Abstract
Triple-negative breast cancer is one of the least responsive breast cancer subtypes to available targeted therapies due to the absence of hormonal receptors, aggressive phenotypes, and the high rate of relapse. Early breast cancer prevention may therefore play an important role in delaying the progression of triple-negative breast cancer. Cancer stem cells are a subset of cancer cells that are thought to be responsible for tumor progression, treatment resistance, and metastasis. We have previously shown that vitamin D compounds, including a Gemini vitamin D analog BXL0124, suppress progression of ductal carcinoma in situ in vivo and inhibit cancer stem-like cells in MCF10DCIS mammosphere cultures. In the present study, the effects of vitamin D compounds in regulating breast cancer stem-like cells and differentiation in triple-negative breast cancer were assessed. Mammosphere cultures, which enriches for breast cancer cells with stem-like properties, were used to assess the effects of 1α,25(OH)2D3 and BXL0124 on cancer stem cell markers in the triple-negative breast cancer cell line, SUM159. Vitamin D compounds significantly reduced the mammosphere forming efficiency in primary, secondary and tertiary passages of mammospheres compared to control groups. Key markers of cancer stem-like phenotype and pluripotency were analyzed in mammospheres treated with 1α,25(OH)2D3 and BXL0124. As a result, OCT4, CD44 and LAMA5 levels were decreased. The vitamin D compounds also down-regulated the Notch signaling molecules, Notch1, Notch2, Notch3, JAG1, JAG2, HES1 and NFκB, which are involved in breast cancer stem cell maintenance. In addition, the vitamin D compounds up-regulated myoepithelial differentiating markers, cytokeratin 14 and smooth muscle actin, and down-regulated the luminal marker, cytokeratin 18. Cytokeratin 5, a biomarker associated with basal-like breast cancer, was found to be significantly down-regulated by the vitamin D compounds. These results suggest that vitamin D compounds may serve as potential preventive agents to inhibit triple negative breast cancer by regulating cancer stem cells and differentiation.
Keywords: Vitamin D, Stem Cells, Mammosphere, Breast Cancer, OCT4
Introduction
Triple-negative breast cancer (TNBC) is characterized by lack of expression of estrogen receptor (ER), progesterone receptor (PR), and HER-2 (ERBB2) (1). Patients with TNBC have poor prognosis due to the lack of effective therapeutic options (2,3). TNBC represents about 10% of all breast cancer cases and about 35% of TNBC patients suffer metastasis within years of diagnosis (4). The overall 5-year survival rate for patients with TNBC is 62% compared to 75% in non-TNBC patients (5).
Breast cancer cells can be classified into three types depending on the three mammary epithelial cell states characterized by cell surface markers: stem-cell-like (CD44hiCD24negEpCAMlo), basal (CD44hiCD24negEpCAMneg) and luminal (CD44loCD24hiEpCAMhi) (6,7). The basal subtype has similar features as the cells that surround the mammary ducts and has a high frequency of TP53 and PIK3CA mutations (8,9). Interestingly, a majority of breast cancers carrying BRCA1 mutations are found to be triple-negative phenotypes (10). Since TNBC is associated with aggressive breast cancer mutations and has no effective targeted therapy available, it is important to explore novel therapeutic options for patients with the disease.
Accumulating evidence suggests that there is a subpopulation of cells in a cancer, known as tumor initiating cells or cancer stem-like cells (CSCs), which are responsible for the development, progression and metastasis of the tumor (11,12). CSCs self-renew through division or give rise to the bulk of tumor cells in the mass through differentiation (13,14). Previous studies have shown that putative breast CSCs are resistant to chemotherapy, and more aggressive (15). Therefore, targeting cancer stem cells may be a useful therapeutic approach in the treatment of TNBC.
We have previously found that vitamin D compounds inhibit mammosphere formation and decrease the expression of putative stem cell markers in ductal carcinoma in situ (DCIS) cells and inhibit the DCIS progression to invasive ductal carcinoma (IDC) (16). The current study investigates the effects of vitamin D compounds with a focus on key stem cell markers involved in early breast cancer formation, stem cell maintenance and differentiation in triple negative breast cancer.
Materials and Methods
Cell Culture and Reagents
1α,25(OH)2D3 and a Gemini vitamin D analog (BXL0124; 1α25-dihydroxy-20R-21(3-hydroxy-3-deuteromethyl-4,4,4-trideuterobutyl)-23-yne-26,27-hexafluorocholecalciferol, >95% purity) were provided by BioXell, Inc. (Nutley, NJ) (17). SUM159 breast cancer cells were obtained from Asterand (Detroit, MI). SUM159 cells were grown in Ham’s F-12 culture medium supplemented with 5% fetal bovine serum, 1% penicillin/streptomycin, 1 μg/ml hydrocortisone and 5 μg/ml insulin at 37°C and 5% CO2.
Mammosphere Forming Assay
SUM159 cells were grown to 50-60% confluence and cells were detached with StemPro Accutase (Life Technologies, CA). Cells were then plated at 2,000 cells/mL in 6-well ultra-low attachment plates and maintained in Mammocult serum-free medium supplemented with hydrocortisone and heparin (Stem Cell Technologies, Vancouver, Canada). Cells were treated with 1α,25(OH)2D3 or BXL0124 for five days for each passage. For secondary and tertiary mammosphere culture, primary mammospheres were collected and enzymatically dissociated using StemPro Accutase (Life Technologies, CA). Then, cells were re-plated at a density of 2,000 cells/mL for subsequent passages. Images of mammospheres were taken, and the number of mammospheres was counted to determine the mammosphere forming efficiency (MFE). The MFE was calculated by dividing the number of mammospheres (≥100 μm) formed by the number of single cells seeded. Experiments were repeated three times.
Western Blot Analysis
Whole cell lysates (15 μg/lane) were resolved in 4% to 20% SDS-PAGE from Bio-Rad (Hercules, CA). Blots were then probed with the indicated antibodies. Primary antibodies against c-Notch1 (4147, 1:1000), cytokeratin 18 (4548, 1:1000) and Oct4 (2750, 1:1000) were from Cell Signaling Technology (Beverly, MA); β-actin (A1978, 1:2000) was from Sigma-Aldrich (St. Louis, MO). Secondary antibodies were from Cell Signaling Technology. Western blot images are quantified by using GeneGnome XRQ chemiluminescence imaging system and analyzed by GeneTools analysis software from Syngene (MD, USA).
Quantitative Polymerase Chain Reaction Analysis
The Taqman® probe- based gene expression system from Applied Biosystems (Foster City, CA) was used to detect the genes of interest. The procedures were followed as described previously (18). Primers used for analysis are GAPDH (Hs02758991), CD44 (Hs01075861), LAMA5 (Hs00966585), CD24 (Hs00175569), NOTCH1 (Hs01062014), JAG1 (Hs00164982), JAG2 (Hs00171432), NFKB1 (Hs00765730), OCT4 (POU5F1) (Hs00999632), NOTCH2 (Hs01050702), NOTCH3 (Hs01128537), HES1 (Hs00172878), KRTN14 (Hs00265033), KRTN18 (Hs02827483), ACTA2 (Hs00426835) and KRTN5 (Hs00361185). Experiments were repeated three times in duplicates.
Statistical analysis
Statistical significance was evaluated using the Student’s t-test.
Results
Inhibition of mammosphere forming efficiency by 1α,25(OH)2D3 and BXL0124 in SUM159 breast cancer cells
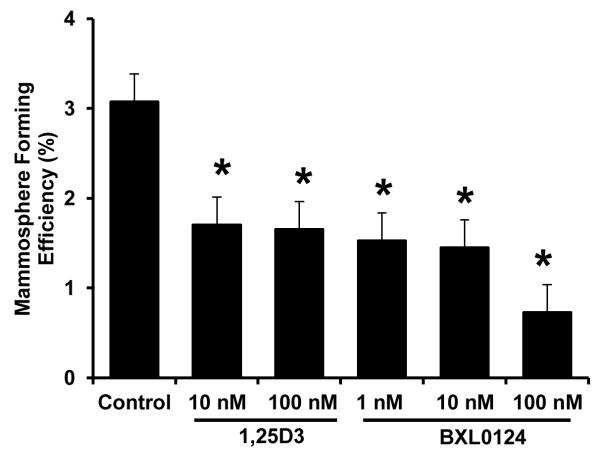
Varying doses of 1α,25(OH)2D3 and BXL0124 were tested for their effectives on mammosphere forming efficiency (MFE) in SUM159 cells. MFE was significantly reduced with 1α,25(OH)2D3 at 10 nM (44.7% inhibition, p<0.05) and 100 nM (46.3% inhibition, p<0.05). BXL0124 reduced the MFE at 1 nM (50.4% inhibition, p< 0.05), 10 nM (52.8% inhibition, p<0.05) and 100 nM (76.4% inhibition, p<0.05) (Figure 1). BXL0124 was more potent than 1α,25(OH)2D3 at the concentrations tested.
Figure 1. Inhibition of mammosphere forming efficiency (MFE) by 1α,25(OH)2D3 and BXL0124 in SUM159 breast cancer cells.
SUM159 cells were plated at a density of 2,000 cells/ml in ultra-low attachment 6-well plates and grown for 5 days in the presence of 1α,25(OH)2D3 (1,25D3, 10 nM and 100 nM) and BXL0124 (1 nM, 10 nM and 100 nM). Mammosphere forming efficiency is calculated by dividing the number of mammospheres (≥100 μm) formed by the number of cells seeded, presenting this as a percentage. Three independent experiments were performed. The data are presented as the mean ± S.E.M. * p<0.05.
Inhibition of mammosphere self-renewal by 1α,25(OH)2D3 and BXL0124
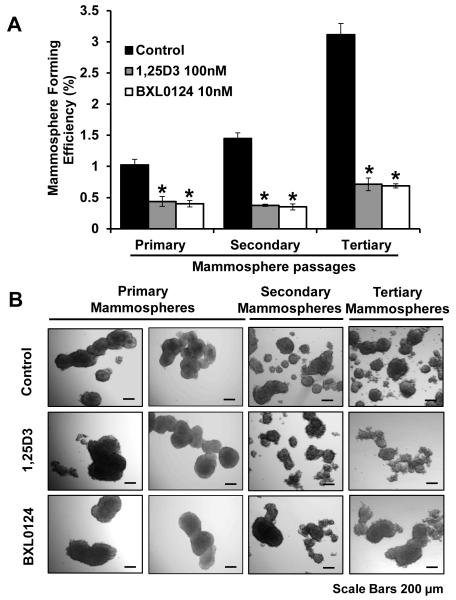
To assess the mammosphere self-renewal capacity as an indicator of stemness, SUM159 spheres were grown in mammosphere cell culture media for three successive passages. MFE was increased from primary to secondary mammospheres (1.03% to 1.45%) and from secondary to tertiary mammospheres (1.45% to 3.11%) in SUM159 cells (Figure 2). Treatment with vitamin D compounds significantly decreased the number of mammospheres in each passage of mammosphere culture. The MFE of primary mammospheres was reduced upon treatment with 100 nM 1α,25(OH)2D3 (60.6% inhibition, p<0.01) or 10 nM BXL0124 (64.7% inhibition, p<0.01). Similarly, the MFE in secondary and tertiary mammospheres was decreased with 1α,25(OH)2D3 (60.7% inhibition, p<0.01 and 69.7% inhibition, p<0.01) and BXL0124 (62.4% inhibition, p<0.01 and 71.6% inhibition, p<0.01) (Figure 2A). Mammospheres treated with vitamin D compounds exhibited more round and smooth edges compared to those of control group (Figure 2B).
Figure 2. Inhibition of mammosphere self-renewal by 1α,25(OH)2D3 and BXL0124 in SUM159 mammospheres.
(A) MFE of primary, secondary and tertiary passages of SUM159 mammospheres are shown. Mammospheres were treated with 1α,25(OH)2D3 (1,25D3, 100 nM) and BXL0124 (10 nM) for 5 days. Three independent experiments were performed. The data are presented as the mean ± S.E.M. *p<0.05. (B) Representative images of SUM159 mammospheres from primary, secondary and tertiary passages are shown for morphological comparison (scale bar 200 μm)
Repression of pluripotency markers and cancer stem cell genes by 1α,25(OH)2D3 and BXL0124
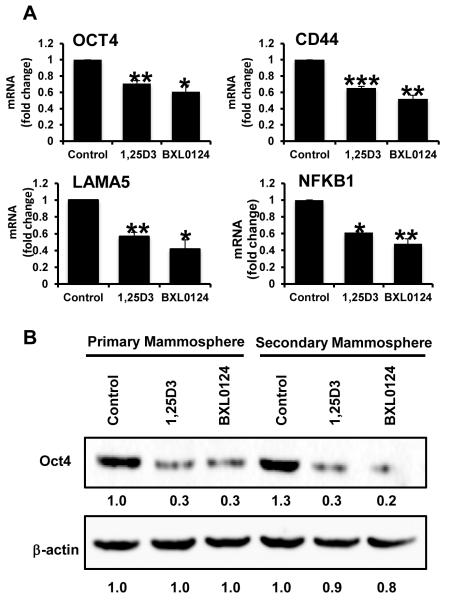
To further investigate the effect of vitamin D compounds on cancer stem-like cells in TNBC, we analyzed SUM159 mammospheres treated with vitamin D compounds for expression of pluripotency and stem cell genes which have been shown to be important in breast cancer progression (19). The pluripotency marker, OCT4, was greatly reduced by treatment with 100 nM 1α,25(OH)2D3 (29%, p<0.01) and 10 nM BXL0124 (39%, p<0.05) (Figure 3). Levels of CD44 and LAMA5, markers associated with stem cell maintenance, were decreased with vitamin D compounds. Levels of CD44 mRNA were decreased by 35% with 1α,25(OH)2D3 (p<0.001) and 48% with BXL0124 (p<0.01). LAMA5 level was decreased by 43% with 1α,25(OH)2D3 (p<0.01) and 59% with BXL0124 (p<0.05). NFκB1, a key molecule involved in stem cell signaling, was significantly reduced by both compounds: 39% by 1α,25(OH)2D3 (p<0.05) and 52% by BXL0124 (p<0.01). Western blot analysis showed that Oct4 was increased from primary to secondary mammospheres in the control group, whereas treatment with vitamin D compounds reduced the protein levels in both primary and secondary mammospheres (Figure 3B).
Figure 3. Repression of pluripotency and stem cell markers by 1α,25(OH)2D3 and BXL0124 in SUM159 mammospheres.
Mammospheres were treated with 1α,25(OH)2D3 (1,25D3, 100 nM) and BXL0124 (10 nM) for 5 days. (A) qPCR analysis was performed on primary mammospheres harvested after five days of growth to assess the gene expression of Oct4, CD44, LAMA5 and NF-κB. Average Ct values are shown in parenthesis for OCT4 (22), CD44 (19), LAMA5 (22), and NF-κB (23). The experiments were repeated three times. *p<0.05, **p< 0.01, ***p<0.001. (B) Western blot analysis of primary and secondary mammospheres treated with 100 nM 1,25D3 and 10 nM BXL0124 detected by anti-Oct4 antibody is shown. β-actin was used as a loading control. Protein levels are quantified by GeneTools analysis software.
Repression of Notch signaling molecules responsible for stem cell maintenance by 1α,25(OH)2D3 and BXL0124
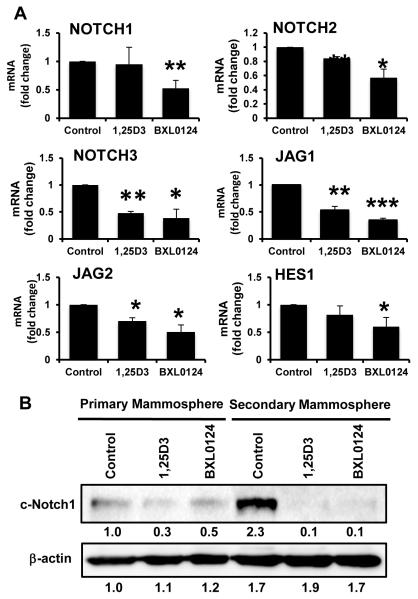
Notch signaling has been shown to play a fundamental role in embryonic development, cell differentiation, tissue homeostasis, and stem cell maintenance (20). In normal breast stem cells, activation of Notch signaling promotes stem cell self-renewal and differentiation of progenitor cells (21). Clarke et al., showed that Musashi1 and Notch1 signaling regulated human breast cancer cells (22). High Notch activity in breast cancer cells increased mammosphere formation and expression of breast cancer stem cell markers (23). In this study, therefore, we investigated whether vitamin D compounds regulate the expression of key molecules in this family involved in stem cell maintenance. The NOTCH1 mRNA level was decreased by 6% and 48% with 1α,25(OH)2D3 and BXL0124 (p<0.01), respectively (Figure 4). NOTCH2 mRNA level was decreased by 16% and 43% with 1α,25(OH)2D3 (p<0.01) and BXL0124 (p<0.05). NOTCH3 mRNA level was decreased by 52% with 1α,25(OH)2D3 (p<0.01) and 62% with BXL0124 (p<0.05). Ligand JAG1 expression was decreased by 48% and 66% with 1α,25(OH)2D3 (p<0.01) and BXL0124 (p<0.001), respectively. Ligand JAG2 expression was also decreased by 30% and 50% with 1α,25(OH)2D3 (p<0.05) and BXL0124 (p<0.05), respectively. HES1 expression was reduced by 19% and 40% with 1α,25(OH)2D3 and BXL0124 (p<0.05). The levels of the activated form of NOTCH1 (cleaved-NOTCH1, c-NOTCH1) were increased from primary to secondary mammospheres in the control group, whereas the vitamin D compounds decreased c-NOTCH1 protein levels in both primary and secondary mammospheres (Figure 4B).
Figure 4. Repression of Notch signaling molecules by 1α,25(OH)2D3 and BXL0124 in SUM159 mammospheres.
Mammospheres were treated with 1α,25(OH)2D3 (1,25D3, 100 nM) and BXL0124 (10 nM) for 5 days. (A) qPCR analysis was performed on primary mammospheres harvested after 5 days of growth to assess the gene expression of markers associated with the Notch signaling pathway – NOTCH1, NOTCH2, NOTCH3, JAG1, JAG2 and HES1. Average Ct values are shown in parenthesis for NOTCH1 (25), NOTCH2 (23), NOTCH3 (23), JAG1 (24), JAG2 (25) and HES1 (23). The data are presented as the mean ± S.E.M. *p<0.05, **p<0.01, ***p<0.001. (B) Western blot analysis of primary and secondary mammospheres treated with 100 nM 1,25D3 and 10 nM BXL0124 detected by c-NOTCH1 antibody. β-actin was used as a loading control. Protein levels are quantified by GeneTools analysis software.
Modulation of mammary epithelial lineage-specific differentiation markers by vitamin D compounds
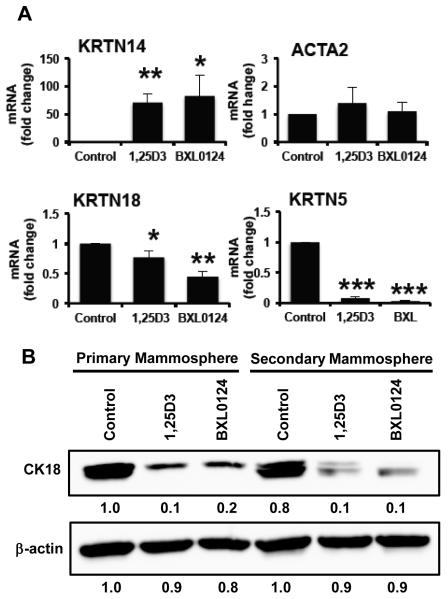
Mammospheres in culture generally fail to express markers associated with breast lineage commitment and differentiation but can be induced to do so with differentiating stimuli, showing the plasticity and stem-like nature of the mammospheres (24). In this study, therefore, we assessed expression of markers associated with myoepithelial/basal phenotype [cytokeratin 14 (CK14) and smooth muscle actin (SMA)] of vitamin D treatment. CK14 (KRTN14) mRNA level was increased by 70-fold with 1α,25(OH)2D3 (p<0.01) and 82-fold with BXL0124 (p<0.05) (Figure 5A). Levels of SMA (ACTA2) mRNA were increased by 1.3-fold with 1α,25(OH)2D3 and 1.1-fold with BXL0124, but these increases were not statistically significant. CK18 (KRTN18), a marker associated with luminal/ductal cells, was down-regulated by 1α,25(OH)2D3 (24% inhibition, p<0.05) and BXL0124 (56% inhibition, p<0.01). Cytokeratin 5 (CK5, KRTN5), a biomarker for basal-like breast cancers and epithelial-mesenchymal transition, was significantly decreased upon treatment with vitamin D compounds. The mRNA levels of KRTN5 were decreased with 100 nM 1α,25(OH)2D3 (92% inhibition, p<0.001) and 10 nM BXL0124 (97% inhibition, p<0.001). Western blot analysis of primary and secondary mammospheres demonstrated that CK18 levels were decreased by the treatment with 1α,25(OH)2D3 and BXL0124 (Figure 5B).
Figure 5. Induction of myoepithelial differentiation by vitamin D compounds.
Mammospheres were treated with 1α,25(OH)2D3 (1,25D3, 100 nM) and BXL0124 (10 nM) for 5 days. (A) qPCR analysis of markers associated with myoepithelial cells (CK14, CK5, and ACTA2) and luminal/ductal cells (CK18) in SUM159 primary mammospheres harvested after 5 days of growth. Average Ct values for CK14 (control #35, 1,25D3 #29, BXL0124 #28); ACTA2 (control #25, 1,25D3 #25, BXL0124 #25); CK18 (control #19, 1,25D3 #20, BXL0124 #20) and CK5 (control #25, 1,25D3 #29, BXL0124 #30). Three independent experiments were performed. The data are presented as the mean ± S.E.M. * p<0.05, ** p<0.01, *** p<0.0001 (B) Western blot analysis of primary and secondary mammospheres treated with 100 nM 1,25D3 and 10 nM BXL0124 detected by CK18 antibody. β-actin was used as a loading control. Protein levels are quantified by GeneTools analysis software.
Discussion
The nutritional importance of vitamin D compounds has long been known but it has also been appreciated for some time that vitamin D and its analogs have anti-proliferative and chemopreventive effects in solid tumors (25). In our previous studies, we have reported that a novel Gemini analog BXL0124 inhibits ErbB2-positive mammary tumor growth and repress CD44 expression in MCF10DCIS.com human breast cancer in vitro and in vivo without causing hypercalcemic toxicity (26,27). Furthermore, oral administration of BXL0124, and a synthetic triterpenoid, CDDO-IM has shown to delay MMTV-ErbB2/neu-induced mammary tumor formation by decreasing activation of ErbB2 and downstream targets including activated-Erk1/2, activated-Akt, Bcl2, CycD1, c-Myc, p21, and PCNA (28). Combination treatment of BXL0124 and CDDO-IM showed stronger efficacy compared to treatment with individual compound alone without hypercalcemic toxicity (28).
Certain vitamin D analogs have been tested in cancer patients as single or as part of combination regimen showing promising responses (29-31). Preclinical studies showing the potentiating effect of vitamin D compounds in tumor inhibition have led to combination studies in clinical trials (32). In a clinical trial of advanced prostate cancer, Beer and colleagues reported that combination of calcitriol and docetaxel exhibit prostate specific antigen (PSA) partial response and increased median survival rate in patients (33). A formulation of calcitriol, DN-101, administered together with Naproxen, delayed early prostate cancer progression and PSA doubling time (34). Given the potent efficacy of BXL0124 in both in vivo and in vitro studies with several models of breast cancer, BXL0124 could be a potentially promising agent to be tested in clinical trials as a single preventive agent or in combination with others.
The cancer inhibitory effects of vitamin D compounds, including 1α,25(OH)2D3 and a Gemini vitamin D analog BXL0124, are mediated through signaling pathways involved in cancer stem cell signaling, cell cycle suppression and differentiation pathways (35). TNBC cell lines, such as SUM159, exhibit predominantly patterns of basal cell surface markers with some minor subpopulations of stem-like or luminal type (6). The sorted stem-like subpopulation, CD44hiCD24negEpCAMlo, of SUM159 cells can readily form tumors when injected into NOD/SCID mice with as few as 100 cells, and this subpopulation of cells expressed high colony forming unit capacity, and elevated spheroid formation, resistance to chemotherapy and ability to reconstitute the parental cell line, which are features of self-renewal characteristics of cancer stem cells and tumorigenicity (7). In this study, we found that vitamin D compounds decreased SUM159 mammosphere formation in association with down-regulation of expression of key markers of cancer stem cell phenotype and maintenance. These findings point to possible mechanistic links between cancer stem cell signaling and VDR pathways regulating the tumor growth, suggesting that vitamin D compounds may be used as chemopreventive agents targeting the cancer stem cell population to prevent tumor development in triple negative breast cancer. More potent dose dependent decrease in MFE in mammospheres treated with BXL0124 compared to 1α,25(OH)2D3, suggesting that BXL0124 is a highly effective and safe agent to selectively target cancer stem cells in triple negative breast cancer.
Oct4 is a critical transcription factor in adult stem cell reprogramming to give rise to induced pluripotent stem cells (36). Interestingly, somatic cell reprogramming and tumorigenesis share common mechanisms (37). Aberrant expression of Oct4 and other key pluripotency markers are associated with abnormal cell growth and tumor formation (38,39). Kumar et al. demonstrated that over-expression of Oct4 gene contributed to de-differentiation of melanoma cells to CSC-like cells, while RNAi knockdown of Oct4 in de-differentiated melanoma cells led to diminished CSC phenotypes (40). In our study, we found that both mRNA and protein levels of Oct4 decreased with vitamin D compound treatment in mammospheres. This indicates an important role for vitamin D compounds in regulating a key transcription factor of cancer stem cells. The critical role of Oct4 in differentiation of breast cancer is currently under further investigation.
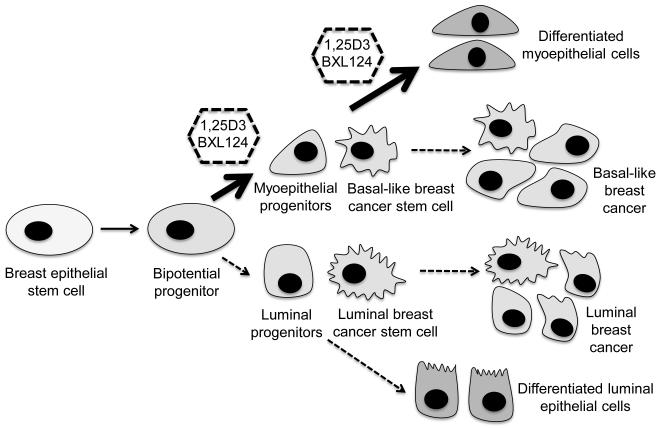
Myoepithelial cells are localized between luminal cells and stroma, maintaining tissue integrity and polarity in normal breast tissue (41). Once breast cells are transformed into tumor cells, normal tissue architecture and polarity are lost. This is followed by a decrease in differentiated myoepithelial cells surrounding the tumor (42,43). Thus, myoepithelial cells appear to play a natural suppressive role limiting tumor growth and invasion (44). Upregulation of myoepithelial markers in SUM159 mammospheres by vitamin D compounds suggest that the tumor inhibitory effects of these compounds are mediated by inducing the cancer stem cells into more mature differentiated cell types (Figure 6).
Figure 6. A proposed scheme of action of vitamin D compounds in cancer stem cells and differentiation pathway.
The lineage diagram of cancer stem cells is modified from a previous publication (51) and the possible action sites of vitamin D compounds are shown.
CD44 is a transmembrane glycoprotein that is involved in malignant progression and metastasis of breast cancer (45). Knockdown of CD44 induces differentiation and drives the breast cancer stem cell-like population toward a non-stem cell-like phenotype (46). We show here that vitamin D compounds reduced the CD44 mRNA transcript levels in mammospheres, suggesting that vitamin D compounds may induce differentiation of breast cancer cells. In addition, LAMA5 is a signature extracellular matrix component in human pluripotent stem cells (47). shRNA knockdown of LAMA5 reduced self-renewal capacity of human pluripotent stem cells (47). Vitamin D compounds decreased LAMA5 levels in mammospheres, indicating that vitamin D compounds may regulate self-renewal of breast cancer stem cells.
Triple-negative breast cancer has a higher rate of relapse and poorer prognosis than other major breast cancer types (48). The basal-like subtype of TNBC is found to exhibit constitutively high level of NF-κB signaling that, in turn, up-regulates JAG1 expression and activates NOTCH signaling, leading to expansion of cancer stem cells (49). Notch signaling is important for normal mammary stem cell maintenance during development. It is required for self-renewal of mammary stem cells and activation of the pathway increased secondary mammosphere formation by 10-fold (50). Interestingly, vitamin D compounds repressed the components of Notch-signaling axis including NF-κB, NOTCH1, NOTCH2, NOTCH3, JAG1, JAG2 and HES1. These findings highlight the therapeutic potential of vitamin D compounds that target Notch signaling in cancer stem cells. Overall, therefore, our study suggests that vitamin D compounds may be useful agents to prevent or impede progression in triple negative breast cancer by targeting cancer stem cell populations.
Highlights.
Vitamin D compounds target cancer stem cells in triple negative breast cancer
Repressive actions on pluripotency markers and Notch signaling pathway
Modulate mammary epithelial differentiation lineage-specific markers
Acknowledgements
The authors would like to thank Dr. Philip Furmanski for his helpful suggestions. This work was supported in part by the National Institutes of Health National Cancer Institute R01 CA127645, the National Institute of Environmental Health Sciences Grant ES005022 and The Trustees Research Fellowship Program at Rutgers, The State University of New Jersey.
Abbreviations
- 1α,25(OH)2D3
1α,25-dihydroxyvitamin D3
- TNBC
triple negative breast cancer
- MFE
mammosphere forming efficiency
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- DCIS
ductal carcinoma in situ
- OCT4
octamer-binding transcription factor 4
- LAMA5
laminin subunit alpha 5
- JAG1
Jagged1
- JAG2
Jagged2
- HES1
hairy and enhancer of split-1
Footnotes
Conflicts of Interest: Authors have no potential conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mayer IA, Abramson VG, Lehmann BD, Pietenpol JA. New strategies for triple-negative breast cancer--deciphering the heterogeneity. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:782–90. doi: 10.1158/1078-0432.CCR-13-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collignon J, Lousberg L, Schroeder H, Jerusalem G. Triple-negative breast cancer: treatment challenges and solutions. Breast cancer (Dove Medical Press) 2016;8:93–107. doi: 10.2147/BCTT.S69488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nature reviews Clinical oncology. 2016;13:674–90. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pogoda K, Niwinska A, Murawska M, Pienkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Medical oncology (Northwood, London, England) 2013;30:388. doi: 10.1007/s12032-012-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal S, Luchtenborg M, Davies EA, Jack RH. The treatment and survival of patients with triple negative breast cancer in a London population. SpringerPlus. 2014;3:553. doi: 10.1186/2193-1801-3-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta Piyush B, Fillmore Christine M, Jiang G, Shapira Sagi D, Tao K, Kuperwasser C, Lander Eric S. Stochastic State Transitions Give Rise to Phenotypic Equilibrium in Populations of Cancer Cells. Cell. 2011;146:633–44. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast cancer research : BCR. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollestelle A, Nagel JH, Smid M, Lam S, Elstrodt F, Wasielewski M, Ng SS, French PJ, Peeters JK, Rozendaal MJ, Riaz M, Koopman DG, Ten Hagen TL, de Leeuw BH, Zwarthoff EC, Teunisse A, van der Spek PJ, Klijn JG, Dinjens WN, Ethier SP, Clevers H, Jochemsen AG, den Bakker MA, Foekens JA, Martens JW, Schutte M. Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat. 2010;121:53–64. doi: 10.1007/s10549-009-0460-8. [DOI] [PubMed] [Google Scholar]
- 10.Collins LC, Martyniak A, Kandel MJ, Stadler ZK, Masciari S, Miron A, Richardson AL, Schnitt SJ, Garber JE. Basal cytokeratin and epidermal growth factor receptor expression are not predictive of BRCA1 mutation status in women with triple-negative breast cancers. Am J Surg Pathol. 2009;33:1093–7. doi: 10.1097/PAS.0b013e31819c1c93. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gangopadhyay S, Nandy A, Hor P, Mukhopadhyay A. Breast cancer stem cells: a novel therapeutic target. Clinical breast cancer. 2013;13:7–15. doi: 10.1016/j.clbc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Wicha MS. Targeting self-renewal, an Achilles' heel of cancer stem cells. Nature medicine. 2014;20:14–5. doi: 10.1038/nm.3434. [DOI] [PubMed] [Google Scholar]
- 14.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. Journal of the National Cancer Institute. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 16.Wahler J, So JY, Cheng LC, Maehr H, Uskokovic M, Suh N. Vitamin D compounds reduce mammosphere formation and decrease expression of putative stem cell markers in breast cancer. The Journal of steroid biochemistry and molecular biology. 2015;148:148–55. doi: 10.1016/j.jsbmb.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maehr H, Lee HJ, Perry B, Suh N, Uskokovic MR. Calcitriol derivatives with two different side chains at C-20. V. Potent Inhibitors of Mammary Carcinogenesis and Inducers of Leukemia Differentiation. Journal of medicinal chemistry. 2009;52:5505–19. doi: 10.1021/jm900780q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ, Liu H, Goodman C, Ji Y, Maehr H, Uskokovic M, Notterman D, Reiss M, Suh N. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochemical pharmacology. 2006;72:332–43. doi: 10.1016/j.bcp.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 19.Bozorgi A, Khazaei M, Khazaei MR. New Findings on Breast Cancer Stem Cells: A Review. Journal of breast cancer. 2015;18:303–12. doi: 10.4048/jbc.2015.18.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development. 2013;140:689–704. doi: 10.1242/dev.080614. [DOI] [PubMed] [Google Scholar]
- 21.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & development. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Developmental Biology. 2005;277:443–56. doi: 10.1016/j.ydbio.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 23.D'Angelo RC, Ouzounova M, Davis A, Choi D, Tchuenkam SM, Kim G, Luther T, Quraishi AA, Senbabaoglu Y, Conley SJ, Clouthier SG, Hassan KA, Wicha MS, Korkaya H. Notch reporter activity in breast cancer cell lines identifies a subset of cells with stem cell activity. Molecular cancer therapeutics. 2015;14:779–87. doi: 10.1158/1535-7163.MCT-14-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer research. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 25.Maund SL, Barclay WW, Hover LD, Axanova LS, Sui G, Hipp JD, Fleet JC, Thorburn A, Cramer SD. Interleukin-1alpha mediates the antiproliferative effects of 1,25-dihydroxyvitamin D3 in prostate progenitor/stem cells. Cancer research. 2011;71:5276–86. doi: 10.1158/0008-5472.CAN-10-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.So JY, Lee HJ, Smolarek AK, Paul S, Wang CX, Maehr H, Uskokovic M, Zheng X, Conney AH, Cai L, Liu F, Suh N. A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Molecular pharmacology. 2011;79:360–7. doi: 10.1124/mol.110.068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HJ, So JY, DeCastro A, Smolarek A, Paul S, Maehr H, Uskokovic M, Suh N. Gemini vitamin D analog suppresses ErbB2-positive mammary tumor growth via inhibition of ErbB2/AKT/ERK signaling. The Journal of steroid biochemistry and molecular biology. 2010;121:408–12. doi: 10.1016/j.jsbmb.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.So JY, Wahler JE, Yoon T, Smolarek AK, Lin Y, Shih WJ, Maehr H, Uskokovic M, Liby KT, Sporn MB, Suh N. Oral administration of a gemini vitamin D analog, a synthetic triterpenoid and the combination prevents mammary tumorigenesis driven by ErbB2 overexpression. Cancer Prev Res (Phila) 2013;6:959–70. doi: 10.1158/1940-6207.CAPR-13-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu G, Wilding G, Staab MJ, Horvath D, Miller K, Dresen A, Alberti D, Arzoomanian R, Chappell R, Bailey HH. Phase II study of 1alpha-hydroxyvitamin D(2) in the treatment of advanced androgen-independent prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:4077–83. [PubMed] [Google Scholar]
- 30.Obara W, Mizutani Y, Oyama C, Akaza H, Ishii N, Kohri K, Namiki M, Okuyama A, Shima H, Yokoyama M, Shuin T, Miki T, Watanabe Y, Fujioka T. Prospective study of combined treatment with interferon-alpha and active vitamin D3 for Japanese patients with metastatic renal cell carcinoma. International journal of urology : official journal of the Japanese Urological Association. 2008;15:794–9. doi: 10.1111/j.1442-2042.2008.02086.x. [DOI] [PubMed] [Google Scholar]
- 31.Trouillas P, Honnorat J, Bret P, Jouvet A, Gerard JP. Redifferentiation therapy in brain tumors: long-lasting complete regression of glioblastomas and an anaplastic astrocytoma under long term 1-alpha-hydroxycholecalciferol. Journal of neuro-oncology. 2001;51:57–66. doi: 10.1023/a:1006437003352. [DOI] [PubMed] [Google Scholar]
- 32.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nature reviews Cancer. 2014;14:342–57. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 33.Beer TM, Eilers KM, Garzotto M, Egorin MJ, Lowe BA, Henner WD. Weekly high-dose calcitriol and docetaxel in metastatic androgen-independent prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:123–8. doi: 10.1200/jco.2003.05.117. [DOI] [PubMed] [Google Scholar]
- 34.Srinivas S, Feldman D. A phase II trial of calcitriol and naproxen in recurrent prostate cancer. Anticancer research. 2009;29:3605–10. [PubMed] [Google Scholar]
- 35.So JY, Suh N. Targeting cancer stem cells in solid tumors by vitamin D. The Journal of steroid biochemistry and molecular biology. 2015;148:79–85. doi: 10.1016/j.jsbmb.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Daley GQ. Common themes of dedifferentiation in somatic cell reprogramming and cancer. Cold Spring Harbor symposia on quantitative biology. 2008;73:171–4. doi: 10.1101/sqb.2008.73.041. [DOI] [PubMed] [Google Scholar]
- 38.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–77. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, Klein B. Embryonic stem cell markers expression in cancers. Biochemical and biophysical research communications. 2009;383:157–62. doi: 10.1016/j.bbrc.2009.02.156. [DOI] [PubMed] [Google Scholar]
- 40.Kumar SM, Liu S, Lu H, Zhang H, Zhang PJ, Gimotty PA, Guerra M, Guo W, Xu X. Acquired cancer stem cell phenotypes through Oct4-mediated dedifferentiation. Oncogene. 2012;31:4898–911. doi: 10.1038/onc.2011.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sopel M. The myoepithelial cell: its role in normal mammary glands and breast cancer. Folia Morphol (Warsz) 2010;69:1–14. [PubMed] [Google Scholar]
- 42.Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. Journal of cell science. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gusterson BA, Warburton MJ, Mitchell D, Ellison M, Neville AM, Rudland PS. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer research. 1982;42:4763–70. [PubMed] [Google Scholar]
- 44.Sternlicht MD, Kedeshian P, Shao ZM, Safarians S, Barsky SH. The human myoepithelial cell is a natural tumor suppressor. Clinical cancer research : an official journal of the American Association for Cancer Research. 1997;3:1949–58. [PubMed] [Google Scholar]
- 45.Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nature reviews Cancer. 2011;11:254–67. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 46.Pham PV, Phan NL, Nguyen NT, Truong NH, Duong TT, Le DV, Truong KD, Phan NK. Differentiation of breast cancer stem cells by knockdown of CD44: promising differentiation therapy. Journal of translational medicine. 2011;9:209. doi: 10.1186/1479-5876-9-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laperle A, Hsiao C, Lampe M, Mortier J, Saha K, Palecek SP, Masters KS. alpha-5 Laminin Synthesized by Human Pluripotent Stem Cells Promotes Self-Renewal. Stem cell reports. 2015;5:195–206. doi: 10.1016/j.stemcr.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann Oncol. 2012;23(Suppl 6):vi7–12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto M, Taguchi Y, Ito-Kureha T, Semba K, Yamaguchi N, Inoue J. NF-kappaB non-cell-autonomously regulates cancer stem cell populations in the basal-like breast cancer subtype. Nature communications. 2013;4:2299. doi: 10.1038/ncomms3299. [DOI] [PubMed] [Google Scholar]
- 50.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast cancer research : BCR. 2004;6:R605–15. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shipitsin M, Polyak K. The cancer stem cell hypothesis: in search of definitions, markers, and relevance. Laboratory investigation; a journal of technical methods and pathology. 2008;88:459–63. doi: 10.1038/labinvest.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]