Supplemental Digital Content is available in the text
Keywords: adverse events, antidepressant, efficacy, meta-analysis, Parkinson disease
Abstract
Background:
Parkinson disease (PD) was considered as the 2nd most prevalent neurodegenerative disorder after Alzheimer disease, while depression is a prevailing nonmotor symptom of PD. Typically used antidepression medication includes tricyclic antidepressants (TCA), selective serotonin reuptake inhibitors (SSRI), serotonin and norepinephrine reuptake inhibitors (SNRI), monoamine-oxidase inhibitors (MAOI), and dopamine agonists (DA). Our study aimed at evaluating the efficacy of antidepressive medications for depression of PD.
Methods:
Web of Science, PubMed, Embase, and the Cochrane library were searched for related articles. Traditional meta-analysis and network meta-analysis (NMA) were performed with outcomes including depression score, UPDRS-II, UPDRS-III, and adverse effects. Surface under the cumulative ranking curve (SUCRA) was also performed to illustrate the rank probabilities of different medications on various outcomes. The consistency of direct and indirect evidence was also assessed by node-splitting method.
Results:
Results of traditional pairwise meta-analysis were performed. Concerning depression score, significant improvement was observed in AD, MAOI, SSRI, and SNRI compared with placebo. NMA was performed and more information could be obtained. DA was illustrated to be effective over placebo concerning UPDRS-III, MAOI, and SNRI. DA demonstrated a better prognosis in UPDRS-II scores compared with placebo and MAOI. However, DA and SSRI demonstrated a significant increase in adverse effects compared with placebo. The SUCRA value was calculated to evaluate the ranking probabilities of all medications on investigated outcomes, and the consistency between direct and indirect evidences was assessed by node-splitting method.
Conclusion:
SSRI had a satisfying efficacy for the depression of PD patients and could improve activities of daily living and motor function of patient but the adverse effects are unneglectable. SNRI are the safest medication with high efficacy for depression as well while other outcomes are relatively poor.
1. Introduction
Parkinson disease (PD) is considered as the 2nd most prevalent neurodegenerative disorder after Alzheimer disease.[1] Age is the greatest risk factor of PD, the incidence rate of PD generally increases with it, peaking at 104.99 per 100,000 persons for female and 132.72 for male between the age of 70 to 79.[2] The onset of PD is related to the degeneration of dopaminergic neurons in the substantia nigra as well as the development of Lewy bodies in dopaminergic neurons. Gradually in a long time of 2 decades or more, pathological changes in neurons may precede into both motor and nonmotor system manifestations.[3] In the motor system, PD is associated with rest tremor, bradykinesia, muscular rigidity, and postural instability. In the nonmotor system, cognitive changes, behavioral or neuropsychiatric changes, pain and fatigue, autonomic dysfunction, psychosis and hallucinations, sleep disorder, depression, and anxiety are also prevailing symptoms of PD patients.[4]
The estimated prevalence of depression as a symptom of PD ranged from 7% to 76%, as a result of inconsistent sampling procedures, assessment techniques, and definitions of depression,[5] and depression greatly eroded the lining quality of PD patients. There is evidence that depression is underrecognized and undertreated in clinical practice, so that the etiology of depression in PD has not been elucidated yet, but exogenously, being diagnosed with a disabling and noncurable disease can be a shock for the patients and results in the state of depression, while depression may also be associated with the neurological changes in the disease process.[6] Currently, treatment for depression of PD includes antidepressive medications, behavioral interventions such as psychotherapy, electroconvulsive therapy, repetitive transcranial magnetic stimulation, and deep brain stimulation.
Typically used antidepression medication includes tricyclic antidepressants (TCA), selective serotonin reuptake inhibitors (SSRI), serotonin and norepinephrine reuptake inhibitors (SNRI), monoamine-oxidase inhibitors (MAOI), and dopamine agonists (DA). Many clinical trials have been conducted to investigate their therapeutic effect on depression of PD. Barone et al[7] reported in their randomized trial that treatment with rasagiline, an MAOI, did not help to improve depressive symptoms in PD patients. Atomoxetine, an SNRI, was reported to be not efficacious for depression of PD, but might help to improve cognitive disorder and daytime sleepiness.[8] Yet pramipexole, a DA was found to be able to improve depressive symptoms in patients with PD, through a direct antidepressant effect.[9] A randomized clinical trial in the USA also found that nortriptyline,[10] a TCA, was efficacious in the treatment of depressive symptoms, but not paroxetine, an SSRI.[10] However, sample sizes of previous studies were relatively limited. The assessment of depression and depression scale were not in consistence with each other. Thus, a large-scaled meta-analysis was needed to help interpret data from previous trials. In the present study, we aimed at evaluating the efficacy of antidepressants on depression of PD patients with 4 endpoints.
2. Material and methods
2.1. Search strategy
Web of Science, PubMed, Embase, and the Cochrane library were searched for related articles concerning the therapeutic value of antidepression drugs for PD. All typical antidepression drugs were enrolled in the screening of relevant articles, including TCA, SSRI, SNRI, MAOI, and DA. Articles published between January 1, 1980 and September 1, 2016 were retrieved in the primary search. The following Mesh terms and their synonyms and abbreviations were used to find relevant studies in PubMed: “Parkinson Disease,” “antidepressive agents,” “tricyclic antidepressants,” “selective serotonin reuptake inhibitors,” “serotonin and norepinephrine reuptake inhibitors,” “dopamine agonists,” and “monoamine-oxidase inhibitors” (Table S1). Two authors independently screened titles and abstracts of retrieved articles to evaluate their qualification according to the inclusion criteria. Reference list of enrolled articles were also reviewed manually to improve the integrity of this study. The analysis was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.[11]
2.2. Evaluated outcomes and inclusion criteria
In the present study, a depression score was applied to evaluate the symptom of depression on patients. The depression score is based on Hamilton depression rating scale, Beck depression inventory, and Unified Parkinson Disease Rating Scale – Mental. Unified Parkinson Disease Rating Scale-Activities of daily living (UPDRS-II) for activities of daily living, Unified Parkinson Disease Rating Scale-Motor (UPDRS-III) for motor function, and adverse effect were evaluated as the secondary outcomes.
Inclusion criteria for retrieved studies was as follows: patients should be diagnosed with idiopathic PD; symptoms of depression were diagnosed clearly, with severity of depression evaluated by Hamilton depression rating scale, Beck Depression Inventory, or Unified Parkinson Disease Rating Scale – Mental; study should be performed with a randomized controlled design; sufficient data for further analysis should be provided in original articles; and patients should not receive irrelevant anti-Parkinson treatment in these studies.
2.3. Data extraction
Two authors extracted relevant data from eligible articles independently. In the current study, information as follows were extracted: last name of first author, year of publication, origin country, study design, number of subjects, time of follow-up (in weeks), age of subjects, duration of PD among the subjects (in years), average Hoehn and Yahr stage of subjects, treatments, and the evaluation scale of depression. A 3rd author would resolve discrepancies after discussion. Depression score was considered as the primary outcome in this study.
2.4. Statistical analysis
A traditional pair-wise meta-analysis was performed in order to evaluate the efficacy of different types of medication on depression. Standard mean deviation (SMD) and corresponding 95% credible interval (CrI) were calculated for depression score, UPDRS-II, and UPDRS-III. And for adverse effect, ORs and 95% CrI were calculated. The heterogeneity was evaluated by I2 test and Q statistics. Fixed-effect model was applied if significant heterogeneity was not observed in the ORs, while ORs with heterogeneity were calculated by random-effect model.
Consequently, Bayesian network meta-analysis (NMA) was performed with a random-effects model using Markov chain Monte Carlo methods in WinBUGS (MRC Bio-statistics Unit, Cambridge, UK) to compare direct and indirect evidence. Depression score, UPDRS-II, and UPDRS-III were represented by SMD and 95% CrI, and adverse effect represented by ORs and 95% CrI.
Besides, surface under the cumulative ranking curve (SUCRA) was created to evaluate the ranking probabilities for different medications on various outcomes.[12] Moreover, the consistency between direct and indirect evidence was assessed by node-splitting method; a P value less than .05 was deemed as inconsistent. STATA 12.0 (Stata Corp, College Station, TX) software was used in our analysis with a 2-side P less than .05 considered as significant.
3. Results
3.1. Study characteristics
A total of 8890 subjects from 45 publications were involved to investigate the efficacy of TCA, SSRI, SNRI, DA, and MAOI in patients with PD.[7–10,13–53] Flow chart in Fig. 1 illustrated the process of study selection. The following-up time of our enrolled studies ranged from 1 to 240 weeks with an average value of 31 weeks. Among the enrolled medications, nortriptyline, amitriptyline, and doxepin were categorized as TCA; fluoxetine, paroxetine, citalopram, sertraline, and desipramine were regarded as SSRI; venlafaxine, atomoxetine, and nefazodone were SNRI; MAOI included selegiline, rasagiline, and lazabemide; DA involved pramipexole, memantine, pergolide, ropinirole, pardoprunox, levodopa, bromocriptine, lisuride, piribedil, and cabergoline. Characteristics of enrolled articles were presented in Table 1. To clarify the comparisons involved in the NMA, a network plot was generated (Fig. 2). Numbers in the circles illustrated the number of subjects. The width of line is proportional to the total number of studies included. As indicated in the figure, MAOI and DA were investigated by large amount of studies, whereas TCA, SSRI, and SNRI obtained significantly fewer samples thus indicating a higher potential deviation in traditional meta-analysis.
Figure 1.
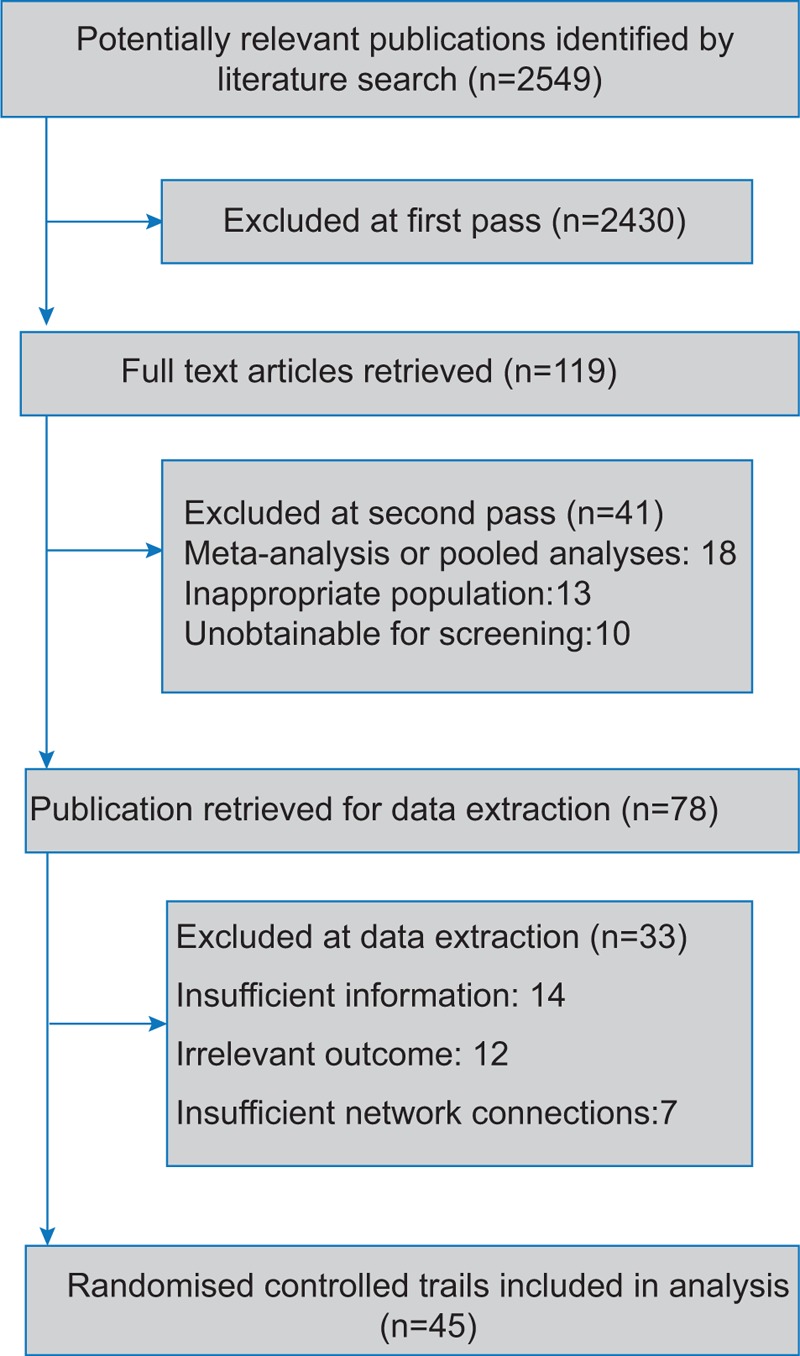
Flow chart.
Table 1.
Characteristics of studies included in the network meta-analysis.

Figure 2.
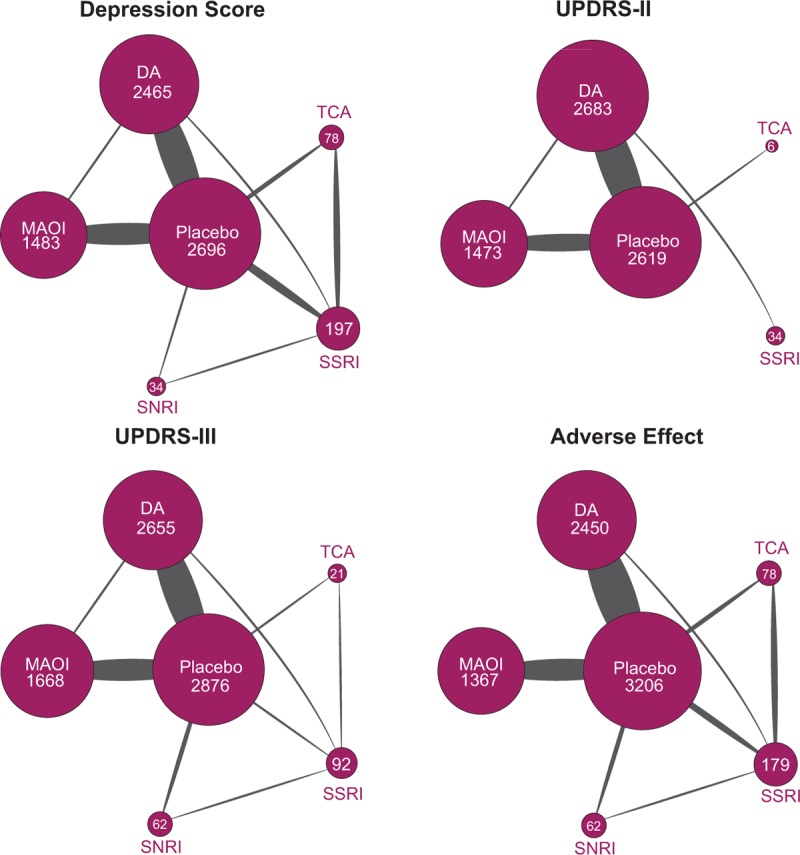
The network of included trials.
3.2. Pairwise meta-analysis results
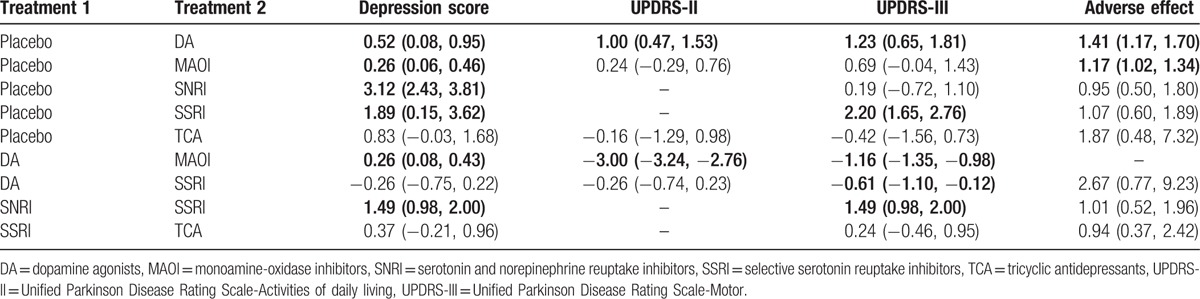
Results of traditional pairwise meta-analysis were listed in Table 2. As illustrated in the table, compared with placebo, patients taking DA were observed to have improvement on depression score, UPDRS-II, and UPDRS-III (SMD = 0.52, 95% CrI: [0.08, 0.95]; SMD = 1.00, 95% CrI: [0.47, 1.53]; and SMD = 1.23, 95% CrI: [0.65, 1.81]). However, the issue of adverse effects remained to be resolved (OR = 1.41, 95% CrI: [1.17, 1.70]). Besides, concerning depression score, significant improvement was also observed in MAOI (SMD = 0.26, 95% CrI: [0.06, 0.46]), SSRI (SMD = 3.12, 95% CrI: [2.43, 3.81]), and SNRI (SMD = 1.89, 95% CrI: [0.15, 3.62]). Moreover, in comparisons between DA and MAOI, significant efficacy of MAOI on depression score over DA was observed (MD = 0.26, 95% CrI: [0.08, 0.43]), whereas the improvement of activities of daily living and motor function was not as powerful as DA (UPDRS-II SMD = −3.00, 95% CrI: [−3.24, −2.76]; UPDRS-III SMD = −1.16, 95% CrI: [−1.35, −0.98]). Also, the results showed that SSRI were more effective than SNRI in relieving depression and impaired motor function (depression score: OR = 1.49, 95% CrI: [0.98, 2.00]; UPDRS-III: SMD = 1.49, 95% CrI: [0.98, 2.00]). MAOI also could lead to an increase in adverse effect (OR = 1.17, 95% CrI: [1.02, 1.34]).
Table 2.
Meta-analysis results for pair-wise comparisons.
3.3. NMA results
In additional to traditional meta-analysis, NMA was performed to promote result validity by merging direct and indirect evidences. Corresponding results were presented in Table 3 and plotted in Fig. 3 and Figure S1. In the assessment of depression score, all medication other than MAOI were observed to be significantly effective in treating depression (DA: SMD = −0.56, 95% CrI [−0.93, −0.2]; MAOI: SMD = −0.38, 95% CrI [−0.81, 0.06]; SNRI: SMD = −1.55, 95% CrI [−2.65, −0.45]; SSRI: SMD = −1.56, 95% CrI [−2.16, −0.96]; and TCA: SMD = −1.5, 95% CrI [−2.31, −0.7]). Interestingly, both SSRI and TCA were more significant than DA and MAOI in NMA, while traditional comparisons were not available as a result of limited sample size. Concerning UPDRS-III that represents improvement of motor function, DA were illustrated to be effective over placebo, MAOI, and SNRI (placebo vs DA: SMD = −4.09, 95% CrI [−5.60, −2.69]; DA vs MAOI: SMD = 3.32, 95% CrI [1.18, 5.50]; DA vs SNRI: SMD = 4.29, 95% CrI [0.46, 8.40]). DA were also observed to be the only medication that demonstrated a better prognosis in UPDRS-II scores for activities of daily living compared with placebo and MAOI (placebo vs DA: SMD = −1.53, 95% CrI: [−2.15, −0.93]; DA vs MAOI: SMD = 1.46, 95% CrI: [0.46, 2.49]). However, DA and SSRI demonstrated a significant increase in adverse effects compared with placebo (OR = 2.29, 95% CrI: [1.67, 3.17]; OR = 2.58, 95% CrI: [1.00, 6.77], respectively).
Table 3.
Network meta-analysis results according to depression score represented by SMD and 95% CrI, UPDRS-II and UPDRS-III represented by MD and 95% CrI, and adverse effect represented by ORs and 95% CrI.
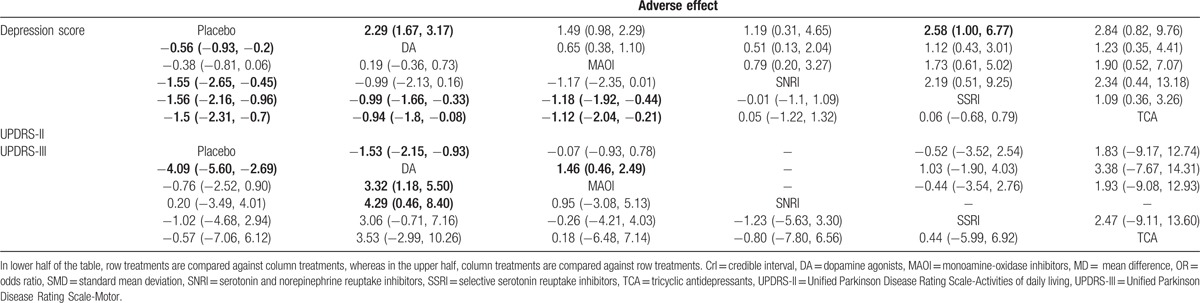
Figure 3.
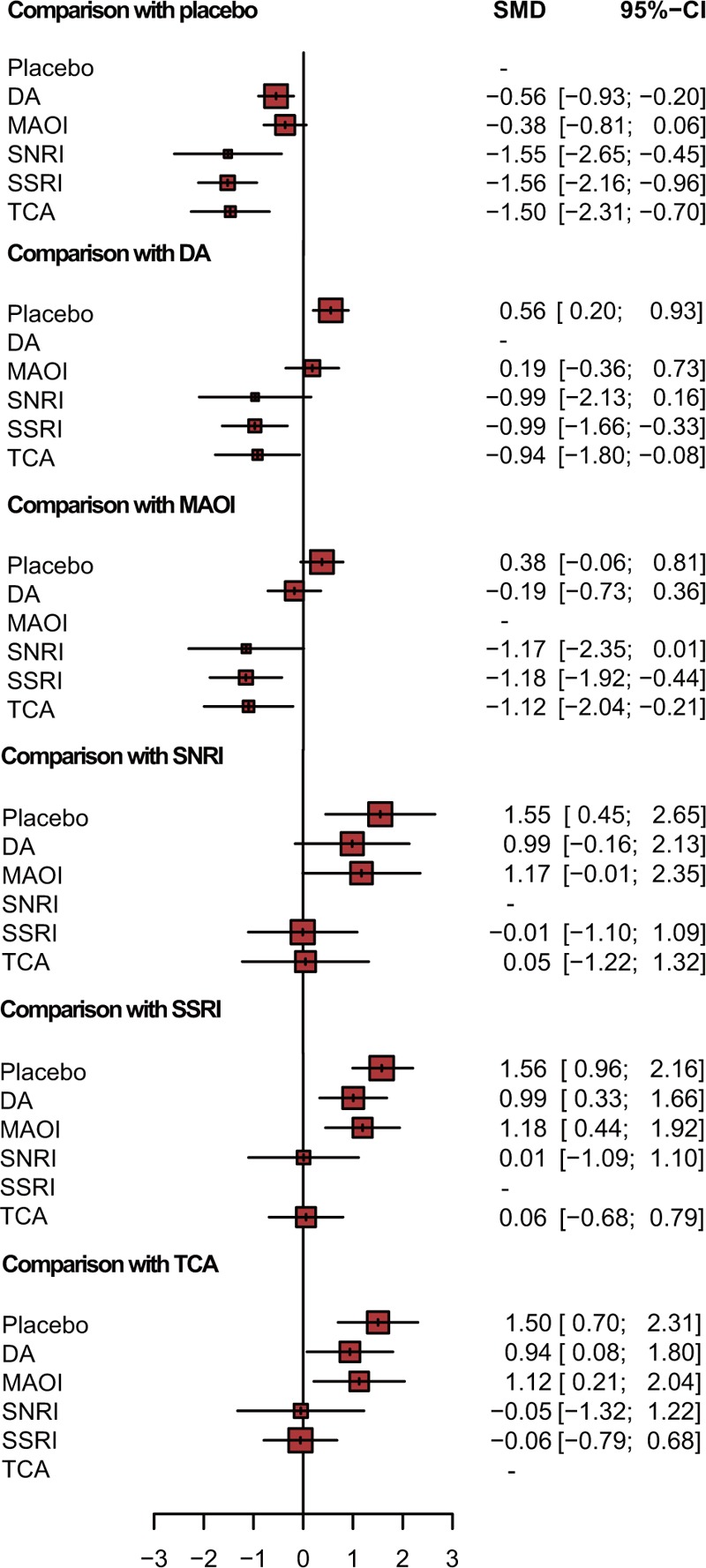
Forest plot for depression score.
3.4. Ranking probability
To better understand the results, the SUCRA value was calculated to evaluate the ranking probabilities of all medications on investigated outcomes. Results were presented in Fig. 4 and Table 4. As suggested by ranking probabilities, SSRI were the most effective medication for depression in patients with PD (0.800), SNRI and TCA were also among the best (0.746 and 0.740 independently). Regarding to the improvement in UPDRS-II and UPDRS-III, DA was the most helpful one (0.873 for UPDRS-II and 0.958 for UPDRS-III). SSRIs ranked the 2nd (0.533 for UPDRS-II and 0.518 for UPDRS-III) and MAOI ranked the 3rd (0.400 for UPDRS-II and 0.492 for UPDRS-III). In the aspect of adverse effect, SNRI were the safest (0.714), whereas patients taking SSRI and TCA were more likely to suffer from adverse effects. A clustered ranking plot based on SUCRA values was also generated and presented NMA results visually in Fig. 5.
Figure 4.
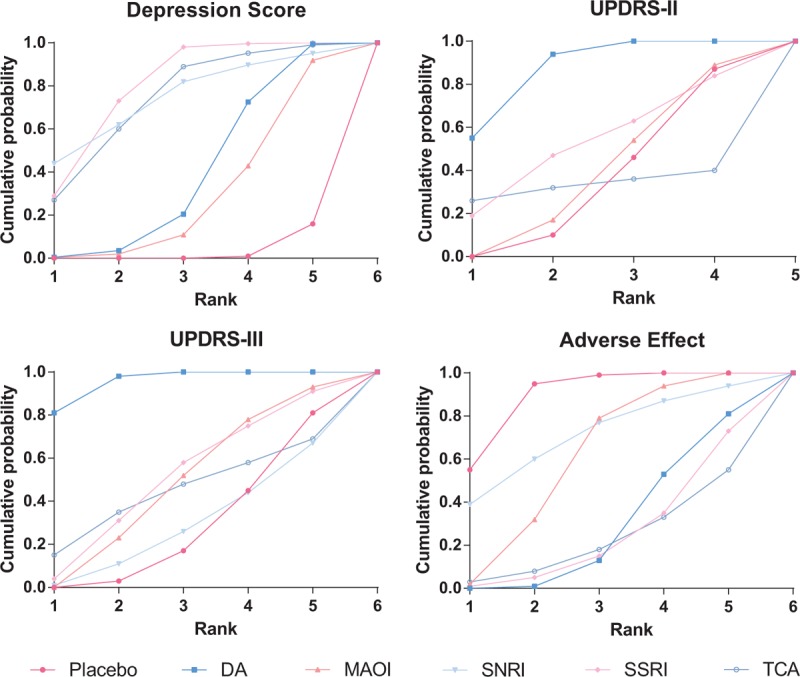
SUCRA of depression score, UPDRS-II, UPDRS-III, and adverse effect. SUCRA = surface under the cumulative ranking curve, UPDRS-II = Unified Parkinson Disease Rating Scale-Activities of daily living, UPDRS-III = Unified Parkinson Disease Rating Scale-Motor.
Table 4.
SUCRA results of depression score, UPDRS-II, UPDRS-III, and adverse effect.
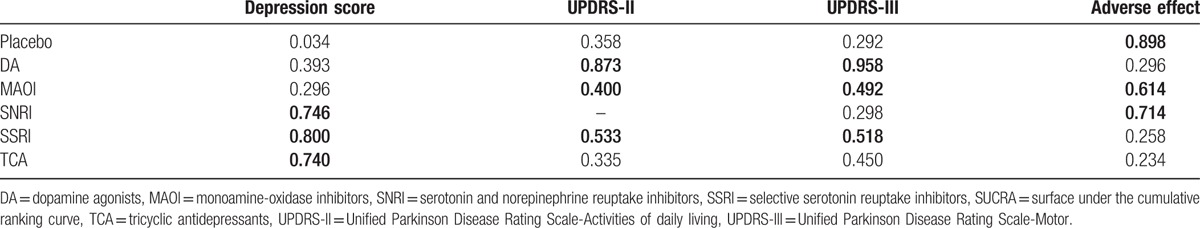
Figure 5.
Clustered ranking plot of the network. The plot is based on cluster analysis of surface under the cumulative ranking curves (SUCRA) values. Each plot shows SUCRA values for 2 outcomes. Each color represents a group of treatments that belong to the same cluster. Treatments lying in the upper right corner are more effective and safe than the other treatments.
3.5. Consistency analysis
The consistency between direct and indirect evidences was evaluated by node-splitting method. As listed in Table 5 and Fig. S2, significant difference between evidences was observed in the comparison on depression score between placebo and SNRI, as well as the comparison between SSRI and SNRI (both P < .001). DA and MAOI also presented significant inconsistency with respect to UPDRS-II. To further clarify the source of inconsistency, the net heat plot was generated and presented in Fig. 6.
Table 5.
Results of direct and indirect comparisons according to depression score.
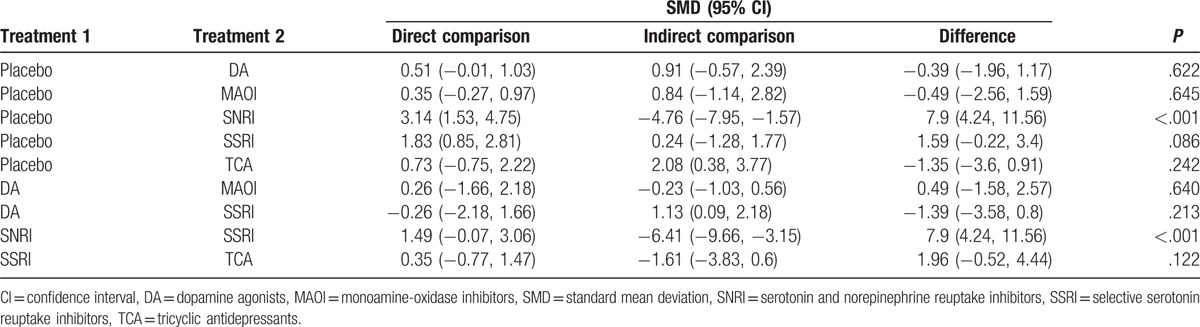
Figure 6.
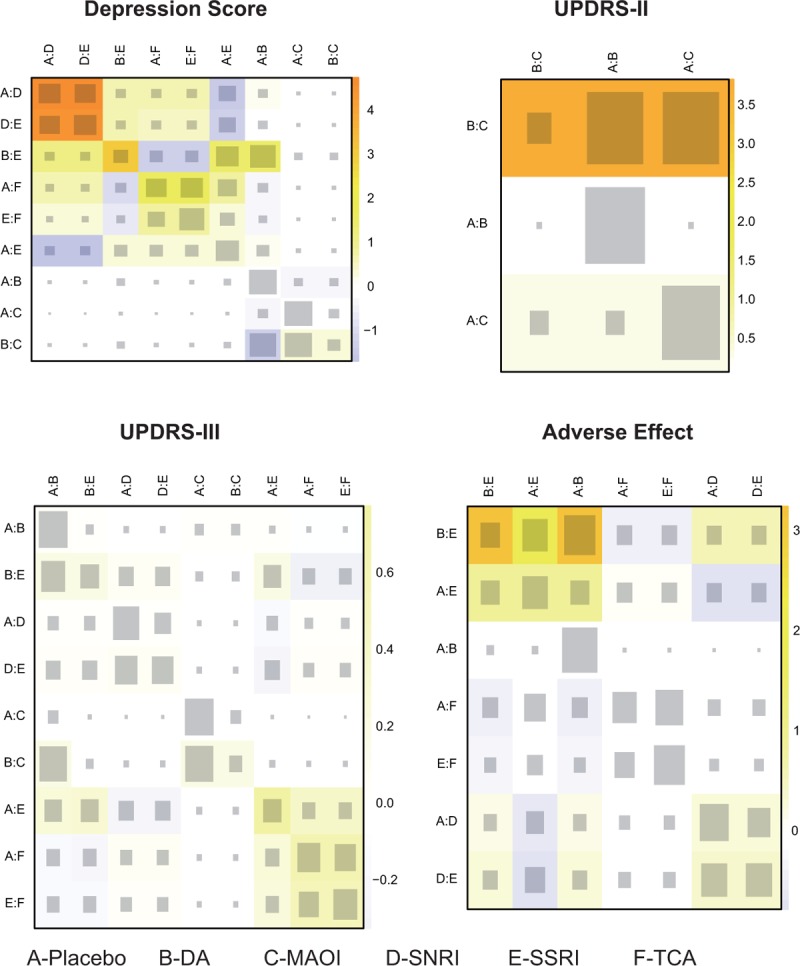
Net heat plot. The size of the gray squares indicates the contribution of the direct evidence (shown in the column) to the network evidence (shown in the row). The colors are associated with the change in inconsistency between direct and network evidence. Blue colors indicate an increase of inconsistency and warm colors indicate a decrease.
4. Discussion
A total of 8890 subjects from 45 studies were enrolled in the analysis, and the therapeutic efficacy of common used antidepressive medication on PD was investigated. And all of the enrolled researches guaranteed the exclusion of subjects who had irrelevant antidepressants and anti-Parkinson treatment before and during the original studies. Antidepressants have been widely used in clinical practice to alleviate depression. Additionally, neural plasticity may also be regulated by antidepressants in the diseased brain, which potentially slows disease progression in PD.[54] The high efficacy in improving depression was observed in SSRI, SNRI, and TCA. However, the adverse effects of SSRI need to be taken into account. SNRI was among the safest medication with few reports of adverse effects, yet it may not help to relieve other symptoms of PD. The efficacy of DA and MAOI was not as significant as other medication, but great improvement in activities of daily living and motor function was observed.
TCA exerts symptomatic benefits in depression score but was reported to be associated with a delay in reaching the end point of need to start dopaminergic therapy,[55] which also resulted in a higher adverse effect in our NMA. DA is proved to function through Nurr1, which plays an essential role in midbrain dopaminergic neurons development and survival, thus being a potential target for PD,[4] and our NMA also demonstrated an excellent performance in improving UPDRS-II and UPDRS-III scores. However, our NMA also suggests DA had a poor efficacy in our primary outcome and could result in a high adverse effect, it has been reported by Kataoka et al[1] that an increased dose of DA could trigger tactile hallucinations.
SSRI exhibited outstanding efficacy in our NMA, not only illustrated an outstanding performance in daily living as well as motor function, but also presented a high curative effect in depression score. It is confirmed by Kostic et al[4] that fluoxetine (SSRI) significantly reduced depression in PD patients while no motor performances were impaired. However, unlike other antidepressants, the use of SSRI was associated with greater apathy,[5] which is agreed by our study that SSRI also presented a high adverse effect. It is demonstrated that prolonged SSRI could provide enduring antidyskinetic effects through 5-HT (1A) receptors and enhance striatal dopamine levels to maintain L-DOPAs anti-Parkinsonian efficacy,[56] which could possibly share the cause of adverse events with DA. Due to their promising performances, SSRI and TCA were the 2 most traditionally administered psychiatric medications for depression and anxiety in PD.
SNRI also presented a good performance in depression score and had no obvious side effects, but its results regarding UPDRS are poor. However, there are cases suggested that SNRI may act as substitution therapy for depression in PD that had inadequate response to SSRI.[3] Accordingly, the latest NMA by Liu et al[57] based on 11 trails concluded that SNRI and TCA had favorable balance between benefits and acceptability, which is concurred with our results. Moreover, MAOI was identified as one of the safest medications, which concur with Frisina et al[58] that selegeline (MAOI) does not produce a risk of substantial side effects or mortality for patients with PD.
Apart from previous results, inconsistency was widely observed among studies. Although uncertainty on SSRI efficacy in depression is reported,[59] this uncertainty was not observed in our consistency analysis. Besides, different studies could draw contradictive conclusions. For example, Bomasang-Layno et al[60] concluded from 13 trials that SSRI could significantly improve depression of PD with high efficacy, which is consistence with our results. Yet Troeung et al[61] observed in their study that the pooled effects of antidepressive medication in PD were insignificant, which involved 9 clinical trials. Rocha et al[62] also found that the results about antidepressant efficacy on depression of PD were unstable, and Frisina et al[58] stated that the SSRI literature in their study might have suffered from sampling error. Although there existed such deviations, with a distinctively larger sample size of 45 trials, our results guaranteed a more robust conclusion, and the accumulation of evidence from randomized clinical trials could lead to a more precise conclusion on the efficacy of antidepressants on PD.
The primary limitation of this analysis relates to the limited sample size of involved drugs and subjects, especially for TCA and SNRI. Also, the average duration of follow-time was 31 weeks, whereas the follow-up time in more than half of the enrolled studies was less than 15 weeks, which is possibly not long enough to show complete effects, thus a longer follow-up time is demanded. Fortunately, short follow-up time in Bodkin and Devos studies is unlikely to influence the accuracy of the conclusion, because there is other evidence of comparison between involved treatments in our current study. In addition, it has been suggested that a more unified depression diagnostic criteria should established to assess depression accurately and indicates an internal inconsistency.[6] Larger and well-designed clinical trials on the efficacy of antidepressant on patients with PD are needed for further investigation.
In conclusion, we observed in our meta-analysis that SSRI had a satisfying efficacy for depression of PD patients. They can also help to improve activities of daily living and motor function of patients, yet the adverse effects were also distinctive. SNRI are the safest medication with high efficacy for depression as well. SNRI and TCA are also good at improving depression scores while DA and MAOI tended to have better performance in other symptoms in PD. Larger clinical trials on the efficacy of antidepressant on patients with PD are needed for further investigation.
Supplementary Material
Footnotes
Abbreviations: CrI = credible interval, DA = dopamine agonists, MAOI = monoamine-oxidase inhibitors, NMA = network meta-analysis, PD = Parkinson disease, SMD = standard mean deviation, SNRI = serotonin and norepinephrine reuptake inhibitors, SSRI = selective serotonin reuptake inhibitors, SUCRA = surface under the cumulative ranking curve, TCA = tricyclic antidepressants.
Declaration of interest: There were no conflicts of interest in the conduct, analysis, and publishing of this manuscript among study authors.
Funding/support: The study is supported by Chinese Postdoctoral Science Foundation (2012M20585 to CJZ) and Science and technology fund of Tianjin Health Bureau (2014KR02 to CJZ).
Ethics Statement: Not necessary.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Kataoka H, Sawa N, Sugie K, et al. Can dopamine agonists trigger tactile hallucinations in patients with Parkinson's disease? J Neurol Sci 2014;347:361–3. [DOI] [PubMed] [Google Scholar]
- [2].Zhang LM, Sun CC, Mo MS, et al. Dopamine agonists exert Nurr1-inducing effect in peripheral blood mononuclear cells of patients with Parkinson's disease. Chin Med J (Engl) 2015;128:1755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Takahashi H, Kamata M, Yoshida K, et al. Remarkable effect of milnacipran, a serotonin-noradrenalin reuptake inhibitor (SNRI), on depressive symptoms in patients with Parkinson's disease who have insufficient response to selective serotonin reuptake inhibitors (SSRIs): two case reports. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:351–3. [DOI] [PubMed] [Google Scholar]
- [4].Kostic V, Dzoljic E, Todorovic Z, et al. Fluoxetine does not impair motor function in patients with Parkinson's disease: correlation between mood and motor functions with plasma concentrations of fluoxetine/norfluoxetine. Vojnosanit Pregl 2012;69:1067–75. [PubMed] [Google Scholar]
- [5].Zahodne LB, Bernal-Pacheco O, Bowers D, et al. Are selective serotonin reuptake inhibitors associated with greater apathy in Parkinson's disease? J Neuropsychiatry Clin Neurosci 2012;24:326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marsh L, McDonald WM, Cummings J, et al. Provisional diagnostic criteria for depression in Parkinson's disease: report of an NINDS/NIMH Work Group. Mov Disord 2006;21:148–58. [DOI] [PubMed] [Google Scholar]
- [7].Barone P, Santangelo G, Morgante L, et al. A randomized clinical trial to evaluate the effects of rasagiline on depressive symptoms in non-demented Parkinson's disease patients. Eur J Neurol 2015;22:1184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weintraub D, Mavandadi S, Mamikonyan E, et al. Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology 2010;75:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barone P, Poewe W, Albrecht S, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:573–80. [DOI] [PubMed] [Google Scholar]
- [10].Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 2009;72:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91–2. [DOI] [PubMed] [Google Scholar]
- [12].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [13].Group PS. DATATOP: a multicenter controlled clinical trial in early Parkinson's disease. Parkinson Study Group. Arch Neurol 1989;46:1052–60. [DOI] [PubMed] [Google Scholar]
- [14].Group TIPS. A multicenter Italian randomised study on early treatment of Parkinson disease: comparison of L-dopa, l-deprenyl and dopaminoagonists. Study design and short term results. The Italian Parkinson Study Group. Ital J Neurol Sci 1992;13:735–9. [DOI] [PubMed] [Google Scholar]
- [15].Allain H, Pollak P, Neukirch HC. Symptomatic effect of selegiline in de novo Parkinsonian patients. The French Selegiline Multicenter Trial. Mov Disord 1993;8(Suppl 1):S36–40. [DOI] [PubMed] [Google Scholar]
- [16].Group PS. A controlled trial of lazabemide (RO19-6327) in untreated Parkinson's disease. Parkinson Study Group. Ann Neurol 1993;33:350–6. [DOI] [PubMed] [Google Scholar]
- [17].Group PS. A controlled trial of lazabemide (Ro 19-6327) in levodopa-treated Parkinson's disease. Parkinson Study Group. Arch Neurol 1994;51:342–7. [DOI] [PubMed] [Google Scholar]
- [18].Dalrymple-Alford JC, Jamieson CF, Donaldson IM. Effects of selegiline (deprenyl) on cognition in early Parkinson's disease. Clin Neuropharmacol 1995;18:348–59. [DOI] [PubMed] [Google Scholar]
- [19].Group PS. Effect of lazabemide on the progression of disability in early Parkinson's disease. The Parkinson Study Group. Ann Neurol 1996;40:99–107. [DOI] [PubMed] [Google Scholar]
- [20].Group PS. Safety and efficacy of pramipexole in early Parkinson disease. A randomized dose-ranging study. Parkinson Study Group. JAMA 1997;278:125–30. [DOI] [PubMed] [Google Scholar]
- [21].Larsen JP, Boas J. The effects of early selegiline therapy on long-term levodopa treatment and parkinsonian disability: an interim analysis of a Norwegian–Danish 5-year study. Norwegian–Danish Study Group. Mov Disord 1997;12:175–82. [DOI] [PubMed] [Google Scholar]
- [22].Wermuth L, Sørensen P, Timm S, et al. Depression in idiopathic Parkinson's disease treated with citalopram: a placebo-controlled trial. Nordic J Psychiatry 1998;52:163–9. [Google Scholar]
- [23].Larsen JP, Boas J, Erdal JE. Does selegiline modify the progression of early Parkinson's disease? Results from a five-year study. The Norwegian–Danish Study Group. Eur J Neurol 1999;6:539–47. [DOI] [PubMed] [Google Scholar]
- [24].Pinter MM, Pogarell O, Oertel WH. Efficacy, safety, and tolerance of the non-ergoline dopamine agonist pramipexole in the treatment of advanced Parkinson's disease: a double blind, placebo controlled, randomised, multicentre study. J Neurol Neurosurg Psychiatry 1999;66:436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bodkin JA, Amsterdam JD. Transdermal selegiline in major depression: a double-blind, placebo-controlled, parallel-group study in outpatients. Am J Psychiatry 2002;159:1869–75. [DOI] [PubMed] [Google Scholar]
- [26].Pogarell O, Gasser T, van Hilten JJ, et al. Pramipexole in patients with Parkinson's disease and marked drug resistant tremor: a randomised, double blind, placebo controlled multicentre study. J Neurol Neurosurg Psychiatry 2002;72:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Serrano-Duenas M. [A comparison between low doses of amitriptyline and low doses of fluoxetin used in the control of depression in patients suffering from Parkinson's disease]. Rev Neurol 2002;35:1010–4. [PubMed] [Google Scholar]
- [28].Shoulson I, Oakes D, Fahn S, et al. Impact of sustained deprenyl (selegiline) in levodopa-treated Parkinson's disease: a randomized placebo-controlled extension of the deprenyl and tocopherol antioxidative therapy of parkinsonism trial. Ann Neurol 2002;51:604–12. [DOI] [PubMed] [Google Scholar]
- [29].Amsterdam JD. A double-blind, placebo-controlled trial of the safety and efficacy of selegiline transdermal system without dietary restrictions in patients with major depressive disorder. J Clin Psychiatry 2003;64:208–14. [DOI] [PubMed] [Google Scholar]
- [30].Navan P, Findley LJ, Jeffs JA, et al. 3-month parallel study of the effects of pramipexole, pergolide, and placebo on Parkinsonian tremor. Mov Disord 2003;18:1324–31. [DOI] [PubMed] [Google Scholar]
- [31].Ziegler M, Castro-Caldas A, Del Signore S, et al. Efficacy of piribedil as early combination to levodopa in patients with stable Parkinson's disease: a 6-month, randomized, placebo-controlled study. Mov Disord 2003;18:418–25. [DOI] [PubMed] [Google Scholar]
- [32].Stern MB, Marek KL, Friedman J, et al. Double-blind, randomized, controlled trial of rasagiline as monotherapy in early Parkinson's disease patients. Mov Disord 2004;19:916–23. [DOI] [PubMed] [Google Scholar]
- [33].Moller JC, Oertel WH, Koster J, et al. Long-term efficacy and safety of pramipexole in advanced Parkinson's disease: results from a European multicenter trial. Mov Disord 2005;20:602–10. [DOI] [PubMed] [Google Scholar]
- [34].Antonini A, Tesei S, Zecchinelli A, et al. Randomized study of sertraline and low-dose amitriptyline in patients with Parkinson's disease and depression: effect on quality of life. Mov Disord 2006;21:1119–22. [DOI] [PubMed] [Google Scholar]
- [35].Barone P, Scarzella L, Marconi R, et al. Pramipexole versus sertraline in the treatment of depression in Parkinson's disease: a national multicenter parallel-group randomized study. J Neurol 2006;253:601–7. [DOI] [PubMed] [Google Scholar]
- [36].Feiger AD, Rickels K, Rynn MA, et al. Selegiline transdermal system for the treatment of major depressive disorder: an 8-week, double-blind, placebo-controlled, flexible-dose titration trial. J Clin Psychiatry 2006;67:1354–61. [DOI] [PubMed] [Google Scholar]
- [37].Pahwa R, Stacy MA, Factor SA, et al. Ropinirole 24-hour prolonged release: randomized, controlled study in advanced Parkinson disease. Neurology 2007;68:1108–15. [DOI] [PubMed] [Google Scholar]
- [38].Watts RL, Jankovic J, Waters C, et al. Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology 2007;68:272–6. [DOI] [PubMed] [Google Scholar]
- [39].Devos D, Dujardin K, Poirot I, et al. Comparison of desipramine and citalopram treatments for depression in Parkinson's disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2008;23:850–7. [DOI] [PubMed] [Google Scholar]
- [40].Group PS. Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease. Arch Neurol 2009;66:563–70. [DOI] [PubMed] [Google Scholar]
- [41].Bronzova J, Sampaio C, Hauser RA, et al. Double-blind study of pardoprunox, a new partial dopamine agonist, in early Parkinson's disease. Mov Disord 2010;25:738–46. [DOI] [PubMed] [Google Scholar]
- [42].Ondo WG, Shinawi L, Davidson A, et al. Memantine for non-motor features of Parkinson's disease: a double-blind placebo controlled exploratory pilot trial. Parkinsonism Relat Disord 2011;17:156–9. [DOI] [PubMed] [Google Scholar]
- [43].Sampaio C, Bronzova J, Hauser RA, et al. Pardoprunox in early Parkinson's disease: results from 2 large, randomized double-blind trials. Mov Disord 2011;26:1464–76. [DOI] [PubMed] [Google Scholar]
- [44].Trenkwalder C, Kies B, Rudzinska M, et al. Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord 2011;26:90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rascol O, Bronzova J, Hauser RA, et al. Pardoprunox as adjunct therapy to levodopa in patients with Parkinson's disease experiencing motor fluctuations: results of a double-blind, randomized, placebo-controlled, trial. Parkinsonism Relat Disord 2012;18:370–6. [DOI] [PubMed] [Google Scholar]
- [46].Richard IH, McDermott MP, Kurlan R, et al. A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology 2012;78:1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rios Romenets S, Creti L, Fichten C, et al. Doxepin and cognitive behavioural therapy for insomnia in patients with Parkinson's disease – a randomized study. Parkinsonism Relat Disord 2013;19:670–5. [DOI] [PubMed] [Google Scholar]
- [48].Zhang Z, Wang J, Zhang X, et al. The efficacy and safety of ropinirole prolonged release tablets as adjunctive therapy in Chinese subjects with advanced Parkinson's disease: a multicenter, double-blind, randomized, placebo-controlled study. Parkinsonism Relat Disord 2013;19:1022–6. [DOI] [PubMed] [Google Scholar]
- [49].Hauser RA, Silver D, Choudhry A, et al. Randomized, controlled trial of rasagiline as an add-on to dopamine agonists in Parkinson's disease. Mov Disord 2014;29:1028–34. [DOI] [PubMed] [Google Scholar]
- [50].Nomoto M, Mizuno Y, Kondo T, et al. Transdermal rotigotine in advanced Parkinson's disease: a randomized, double-blind, placebo-controlled trial. J Neurol 2014;261:1887–93. [DOI] [PubMed] [Google Scholar]
- [51].Antonini A, Bauer L, Dohin E, et al. Effects of rotigotine transdermal patch in patients with Parkinson's disease presenting with non-motor symptoms – results of a double-blind, randomized, placebo-controlled trial. Eur J Neurol 2015;22:1400–7. [DOI] [PubMed] [Google Scholar]
- [52].Rascol O, Zesiewicz T, Chaudhuri KR, et al. A randomized controlled exploratory pilot study to evaluate the effect of rotigotine transdermal patch on Parkinson's disease-associated chronic pain. J Clin Pharmacol 2016;56:852–61. [DOI] [PubMed] [Google Scholar]
- [53].Weintraub D, Hauser RA, Elm JJ, et al. Rasagiline for mild cognitive impairment in Parkinson's disease: a placebo-controlled trial. Mov Disord 2016;31:709–14. [DOI] [PubMed] [Google Scholar]
- [54].Paumier KL, Sortwell CE, Madhavan L, et al. Tricyclic antidepressant treatment evokes regional changes in neurotrophic factors over time within the intact and degenerating nigrostriatal system. Exp Neurol 2015;266:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Paumier KL, Siderowf AD, Auinger P, et al. Tricyclic antidepressants delay the need for dopaminergic therapy in early Parkinson's disease. Mov Disord 2012;27:880–7. [DOI] [PubMed] [Google Scholar]
- [56].Conti MM, Ostock CY, Lindenbach D, et al. Effects of prolonged selective serotonin reuptake inhibition on the development and expression of L-DOPA-induced dyskinesia in hemi-parkinsonian rats. Neuropharmacology 2014;77:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Liu J, Dong J, Wang L, et al. Comparative efficacy and acceptability of antidepressants in Parkinson's disease: a network meta-analysis. PLoS One 2013;8:e76651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Frisina PG, Tenenbaum HR, Borod JC, et al. The effects of antidepressants in Parkinson's disease: a meta-analysis. Int J Neurosci 2008;118:667–82. [DOI] [PubMed] [Google Scholar]
- [59].Skapinakis P, Bakola E, Salanti G, et al. Efficacy and acceptability of selective serotonin reuptake inhibitors for the treatment of depression in Parkinson's disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurol 2010;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bomasang-Layno E, Fadlon I, Murray AN, et al. Antidepressive treatments for Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 2015;21:833–42. discussion 833. [DOI] [PubMed] [Google Scholar]
- [61].Troeung L, Egan SJ, Gasson N. A meta-analysis of randomised placebo-controlled treatment trials for depression and anxiety in Parkinson's disease. PLoS One 2013;8:e79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rocha FL, Murad MG, Stumpf BP, et al. Antidepressants for depression in Parkinson's disease: systematic review and meta-analysis. J Psychopharmacol 2013;27:417–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


