer general and angina-related functional status and lower quality of life at baseline, which may compound the effects of psychosocial risk factors such as depression and stress.
The study design precluded us from evaluating sex differences in hospital mortality, which was <1% in VIRGO. Because VIRGO enrolled patients who survived long enough to be admitted and consented into the study, these patients likely represent a healthier cohort than all young and middle-aged patients with AMI. Although there are few nationally representative studies of the US by which to compare our patient profile, a study of young women with AMI using administrative claims data from the Health Care Utilization Project National Inpatient Sample (HCUP-NIS) offers some insight into the representativeness of our sample.10 In general, the composition of patients in VIRGO was similar to that of the HCUP-NIS sample, which showed a higher proportion of black patients and higher rates of hypertension, diabetes, congestive heart failure, cerebrovascular disease, renal failure, and COPD in women than men. These findings support the representativeness of the cohort to the broader U.S. population of young patients with AMI.
Several studies lend support to a biological mechanism for sex differences in clinical presentation and prognosis in young patients with AMI. Given the cardioprotective effects of estrogen, a greater risk factor burden may be needed to incur an AMI, which may explain the differences in baseline risk factors between men and women at the time of presentation for AMI.30 Alternatively, young women who develop atherosclerosis early in life may be predisposed to more aggressive forms of CAD.11 In fact, prior studies have found that young women likely develop CAD via different physiologic pathways than older women or men.29 Data from the Women’s Ischemia Syndrome Evaluation (WISE) study group have shown that ischemic heart disease in women is characterized by more diffuse coronary disease and fewer obstructive lesions than men.31,32 In particular, young women with AMI have significantly less narrowing of the coronary arteries33,34 and are more likely to experience disease of coronary microvasculature35 or spontaneous coronary artery dissection.36 These differences in the pathophysiology of CAD may explain why women in VIRGO had more atypical symptoms, lower peak biomarker (troponin and creatine kinase-MB) levels, and fewer diagnostic findings (LBBB, ST-elevation, and Q-waves) on ECG, and why clinicians were less likely to attribute women’s AMI symptoms to the heart. Such delays in symptom recognition combined with fewer diagnostic lab and ECG findings may explain why women were less likely to receive fibrinolytic therapy or PCI and were more likely to experience delays in reperfusion.
Our study has several implications for future research evaluating sex disparities in AMI outcomes in young patients. First, we found that young and middle-aged women and men with AMI represent two very different populations of patients with respect to pre-hospital risk. Compared with men, young women had more demographic and psychosocial risk factors, greater comorbidity, and poorer functional status and quality of life at baseline. Additional studies are needed to understand how these risk factors contribute to the onset, development, and prognosis of AMI in young women and whether the pathophysiology of AMIs in young women is fundamentally distinct from that in men. Specific attention should be paid to these nontraditional risk factors given the high rates of psychosocial risk factors such as depression and financial strain. Second, young women had longer delays to presentation, more atypical symptoms, and fewer diagnostic laboratory and ECG findings, which may have contributed to delays in diagnosis and treatment. Although prior studies have identified such delays in older women as well,37 future studies should investigate the timing and process of AMI diagnosis in young patients in order to identify systems-level strategies for reducing delays in care.
Nevertheless, this study has some limitations. Because only patients who survived long enough to be admitted to the hospital and consented were enrolled in VIRGO, we lacked information on patients who died prior to arrival and thus are unable to draw conclusions about sex differences in sudden or early cardiac deaths. Nevertheless, we might expect sex differences in risk factors and clinical presentation to be even more pronounced in the entire cohort if young women had higher rates of in-hospital mortality as suggested by prior studies.1,2 Similarly, because in-hospital interviews were conducted after the AMI, patients may have recalled their experiences differently than they would have prior to the event. In addition, patients may have been inclined to answer more negatively on some questionnaire items, such as the stress, health status, and quality of life measures, given their recent experiences. As such, patient responses to these items may not accurately reflect their pre-hospital state. However, we do not anticipate differential recall by sex.
In summary, we identified important sex differences in demographic, psychosocial, and clinical risk factors for AMI, which suggest that young and middle-aged women and men with AMI represent distinct populations with different risk profiles. These differences in pre-hospital risk and clinical presentation may be due to differences in the etiology and pathophysiology of AMI in young women, which may also contribute to their poorer prognosis after AMI. Although men constitute the majority of patients in other studies of young patients with AMI, our findings suggest that young women with AMI represent a unique population with different experiences from those of men and warrant particular attention. Additional research is needed to better understand these sex differences in young patients with AMI and their implications for prevention, diagnosis, and treatment of AMI.
Supplementary Material
Translational Perspective.
Young women and men with AMI have distinct risk factor profiles and clinical presentations. In general, young women with AMI have a higher prevalence of cardiovascular risk factors, comorbidities, and psychosocial risk factors, and they are more likely to present with longer delays to presentation, atypical symptoms, fewer diagnostic findings on ECG, and poorer clinical risk scores. Additional studies are needed to understand how sociodemographic and clinical risk factors contribute to the onset, development, and prognosis of AMI in young women and whether the pathophysiology of AMIs in young women is fundamentally distinct from that in young men. In addition, future research should investigate delays in diagnosis and treatment in young in order to identify systems-level strategies for reducing delays in care.
Acknowledgments
Funding: This work was supported by grant the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services [R01 HL081153-01A1K, F30HL120498-01A1 to E.M.B, and U01 HL105270-04 to the Center for Cardiovascular Outcomes Research at Yale University]. IMJOVEN (Spanish component of VIRGO) is supported in Spain by the Fondo de Investigaciones Sanitarias del Institute Carlos III, Ministry of Science and Technology [PI 081614], and additional funds from the Centro Nacional de Investigaciones Cardiovasculares (CNIC).
None.
Footnotes
Disclosures: HMK has received research grants from Medtronic, Inc and Johnson and Johnson through Yale University for the purpose of disseminating clinical trials and chairs the Cardiac Scientific Advisory Board for United Health. JB reports personal fees from Servier, Pfizer, and Bristol Meyers Squibb. STL is supported by a career development award from the National Institute of Aging, the Chicago Core on Biomeasures in Population-Based Health and Aging at the NORC-University of Chicago, and an additional individual philanthropic gift. No other relevant disclosures are reported.
References
- 1.Vaccarino V, Horwitz RI, Meehan TP, Petrillo MK, Radford MJ, Krumholz HM. Sex differences in mortality after myocardial infarction: evidence for a sex-age interaction. Arch Int Med. 1998;158:2054–62. doi: 10.1001/archinte.158.18.2054. [DOI] [PubMed] [Google Scholar]
- 2.Izadnegahdar M, Singer J, Lee MK, Gao M, Thompson CF, Kopec J, Humphries KH. Do younger women fare worse? Sex differences in acute myocardial infarction hospitalization and early mortality rates over ten years. J Women’s Health. 2014;23:10–7. doi: 10.1089/jwh.2013.4507. [DOI] [PubMed] [Google Scholar]
- 3.Champney KP, Frederick PD, Bueno H, Parashar S, Foody J, Merz CNB, Canto JG, Lichtman JH, Vaccarino V. The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart. 2009;95:895–9. doi: 10.1136/hrt.2008.155804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck CA, Joseph L, Belisle P, Pilote L, Investigators Q Predictors of quality of life 6 months and 1 year after acute myocardial infarction. American heart journal. 2001;142:271–9. doi: 10.1067/mhj.2001.116758. [DOI] [PubMed] [Google Scholar]
- 5.Garavalia LS, Decker C, Reid KJ, Lichtman JH, Parashar S, Vaccarino V, Krumholz HM, Spertus JA. Does health status differ between men and women in early recovery after myocardial infarction? J Women’s Health. 2007;16:93–101. doi: 10.1089/jwh.2006.M073. [DOI] [PubMed] [Google Scholar]
- 6.Schweikert B, Hunger M, Meisinger C, Konig HH, Gapp O, Holle R. Quality of life several years after myocardial infarction: comparing the MONICA/KORA registry to the general population. Eur Heart J. 2009;30:436–43. doi: 10.1093/eurheartj/ehn509. [DOI] [PubMed] [Google Scholar]
- 7.Egiziano G, Akhtari S, Pilote L, Daskalopoulou SS. Sex differences in young patients with acute myocardial infarction. Diab Med. 2013;30:e108–14. doi: 10.1111/dme.12084. [DOI] [PubMed] [Google Scholar]
- 8.Nazzal C, Alonso FT. Younger women have a higher risk of in-hospital mortality due to acute myocardial infarction in Chile. Rev Esp Cardiol. 2013;66:104–9. doi: 10.1016/j.rec.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Fang J, Gillespie C, Wang G, Hong Y, Yoon PW. Age-specific gender differences in in-hospital mortality by type of acute myocardial infarction. Amer J Cardiol. 2012;109:1097–103. doi: 10.1016/j.amjcard.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D’Onofrio GD, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Amer Coll Cardiol. 2014;64:337–45. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, Kiefe CI, Frederick PD, Sopko G, Zheng ZJ. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307:813–22. doi: 10.1001/jama.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawesson SS, Stenestrand U, Lagerqvist B, Wallentin L, Swahn E. Gender perspective on risk factors, coronary lesions, and long-term outcome in young patients with ST-elevation myocardial infarction. Heart. 2010;96:453–9. doi: 10.1136/hrt.2009.175463. [DOI] [PubMed] [Google Scholar]
- 13.Choi J, Daskalopoulou SS, Thanassoulis G, Karp I, Pelletier R, Behlouli H, Pilote L. Sex- and gender-related risk factor burden in patients with premature acute coronary syndrome. Can J Cardiol. 2014;30:109–17. doi: 10.1016/j.cjca.2013.07.674. [DOI] [PubMed] [Google Scholar]
- 14.Khan NA, Daskalopoulou SS, Karp I, Eisenberg MJ, Pelletier R, Tsadok MA, Dasgupta K, Norris CM, Pilote L. Sex differences in acute coronary syndrome symptom presentation in young patients. JAMA Int Med. 2013;173:1863–71. doi: 10.1001/jamainternmed.2013.10149. [DOI] [PubMed] [Google Scholar]
- 15.Leung Yinko SS, Pelletier R, Behlouli H, Norris CM, Humphrise KH, Pilote L. Health-related quality of life in premature acute coronary syndrome: does patient sex or gender really matter? J Amer Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelletier R, Lavoie KL, Bacon SL, Thanassoulis G, Khan NA, Pilote L. Depression and disease severity in patients with premature acute coronary syndrome. Amer J Med. 2014;127:87–93. e1–2. doi: 10.1016/j.amjmed.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 17.Lichtman JH, Lorenze NP, D’Onofrio G, Spertus JA, Lindau ST, Morgan TM, Herrin J, Bueno H, Mattera JA, Ridker PM, Krumholz HM. Variation in recovery: Role of gender on outcomes of young AMI patients(VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3:684–93. doi: 10.1161/CIRCOUTCOMES.109.928713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–95. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell PH, Powell L, Blumenthal J, Norten J, Ironson G, Pitula CF, Froelicher ES, Czajkowski S, Youngblood M, Huber M, Berkman LF. A short social support measure for patients recovering from myocardial infarction: the ENRICHD Social Support Inventory. J Cardiopulm Rehabil Prev. 2003;23:398–403. doi: 10.1097/00008483-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 22.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Amer Coll Cardiol. 1995;25:333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 24.EuroQol G. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 25.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA. Predictors of hospital mortality in the global registry of acute coronary events. Arch Int Med. 2003;163:2345–53. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 26.Vaccarino V, Berkman LF, Krumholz HM. Long-term outcome of myocardial infarction in women and men: a population perspective. Amer J Epidemiol. 2000;152:965–73. doi: 10.1093/aje/152.10.965. [DOI] [PubMed] [Google Scholar]
- 27.Smolderen KG, Strait KM, Dreyer RP, D’Onofrio G, Zhou S, Lichtman JH, et al. Depressive symptoms in younger women and men with acute myocardial infarction: insights from the VIRGO study. J Am Heart Assoc. 2014;4:e001424. doi: 10.1161/JAHA.114.001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thombs BD, Bass EB, Ford DE, Stewart KJ, Tsilidis KK, Patel U, Fauerbach JA, Bush DE, Ziegelstein RC. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med. 2006;21:30–8. doi: 10.1111/j.1525-1497.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finks SW. PSAP VII Cardiology. American College of Clinical Pharmacy; Lenexa: 2010. Cardiovascular disease in women; pp. 179–199. [Google Scholar]
- 30.Huxley VH. Sex and the cardiovascular system: the intriguing tale of how women and men regulate cardiovascular function differently. Adv Physiol Educ. 2007;31:17–22. doi: 10.1152/advan.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quyyumi AA. Women and ischemic heart disease: pathophysiologic implications from the Women’s Ischemia Syndrome Evaluation (WISE) study and future research steps. J Am Coll Cardiol. 2006;47:566–71. doi: 10.1016/j.jacc.2004.11.075. [DOI] [PubMed] [Google Scholar]
- 32.Bairey Merz CN, Shaw LF, Reis SE, et al. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based athophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:521–9. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 33.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998;97:2110–6. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 34.Johansson S, Bergstrand R, Schlossman D, Selin K, Vedin A, Wilhelmsson C. Sex differences in cardioangiographic findings after myocardial infarction. Eur Heart J. 1984;5:374–81. doi: 10.1093/oxfordjournals.eurheartj.a061671. [DOI] [PubMed] [Google Scholar]
- 35.Bellasi A, Raggi P, Merz CN, Shaw LJ. New insights into ischemic heart disease in women. Clev Clin J Med. 2007;74:585–94. doi: 10.3949/ccjm.74.8.585. [DOI] [PubMed] [Google Scholar]
- 36.Thompson EA, Ferraris S, Gress T, Ferraris V. Gender differences and predictors of mortality in spontaneous coronary artery dissection: a review of reported cases. J Invasive Cardiol. 2005;17:59–61. [PubMed] [Google Scholar]
- 37.Thylen I, Ericsson M, Angerud KH, Isaksson RM, Lawesson SS. First medical contact in patients with STEMI and its impact on time to diagnosis; an explorative cross-sectional study. BMJ Open. 2015;5:e007059. doi: 10.1136/bmjopen-2014-007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


