Abstract
Background
Solution blow spinning is a technique for depositing polymer fibers with promising potential use as a surgical sealant. This study assessed the feasibility and efficacy of solution blow spun polymer (BSP) for sealing bowel perforations in a mouse model of partial cecal transection. We then evaluated its use for reinforcing a surgical anastomosis in a pre-clinical piglet model.
Methods
Three commercially available surgical sealants (fibrin glue, polyethylene glycol (PEG) hydrogel, and cyanoacrylate) were compared to BSP in the ability to seal partially transected cecum in mice. For anastomosis feasibility testing in a piglet model, piglets were subjected to small bowel transection with sutured anastomosis reinforced with BSP application. Outcome measures included anastomotic burst pressure, anastomotic leak rate, 14-day survival, and complication rate.
Results
For the mouse model, the survival rates for the sealants were 30% for fibrin glue, 20% for PEG hydrogel, 78% for cyanoacrylate, and 67% for BSP. Three of 9 mice died after BSP administration due to perforation leak, failure to thrive with partial obstruction at the perforation site, and unknown causes. All other mice died of perforation leak. The mean burst pressure at 24 hours was significantly higher for BSP (81mmHg) when compared to fibrin glue (6mmHg, p=0.047) or PEG hydrogel (10mmHg, p=0.047), and comparable to cyanoacrylate (64mmHg, p=0.91). For piglets, 4 of 4 animals survived at 14 days. Mean burst pressures at time of surgery were 37±5mmHg for BSP and 11±9mmHg for suture-only controls (p=0.09).
Conclusions
Solution blow spinning may be an effective technique as an adjunct for sealing of gastrointestinal anastomosis. Further preclinical testing is warranted to better understand BSP properties and alternative surgical applications.
Keywords: surgical sealant, bowel anastomosis
Introduction
Surgical sealants can be used to reinforce bowel anastomoses with aims of minimizing the risk of anastomotic leak. However, studies provide conflicting evidence in support of their use.[1,2] Commercially available sealants each have drawbacks that may prohibit their efficacy and are specific to their composition. Fibrin glues are based on biologically derived material consisting of fibrinogen and thrombin and are the only class of product that is FDA-approved as a sealant, adhesive, and hemostatic agent.[3] While they are absorbable and relatively easy to use, fibrin glues are costly, complex to prepare, difficult to apply across broad surfaces, and can carry the risk of viral disease transmission.[3] Synthetic polyethylene glycol (PEG) hydrogel sealants circumvent the biologic risks associated with fibrin glues and are absorbable and non-cytotoxic. However, PEG hydrogel sealants are expensive, require multiple step preparations to reconstitute freeze-dried components, and can undergo swelling that may cause injury to neighboring structures.[4] Cyanoacrylate products, better known as “superglue”, are fast acting and have the advantage of high adhesive strength to tissue. Use of these products is generally limited to external surfaces due to their rigidity and propensity to cause intense local inflammation.[5,6] A surgical sealant that minimizes all of these problems and could be used internally in the human body may have large-scale clinical impact in improving surgical outcomes and minimizing the occurrence of complications in technically complex procedures such as an anastomosis.
Solution blow spinning is a technique that allows for the direct deposition of polymer fibers on any substrate. This enables the on-demand design of materials that conform to specific anatomical geometries, thus enhancing surgical usability. Solution blow spun polymer (BSP) offers advantages over pre-formed nanofiber mats, meshes, and scaffolds that are commonly used in surgical applications. While other methods of polymer application can be complex and costly, solution blow spinning only requires a concentrated polymer solution, commercial airbrush, and compressed gas source. We previously reported the feasibility of in situ deposition of nanofibers using this method.[7] A polymer blend composed of poly(lactic-co-glycolic acid) (PLGA) and PEG is used with this method due to its absorbability and unique property of transition from a fiber mat into a conformal film when the material is warmed from room temperature to body temperature (tissue >31° C).[8] The optimization of the polymer blend composition by combining PLGA and PEG allowed for an increase in adhesive behavior and may offer superior properties to currently available commercial products. Furthermore, biocompatibility of this material and technique using mouse fibroblasts and human coronary arterial endothelial cells has been demonstrated.[7,8] A concentrated polymer solution containing this polymer blend can be prepared ahead of time, sterilized with UV light, and stored at room temperature, reducing complexity of preparation. Additionally, PLGA and PEG are polymers that are produced in high volume for medical applications. This greatly reduces the potential cost relative to biologic or synthetic hydrogel sealants that incorporate additional expense through the use of proteins or crosslinking chemistries.
In our previous work, a pilot study was performed in a cecal bowel anastomosis mouse model where the polymer blend was used to supplement sutures.[8] This resulted in a 40% increase in survival versus the suture-only control. Given the results of this study, we aimed to further test the sealing capability of BSP in a mouse model of intestinal perforation without sutures and subsequently in a more clinically relevant piglet model of intestinal anastomosis with sutures. We chose a sutureless model to test the efficacy of BSP as an intestinal sealant and compare its ability to seal a relatively small perforation to other commercially available sealants. We hypothesized that BSP would increase burst pressures and improve survival rates relative to other surgical sealants in a mouse model of intestinal perforation and thus could be used to reinforce a sutured anastomosis. We therefore examined the effect of BSP on burst pressures and survival rates in mice with partial cecal transection relative to other surgical sealants. As proof of principle, we then tested BSP for the first time on a sutured small bowel anastomosis in a piglet survival model to further test the feasibility and efficacy of this technology in a larger pre-clinical animal in order to model physiology and common practice in pediatric bowel anastomoses.
1. Material and Methods
1.1 Mouse model
All surgical procedures were approved by the Institutional Animal Care and Use Committee of Children’s National Health System, Washington DC. 16-week old CL57BL6/J female mice (body weight ~20g) were subjected to anesthesia using an intraperitoneal injection of ketamine (100 mg/kg)/xylazine (10mg/kg). After standard skin preparation, a 1–2 cm mini-laparotomy incision was made, and the cecum was identified. The cecum was partially transected, leaving the mesenteric sidewall intact in order to preserve the blood supply. The transected cecum was then reapproximated using surgical sealant product without suturing. The 4 groups of sealant products included a commercial fibrin glue sealant (Tisseel, Baxter International Inc., Deerfield IL), PEG hydrogel (Progel, Bard Davol Inc., Warwick RI), cyanoacrylate (Omnex, Ethicon Inc, Johnson & Johnson, Somerville, NJ), and BSP. The commercially available sealants were prepared and applied according to the product insert directions. A no-suture control group was not performed for ethical concerns over animal pain and suffering from an inevitable bowel leak in which previous reports demonstrate 100% mortality. The sealants were allowed to dry for 2 minutes for all groups; this was the recommended time for 2 of the 3 commercial sealants and over the time recommended for the third commercial sealant. The bowel was placed back into the abdomen, and the skin was closed in one layer using a 4-0 polyglactin suture (Vicryl, Ethicon Inc) in a running fashion. All surgeries were performed by one surgeon who was blinded to the treatment group until product administration. Animals were given standard mouse chow and water ad libitum before and after the procedure. A total of 60 mice were included in these experiments, 40 in the survival study and 20 in the functional study as described in subsequent sections. Given the mouse bowel would not differ based on gender, only female mice were used for consistency and simplicity purposes.
1.2 Mouse Outcomes
Survival Study
Surgery was performed on 10 mice in each of the 4 groups. Each animal was weighed preoperatively, and serial weights were performed every other day until day of sacrifice. Animals were allowed to survive for 14 days and were sacrificed, followed by necropsy and harvesting of the transected bowel segment. Animals were monitored daily and were sacrificed prior to 14 days if they showed signs of distress due to sepsis. Necropsy was performed to determine the cause of sepsis/death. The tissue specimens were placed in phosphate buffered saline, fixed, and later embedded in paraffin blocks. After sectioning, hematoxylin and eosin (H&E) staining was performed and evaluated under standard light microscopy. Histology slides were evaluated and compared by a blinded pathologist.
Functional Study
Surgery was performed on 5 different mice in each of the 4 groups. Anastomotic burst pressure measurements were performed 24 hours after surgery on 4 to 5 mice in each group, pending 24-hour survival rate. Mice were re-anesthetized, and after exploratory laparotomy, the cecum was identified, and burst pressures were determined. The proximal and distal bowel was clamped to obtain a closed loop, and an angiocatheter was placed proximal to the cecal bowel segment. The angiocatheter was connected to tubing with a three-way stopcock, allowing for methylene blue infusion through the third port and pressure measurements using a digital pressure monitor. Burst pressure was measured at the highest pressure reached prior to leakage of the methylene blue solution at the anastomotic site. Mice were sacrificed after the burst pressure analysis.
1.3 Piglet model
Four 3–4 week old female Yorkshire swine (body weight ~6 kg) were fasted the night prior to surgery and then were subjected to anesthesia using ketamine (15–20 mg/kg)/xylazine (0.5–3 mg/kg) and fentanyl (10 mcg/kg) on the day of surgery. Following sedation and intubation, piglets were prepped and draped. A 3 cm exploratory laparotomy incision was made, and a small bowel segment was brought into the field of view. Following full thickness bowel transection, the bowel was initially reapproximated using 6 equally spaced, simple interrupted sutures with 4-0 polyglactin suture. A sutureless model was not attempted on piglets for ethical reasons given the concern that the piglet bowel would separate or leak and result in peritoneal sepsis. We also do not feel that BSP should be used as a stand-alone agent for surgical anastomosis in a clinical setting. Anastomotic burst pressure of the suture-only anastomosis was performed by clamping off the bowel proximal and distal to the anastomotic segment. One angiocatheter was placed proximally to infuse sterile saline into the bowel segment, and a second angiocatheter was placed distally for pressure readings with a digital pressure monitor. Burst pressure was measured at the highest pressure reached prior to leakage of saline at the closure site. Following this, the anastomotic segment was dried, and it was ensured that the previously placed sutures remained intact. BSP was then deposited onto the anastomosis and was allowed to warm and dry for 5 minutes (Figure 1). The dry time was increased to 5 minutes for the piglets due to the larger surface area of the piglet bowel and the time needed to warm to body temperature to optimize the chemical properties of BSP. Burst pressure was then repeated in the same fashion.
Figure 1.
Gross images of BSP application to piglet bowel anastomosis. A. Application of the BSP using a commercial airbrush. B. Initial application of BSP reveals a white coating of polymer fibers to tissue. C. Once BSP warms to body temperature, it becomes a transparent film.
After burst pressure testing, the anastomotic bowel segment was resected, and a new anastomosis using 8 simple interrupted sutures plus application of BSP was completed for the survival analysis. The bowel was placed back into the abdomen, and the fascia was closed using a 0 polyglactin suture in a running fashion. The skin was closed using a nylon suture in a running inter-locking fashion.
Animals were given standard food and water ad libitum after the procedure and were monitored daily. After 14 days of survival, the piglets were sacrificed after harvesting the anastomotic bowel segment for histology. The abdominal cavity was inspected for anastomotic leak and perianastomotic abscess formation. Frozen sections were performed on the bowel segment and were stained with H&E.
1.4 Statistical analysis
Data is expressed as mean ± standard error. Student t-test was used for continuous variables, and chi-square was used for categorical variables. Multiple group comparisons were measured using one-way ANOVA with post hoc analysis using a Tukey-adjusted significance level. All analyses were performed in SAS 9.3 (SAS Institute Inc., Cary, NC). Differences were regarded as significant at p<0.05.
2. Results
2.1 Mouse Survival Study
Of the 40 mice that underwent surgery for the survival study, 2 mice were excluded from analysis. Cyanoacrylate was unable to be applied properly and circumferentially to the cecal transection of one mouse after 3 failed attempts. The product was found to adhere to surrounding tissue, and when attempts were made to release the adhesion, the product would tear off the anastomotic site. One mouse in the BSP group was excluded due to death on post-operative day 5 from bowel evisceration and strangulation. Wound dehiscence occurred due to mice eating at the incision site. On necropsy of this mouse, the cecal bowel segment was intact, and there was no evidence of bowel perforation.
Of the remaining mice, Figure 2 demonstrates survival after 14 days. Fibrin glue and PEG hydrogel mice demonstrated 30% and 20% survival, respectively. BSP and cyanoacrylate demonstrated 67% and 78% survival, respectively. Only the survival difference between cyanoacrylate and PEG hydrogel reached statistical significance (p=0.02); the survival difference between BSP and PEG hydrogel did not reach statistical significance (p=0.07). Of the 3 deaths from the BSP group, 1 mouse died of perforation leak on day 1, 1 mouse died of failure to thrive on day 9 and likely had a partial obstruction at the transection site on necropsy, and 1 mouse died on day 7 of unknown causes. On necropsy, the perforation site was intact and without evidence of bowel leakage. There was no abscess formation present, and the intraabdominal cavity appeared normal. All other deaths in the 3 remaining groups were due to stool leakage at the perforation site.
Figure 2.
14-day survival for 4 groups of mice (n=9 to 10 in each group).
On histologic evaluation, there was a minimal inflammatory response in the serosal tissue for the fibrin glue and BSP; however the PEG hydrogel and cyanoacrylate produced a larger inflammatory response (Figure 3).
Figure 3.
Representative images of H&E histology (10× magnification) of mouse bowel at 14 days. A. Fibrin glue. B. PEG hydrogel. C. Cyanoacrylate. D. BSP. Arrows represent areas of increased inflammation.
2.2 Mouse Functional Study
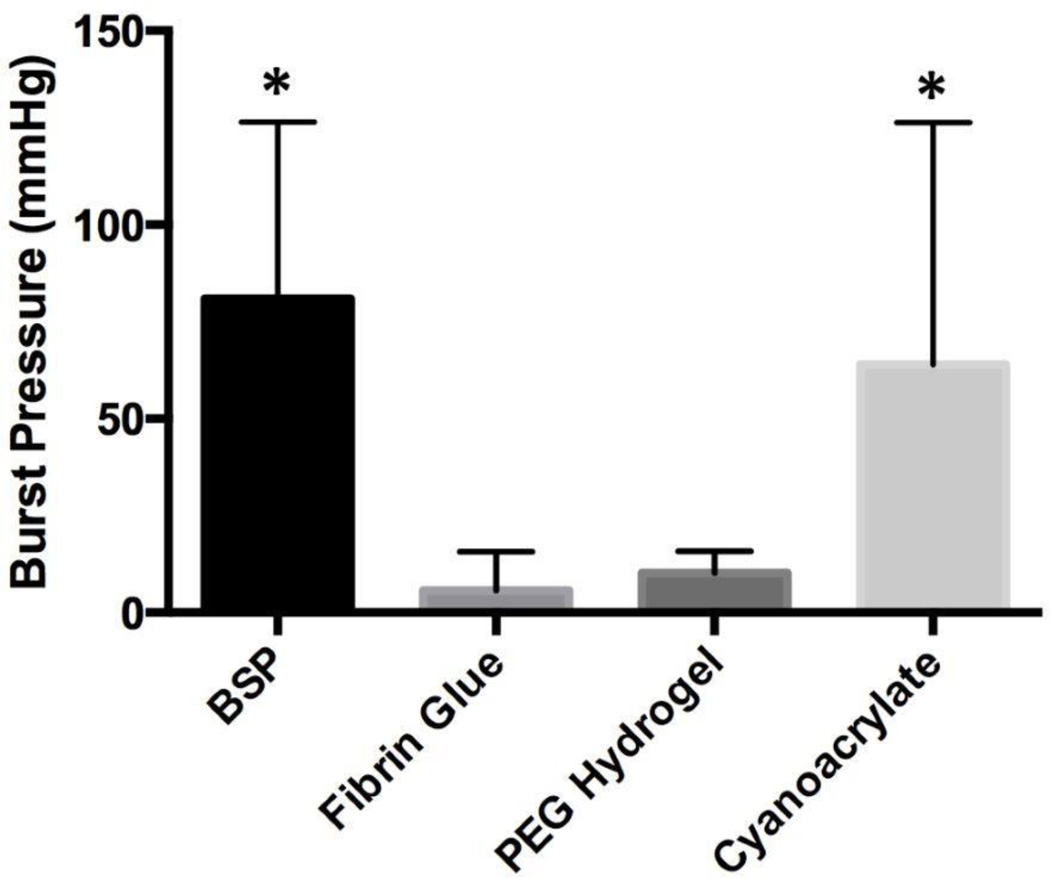
Of the 20 mice that underwent surgery for the non-survival study, 2 mice were excluded from analysis due to death prior to testing at 24 hours (1 from the fibrin glue group and 1 from the cyanoacrylate group). While the burst pressures for BSP and cyanoacrylate were comparable (mean pressure of 81.0±20.3 mmHg and 64.0±31.2 mmHg respectively, p=0.91), a statistical difference was seen between BSP and both fibrin glue and PEG hydrogel (mean pressure of 5.7±5.0 mmHg and 10.2±2.5 mmHg respectively, p=0.047 and p=0.047) (Figure 4). On observation at the time of necropsy for the non-survival study, stool was clearly visualized outside the cecum for 5 of the 5 PEG hydrogel mice.
Figure 4.
24-hour burst pressure measurements in 4 groups of mice (n=4 to 5 in each group). Error bars represent standard error. Asterisks represent statistically significant differences from the groups without asterisks.
2.3 Piglet Feasibility Study
Of the 4 piglets that underwent surgery, all 4 piglets survived and were healthy after 14 days. One piglet demonstrated a small bowel herniation at the incision site, and another piglet had a localized wound infection with abscess formation above the fascia. No piglet demonstrated anastomotic leak or evidence of peri-anastomotic abscess. Bowel was harvested at day 0 and day 14 of surgery. H&E histology revealed an increased inflammatory reaction at day 14 compared to day 0 (Figure 5).
Figure 5.
Representative images of H&E histology (10× magnification) of piglet bowel. A. Histology at day 0 of surgery demonstrated clear border of BSP fibers on serosal surface. B. Histology at day 14 of surgery demonstrated increased inflammatory reaction (arrow).
For burst pressures performed at the time of surgery, suture-only anastomoses demonstrated a mean burst pressure of 10.9±9.0 mmHg, while suture plus BSP anastomoses revealed an almost 4 fold higher burst pressure with a mean pressure of 36.5±4.6 mmHg (p=0.09).
3. Discussion
In a systematic review from 2013 evaluating tissue adhesives for gastrointestinal anastomoses, surgical sealants including fibrin glue showed some benefit for intestinal anastomoses in animal models. The products increased anastomotic burst pressures compared to suture controls but showed no major difference in clinical outcomes including anastomotic leak rate.[1] Furthermore, cyanoacrylate had conflicting results for small and large bowel anastomoses compared to controls, but had no significant benefit and an increased rate of inflammatory reaction and necrosis of tissue.[1]
Understanding the drawbacks of these commercially available products, we aimed to pre-clinically evaluate the feasibility and efficacy of BSP as a surgical sealant for bowel anastomoses. Using surgical sealants as an adjunct to sutured bowel anastomoses, we previously found the BSP performed as well as fibrin glue regarding survival and superior to PEG hydrogel and sutured control mice.[8] We also found a statistically significant increase in anastomotic burst pressure (over 3 fold higher) with BSP compared to fibrin glue, PEG hydrogel, and suture-only controls (p<0.05). In this current study as “proof of concept”, we further demonstrated that when no sutures were used, neither fibrin glue nor PEG hydrogel were able to provide sufficient support for partially transected cecum, while BSP and cyanoacrylate resulted in improved survival rates. The relatively higher anastomotic burst pressures of BSP and cyanoacrylate groups compared to the other sealants supported and correlated with improved survival outcomes. The results from the piglet model conceptually confirmed the practicality and feasibility of BSP as a surgical sealant for gastrointestinal anastomoses. Ultimately the aim would be to translate these findings to improve surgical care of infants and children undergoing bowel surgery.
Several clinical observations were made comparing the various surgical sealants. Whereas the application of the BSP using a simple airbrush is quite targeted and easily applied, the three commercially available products used were relatively difficult to apply to a small, specific area. The PEG hydrogel was noted to be too fluid and ran off tissue surfaces easily, while the fibrin glue product tended to set quickly and adhere poorly to the tissue. Both of these sealants lacked significant adhesiveness to the damp tissue of bowel serosa. Additionally, while cyanoacrylate glue was found to be adhesive to tissue, it also would adhere to unwanted surrounding structures. It was quite difficult to separate the unwanted adhesions without significant tissue damage. The BSP showed good adherence to slightly damp tissue that increased as the polymer warmed to body temperature. Furthermore, if unwanted polymer was applied, BSP could be easily peeled off tissue surfaces.
Another significant problem with both the cyanoacrylate and PEG hydrogel sealants was the increased inflammatory reaction seen on mouse bowel histology at 14 days. While it is unclear as to why an increased reaction was seen with synthetic PEG hydrogel (other than the nature of foreign bodies), an increased inflammatory reaction is a common observation with cyanoacrylate.[6,11,12] Notably, increased inflammation was present on the serosal surface of piglet bowel at 14 days compared to 0 days with the BSP, but no comparisons have currently been performed with other sealants or controls. Therefore, it is unknown as of yet if the increased inflammation is consistent with normal healing from a sutured anastomosis or if BSP caused excess inflammation independent to normal intestinal healing. Future studies on inflammatory reaction are certainly warranted to characterize this outcome.
We have attempted to identify limitations of the solution blow spun polymer blend for surgical use. Similar to most sealants, the PLGA/PEG polymer blend in BSP is unable to adhere to completely wet surfaces; however it is able to adhere to slightly damp surfaces such as the serosa of bowel. Further optimization of the polymer blend may allow for increased adhesiveness to wet surfaces. Additionally, from the mouse survival study, one mouse died of failure to thrive and what appeared to be obstruction at the perforation site with dilation of bowel proximally. Although BSP is a light-weight substance, it is possible that adhesions to this site may have resulted in small bowel obstruction.
4. Conclusions
Solution blow spun polymer may be a feasible, clinically effective, and straight-forward technique for use as a surgical sealant for intestinal anastomoses. Future studies are still warranted to compare this technology with currently available products in pre-clinical large animal intestinal anastomosis. Additionally, with the versatility of BSP as a sealant, further clinical uses should be explored for surgical application.
Acknowledgments
Financial Support/Disclosures: Research reported in this publication is supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number R01EB019963. This work is also supported by grants from Sheikh Zayed Institute for Pediatric Surgical Innovation and Joseph E. Robert Endowment at Children’s National Health System. The funders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. A.M.B. was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award No. F31EB019289. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- BSP
solution blow spun polymer
- PEG
polyethylene glycol
- PLGA
poly(lactic-co-glycolic acid)
- H&E
hematoxylin and eosin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vakalopoulos KA, Daams F, Wu Z, Timmermans L, Jeekel JJ, Kleinrensink GJ, et al. Tissue adhesives in gastrointestinal anastomosis: a systematic review. J Surg Res. 2013;180:290–300. doi: 10.1016/j.jss.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 2.Slieker JC, Vakalopoulos KA, Komen NA, Jeekel J, Lange JF. Prevention of leakage by sealing colon anastomosis: experimental study in a mouse model. J Surg Res. 2013;184:819–824. doi: 10.1016/j.jss.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Spotnitz WD, Burks S. Hemostats, sealants, and adhesives III: a new update as well as cost and regulatory considerations for components of the surgical toolbox. Transfusion. 2012;52:2243–2255. doi: 10.1111/j.1537-2995.2012.03707.x. [DOI] [PubMed] [Google Scholar]
- 4.Spotnitz WD, Burks S. Hemostats, sealants, and adhesives: components of the surgical toolbox. Transfusion. 2008;48:1502–1516. doi: 10.1111/j.1537-2995.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 5.Traver MA, Assimos DG. New generation tissue sealants and hemostatic agents: Innovative urologic applications. Reviews in urology. 2006;8:104–111. [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z, Boersema GSA, Vakalopoulos KA, Daams F, Sparreboom CL, Kleinrensink G, et al. Critical analysis of cyanoacrylate in intestinal and colorectal anastomosis. J Biomed Mater Res Part B Appl Biomater. 2014;102B:635–642. doi: 10.1002/jbm.b.33039. [DOI] [PubMed] [Google Scholar]
- 7.Behrens AM, Casey BJ, Sikorski MJ, Wu KL, Tutak W, Sandler AD, et al. In situ deposition of PLGA nanofibers via solution blow spinning. ACS Macro Lett. 2014;3:249–254. doi: 10.1021/mz500049x. [DOI] [PubMed] [Google Scholar]
- 8.Behrens AM, Lee NG, Casey BJ, Srinivasan P, Sikorski MJ, Daristotle JL, et al. Biodegradable-Polymer-Blend-Based Surgical Sealant with Body-Temperature-Mediated Adhesion. Adv Mater. 2015 doi: 10.1002/adma.201503691. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zilling TL, Jansson O, Walther BS, Ottosson A. Sutureless small bowel anastomoses: experimental study in pigs. Eur J Surg. 1999;165:61–68. doi: 10.1080/110241599750007522. [DOI] [PubMed] [Google Scholar]
- 10.Kanellos I, Mantzoros I, Demetriades H, Kalfadis S, Sakkas L, Kelpis T. Sutureless colonic anastomosis in the rat: a randomized controlled study. Tech Coloproctol. 2002;6:143–146. doi: 10.1007/s101510200033. [DOI] [PubMed] [Google Scholar]
- 11.Nursal TZ, Anarat R, Bircan S, Yildirim S, Tarim A, Haberal M. The effect of tissue adhesive, octyl-cyanoacrylate, on the healing of experimental high-risk and normal colonic anastomoses. Am J Surg. 2004;187:28–32. doi: 10.1016/j.amjsurg.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Kayaoglu HA, Ersoy OF, Ozkan N, Celik A, Filiz NO. Effect of n-butyl-2-cyanoacrylate on high-risk colonic anastomoses. Kaohsiung J Med Sci. 2009;25:177–183. doi: 10.1016/S1607-551X(09)70058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]