Supplemental Digital Content is available in the text
Keywords: bone mineral density, inflammatory bowel diseases, osteoporosis, prevention, screening
Abstract
Low bone mineral density (BMD) and osteoporosis remain frequent problems in patients with inflammatory bowel diseases (IBDs). Several guidelines with nonidentical recommendations exist and there is no general agreement regarding the optimal approach for osteoporosis screening in IBD patients. Clinical practice of osteoporosis screening and treatment remains insufficiently investigated.
In the year 2014, a chart review of 877 patients included in the Swiss IBD Cohort study was performed to assess details of osteoporosis diagnostics and treatment. BMD measurements, osteoporosis treatment, and IBD medication were recorded.
Our chart review revealed 253 dual-energy x-ray absorptiometry (DXA) scans in 877 IBD patients; osteoporosis was prevalent in 20% of tested patients. We identified widely differing osteoporosis screening rates among centers (11%–62%). A multivariate logistic regression analysis identified predictive factors for screening including steroid usage, long disease duration, and perianal disease; even after correction for all risk factors, the study center remained a strong independent predictor (odds ratio 2.3–21 compared to the center with the lowest screening rate). Treatment rates for patients with osteoporosis were suboptimal (55% for calcium, 65% for vitamin D) at the time of chart review. Similarly, a significant fraction of patients with current steroid medication were not treated with vitamin D or calcium (treatment rates 53% for calcium, 58% for vitamin D). For only 29% of patients with osteoporosis bisphosphonate treatment was started. Treatment rates also differed among centers, generally following screening rates. In patients with longitudinal DXA scans, calcium and vitamin D usage was significantly associated with improvement of BMD over time.
Our analysis identified inconsistent usage of osteoporosis screening and underuse of osteoporosis treatment in IBD patients. Increasing awareness of osteoporosis as a significant clinical problem in IBD patients might improve patient care.
1. Introduction
Osteoporosis is a clinically relevant and frequent complication in patients with inflammatory bowel disease (IBD).[1–3] Compared to controls, the fracture risk for IBD patients is increased by approximately 40% to 60%.[4,5] Risk factors for osteoporosis and osteopenia in IBD patients include activity and severity of gut inflammation, perianal disease including fistulae, systemic steroid usage, intestinal malabsorption leading to calcium and vitamin D deficiency, low body mass index, and advanced age.[1,6–22]
Bone mineral density (BMD) remains a widely accepted parameter to quantify osteopenia and osteoporosis. Dual-energy x-ray absorptiometry (DXA) is normally used to assess BMD. BMD can predict fracture risk[23,24] and a BMD of one standard deviation below the age adjusted mean increases the relative fracture risk by 1.6 to 2.6.[23]
Current guidelines recommend screening for osteoporosis in high-risk individuals[22,25–33] (Table 1). For IBD patients recommendations differ in published guidelines by the European Crohn and Colitis Organization, the American College of Gastroenterology (ACG), the American Gastroenterological Association (AGA), and the British Society of Gastroenterology.[22,25–33] Although all guidelines recommend a DXA scan in individuals with significant steroid use and/or recurrent or persistently active disease, each guideline mentions specific additional risk situations. Applicability of these guidelines and compliance with osteoporosis screening for IBD has been insufficiently studied.
Table 1.
Recommendations regarding osteoporosis screening in IBD patients according to current guidelines.
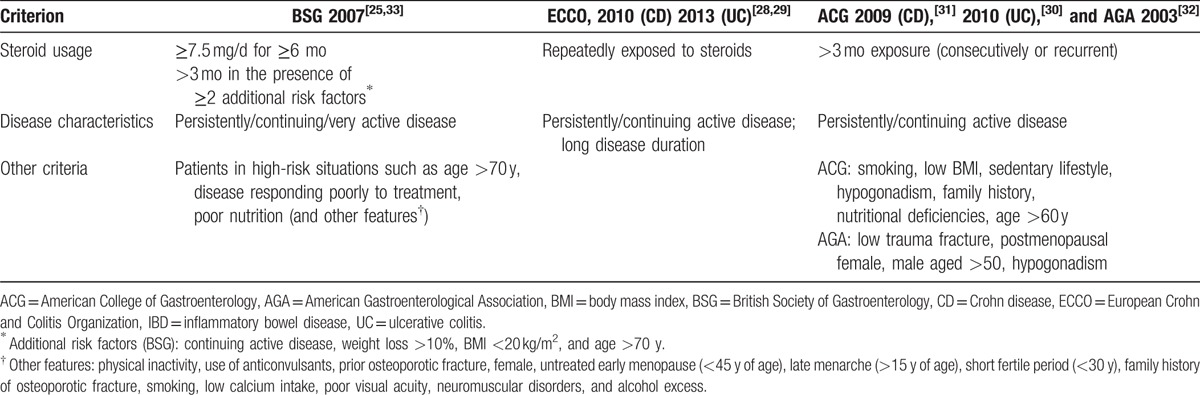
Adequate treatment can reverse osteoporosis and prevent osteoporotic fractures even in high-risk individuals including postmenopausal women, older men with osteoporosis, or glucocorticoid-treated patients.[34–36] There is general agreement that high-risk individuals with decreased BMD should have adequate dietary calcium intake (1000–1200 mg per day), otherwise calcium supplements should be prescribed. Similarly, an intake of 800 to 1000 international units (IU) of vitamin D per day is recommended.[35,37,38] Furthermore, individuals with osteoporosis should receive osteoporosis medication in addition to calcium and vitamin D treatment (eg, bisphosphonates, parathyroid hormone [PTH] analogues, and estrogens).[35,37,38] During systemic glucocorticoid therapy calcium and vitamin D intake should be adequate, and depending on age, hormonal state, and BMD, additionally bisphosphonates or PTH analogues are recommended.[35,37–41] Several guidelines with similar recommendations exist for the treatment of patients with osteoporosis and IBD.[1,18,27,42] It remains unclear, how these guidelines are applied in clinical practice.
To study screening and treatment of low BMD in IBD patients we used data of the Swiss IBD cohort study (SIBDCS), a prospective long-term study of well characterized IBD patients. Our data indicate divergent screening and treatment rates for IBD patients and possibilities to improve patient care.
2. Patients and methods
The SIBDCS is a prospective cohort study of IBD patients. General information regarding the presence of osteopenia/osteoporosis is recorded in the data base but the dates of various DXA scans as well as T scores and Z scores are not. Therefore, a manual review of patient charts was performed in the year 2014. Our chart review covered 4 tertiary care hospitals (providing care from specialists in a large hospital) and 2 secondary care centers. For each available DXA scan T scores and Z scores for hip and lumbar spine were retrieved. Any additional information regarding osteoporosis and osteopenia in the patient chart was also recorded and evaluated as specified below. In addition, information regarding steroid usage ≥10 mg/day, treatment with biologicals (Infliximab, Adalimumab, and Certolizumab pegol) and osteoporosis treatment (calcium, vitamin D, and bisphosphonate medication) was noted. For all treatment parameters, both current usage and any usage within patient history were recorded. For the analysis of the association of steroid treatment with osteoporosis, osteopenia, and normal BMD, treatment information was retrieved from SIBDC data base.
DXA measurements were performed in the femur (femoral neck and/or total hip) and/or lumbar spine. For the T score data were compared to the BMD of a sex-matched young adult reference population while for the Z scores data were compared to an age-, sex-, and ethnicity-matched reference population.[35] In postmenopausal women and in men ≥50 years, osteoporosis and osteopenia were defined by a T score ≤−2.5 and <−1, respectively, in lumbar spine, total hip, or femoral neck.[35] For premenopausal women and younger men, the diagnosis of osteoporosis is not possible on BMD values alone but a Z score of ≤−2 is a helpful parameter.[35,43]
A total of 877 patient charts from 6 centers were reviewed for evidence of one or more past DXA scans.[22] Diagnosis or exclusion of osteoporosis and osteopenia was done as described in our previous study,[22] in brief: osteoporosis was defined as T scores ≤−2.5 and Z scores ≤−2, whereas osteopenia was diagnosed at T scores <−1 and >−2.5 and Z scores <−1 and >−2. If scores for both, hip and spine were available, the lowest scores were considered. For our diagnostic procedure the following hierarchy was used: if available, the T score was used. If no T score was available Z score was used. Without information of DXA scores the diagnosis of osteoporosis/osteopenia in the patient chart was considered. For 6 patients an unambiguous reference to a DXA scan was found but no score and no interpretation was available; these patients were only used for the analysis of screening rates but not for statistics about diagnosis and treatment. For the calculations of screening rates evidence for either osteoporosis/osteopenia within the SIBDCS data base or any documentation regarding a DXA scan within patient charts (see above) were taken into account.
2.1. Data analysis
For the multivariate analysis the following variables were considered: IBD subtype (Crohn disease [CD] vs ulcerative colitis/indeterminate colitis), gender, last body mass index, last smoking status, steroid use, presence of intestinal stenosis, perianal disease, prior intestinal surgery, presence of malabsorption syndrome, presence of extraintestinal disease manifestations, age at last follow-up, childhood diagnosis of IBD, disease duration, family history of IBD, alcohol consumption more than once a day, sport at least once a week, last Activity Index, and study center, similar to a previous study.[22] For the calculation of the Activity Index, disease activity was normalized to a parameter ranging from 0 (no activity) to 100 (strongest activity). Thereby, for CD patients the Crohn disease activity index was divided by 5; for ulcerative colitis/indeterminate colitis patients the modified Truelove and Witts severity index was divided by .21.[22]
Univariate and multivariate logistic regression analyses were used to determine the association of clinical variables with osteoporosis screening in IBD patients. We first performed univariate regressions with each factor mentioned above. We then fit together all variables such that the corresponding P-value in univariate regressions was less than .2. A step-wise approach was finally used to select a model with predictors whose P-value were less than .157.[44] For this analysis the Stata software was used (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StatCorp LP).
For the statistical analysis of screening and treatment rates and trends in BMD according to treatments Fisher exact test and a linear regression analysis, respectively, were performed, using appropriate modules of GraphPad Prism, version 6.0d. A P value at or below .05 was prospectively defined as significant.
2.2. Ethical considerations
The SIBDCS protocol has been approved as a multicenter study by the ethics committee of Zurich County (KEK-ZH). Patients provided written informed consent to data acquisition and analysis during inclusion into the SIBDCS. Data analysis was performed according to the declaration of Helsinki.
3. Results
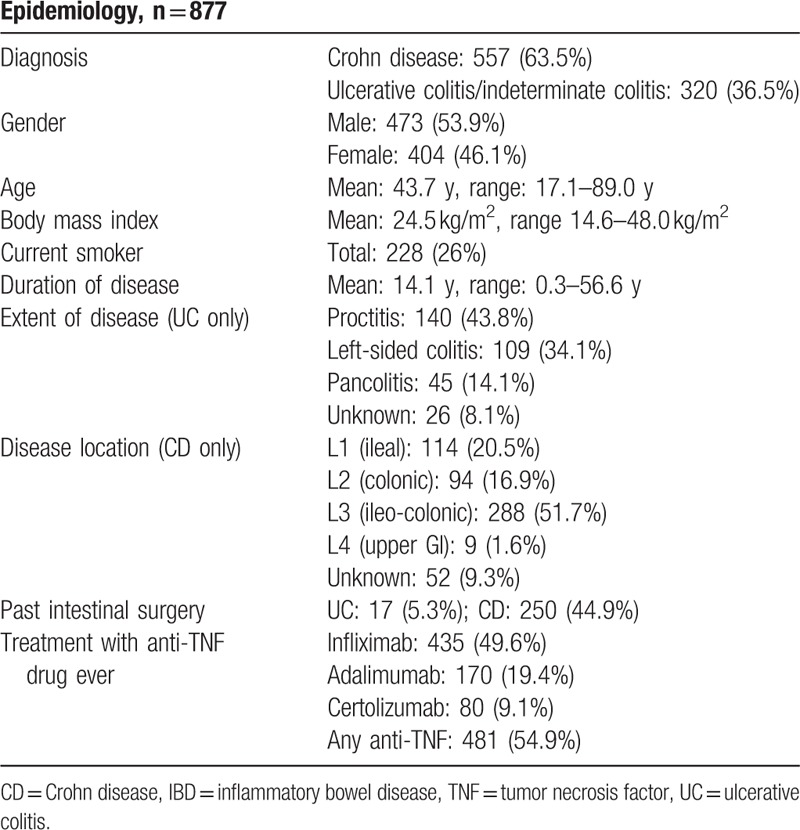
For our analysis, we used a subgroup of SIBDCS, a large prospective cohort study of well-characterized Swiss IBD patients. A chart review was performed in 6 centers. Altogether, data for 877 IBD patients could be retrieved. These patients represent a mixed IBD cohort from tertiary and secondary referral centers with expected epidemiological characteristics regarding age and gender distribution as well as IBD characteristics (Table 2).
Table 2.
Epidemiological characteristics of our IBD patients in 2014 from 6 Swiss secondary or tertiary health care centers.
3.1. Prevalence of osteoporosis screening
In 259 of the 877 patients (30%), osteoporosis screening was performed. Screening rates differed strongly between centers ranging from 11% to 62% (Fig. 1). For example, in center A, 90 out of 146 patients (62%) have had a DXA scan; in center B, 83 out of 231 (36%) were screened by DXA whereas in center F only 25 out of 237 patients (11%) have had a DXA scan. Overall, screening rates for osteoporosis tended to be slightly higher in tertiary referral centers compared to secondary centers (30.2% compared to 24.5%, not significant). However, pronounced differences were also observed within the group of tertiary care centers (Fig. 1, centers A, B, E, F).
Figure 1.
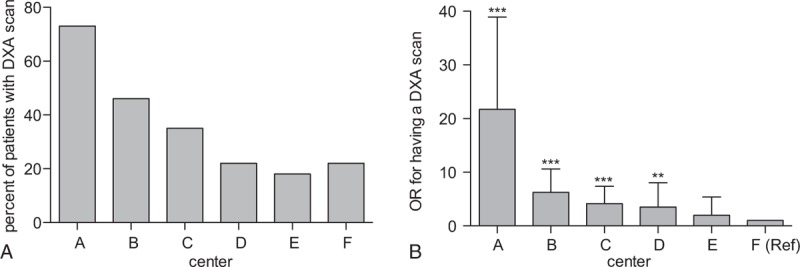
Screening for osteoporosis in 6 Swiss IBD Cohort Study centers from inclusion into the study until year 2014. (A) Screening rates per center. In a conservative approach, screening rates were defined as evidence of osteoporosis/osteopenia in the cohort documentation and/or the patient chart. (B) OR for having a DXA scan in various centers (compare Table 3). Multivariate analysis: ∗∗∗P < .001, ∗∗P < .01, ∗P < .05. DXA = dual-energy x-ray absorptiometry, IBD = inflammatory bowel disease, OR = odds ratio.
In a multivariate analysis considering multiple risk factors[22] (see Patients and methods), the study center remained a strong independent and significant predictive factor for the performance of a DXA scan (Table 3). The following clinical variables were significantly associated with performance of a DXA scan: presence of perianal disease (odds ratio 1.52; confidence interval [CI]: 1.04–2.2; P = .032) and usage of any steroid at last visit, including budesonide (odds ratio 2.2; CI: 1.5–3.2; P < .001). Treatment with budesonide on its own (instead of all steroids) was also significantly associated with osteoporosis screening; however, the association of all steroids including budesonide with osteoporosis screening was stronger (ie, resulted in better model characteristics; not shown). Age at diagnosis, disease duration, gender, and presence of primary sclerosing cholangitis did not significantly influence the decision to screen in this multivariate analysis.
Table 3.
Multivariate model for having a DXA scan.
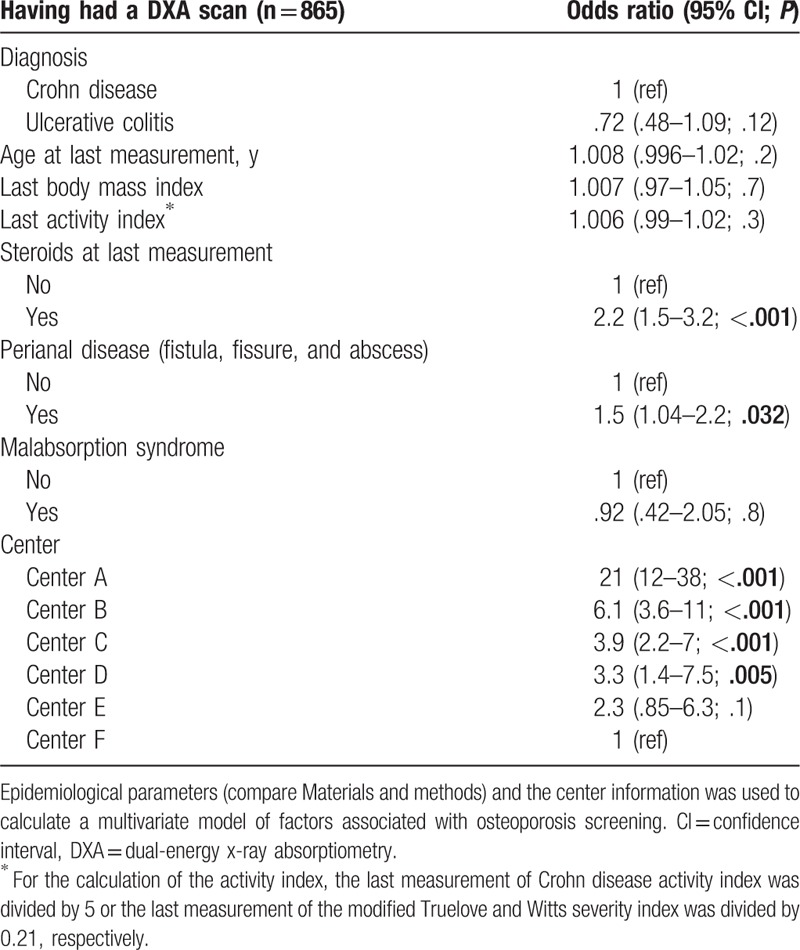
These results suggest that clinicians considered clinical parameters for their decision to order a DXA scan but clinical practice differed strongly among centers.
3.2. Prevalence of osteoporosis and osteoporosis risk factors
Overall, 169 of 877 patients (19.3%) had documented decreased BMD. When only the 253 patients with available DXA scans were considered, osteopenia was found in 57% and osteoporosis in 20%. Looking at the different centers separately, among patients with DXA scans rates for reduced BMD ranged from 43% to 82% and for osteoporosis from 9% to 30% (Fig. 2). Patients aged ≥50 years (103 out of 253) showed higher rates of osteoporosis compared to younger patients (29.1% vs 12%, P = .001); however, the rates of osteopenia did not differ (45.6% in patients ≥50 years vs 45.3% in patients <50 years). In patients with a disease duration of ≥15 years (141 out of 253), rates of osteopenia and osteoporosis did not differ significantly (osteopenia: 49.6% vs 40.2%; osteoporosis: 20.6% vs 16.1%, ns).
Figure 2.
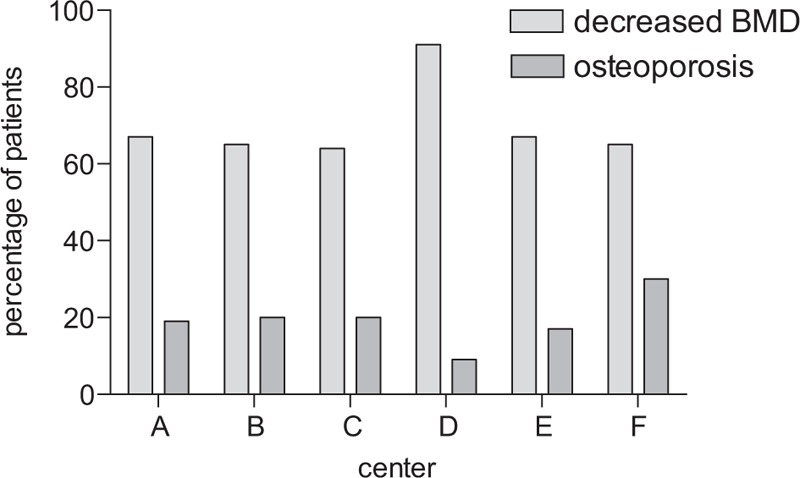
Fraction of dual-energy x-ray absorptiometry (DXA) scans diagnostic for osteopenia or osteoporosis in 6 Swiss study centers from inclusion into the study until year 2014. Percentage of DXA scans diagnostic for osteoporosis or osteopenia are shown. Rates of positive findings did not differ significantly (Chi-square test).
Table 4 provides a comparison of patients with osteoporosis, osteopenia, and normal BMD. Patients with osteoporosis were elder, more likely to suffer from CD and more likely to be male compared to patients with osteopenia and normal BMD, while disease duration and prevalence of primary sclerosing cholangitis did not differ significantly (Table 4). Rates of steroid treatment at last visit were highest for osteoporosis (79.2%), intermediate for osteopenia (62.6%), and lowest for normal BMD (42.2%; P < .001). Rates of budesonide treatment at last visit also differed according to BMD but this trend failed to reach significance. Interestingly, the percentage of positive DXA scans was not related to the screening rate (compare Figs. 1 and 2; no significant association in a Spearmen correlation analysis).
Table 4.
Risk factors for osteoporosis in 253 patients with known BMD.
3.3. Treatment of osteoporosis
Treatment of reduced BMD differed within our cohort. This analysis was restricted to 253 patients with known BMD. Overall, 106 of 253 patients (42%) were treated with calcium supplementation, 140 (55%) with vitamin D, and 14 (6%) with bisphosphonates at the time of chart review. Treatment differed according to BMD: 28/51 (55%) of patients with osteoporosis, 67/143 (47%) of patients with osteopenia, and 26/84 (31%) of patients with normal BMD were treated with calcium (P = .013, Chi square test for the whole group, compare Fig. 3A). Similar but nonsignificant effects on treatment rates for vitamin D were recorded and 33/51 (65%) of patients with osteoporosis, 84/143 (59%) of patients with osteopenia, and 40/84 (48%) of patients with normal BMD received vitamin D supplementation (P = .112). The fraction of patients that did not receive any osteoporosis treatment at the time of chart review was 27% for osteoporosis and 36% for osteopenia.
Figure 3.
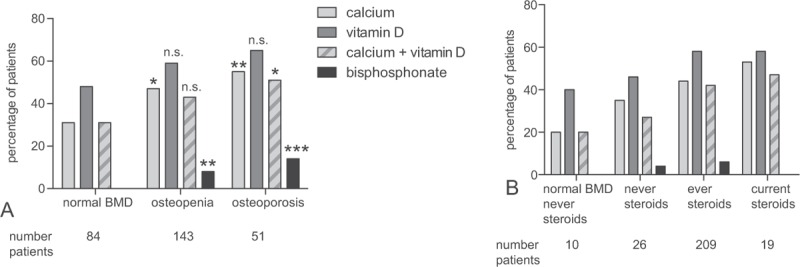
Osteoporosis treatment. (A) Percentage of patients with osteoporosis treatment at the time of chart review according to results of DXA scans. For the statistical analysis patients with osteoporosis/osteopenia were compared to patients with normal BMD. Fisher exact test: ns, ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. (B) Treatment in patients depending on their history of steroid therapy. For the statistical analysis patients which never received steroids were compared to patients with current or any steroid treatment. No significant differences were found. BMD = bond mineral density, DXA = dual-energy x-ray absorptiometry, ns = not significant.
When the complete treatment history of the patient was considered, rates for patients ever treated with calcium/vitamin D increased to 92%/90% for patients with osteoporosis, 83%/87% for patients with osteopenia, and 61%/70% for patients with normal BMD.
Centers with higher rates of osteoporosis screening also showed higher rates for osteoporosis treatment. For the 3 centers with the highest screening rates (center A–F, Fig. 1) treatment rates followed screening rates with calcium/vitamin D medication in 54%/71% in center A, 39%/54% in center B, and 27%/34% in center C. The trend remained robust if subgroups of patients with osteoporosis or osteopenia were considered.
Patients currently treated with steroids tended to receive calcium and vitamin D supplementation slightly more frequently than patients without steroids (10/19; 53% for calcium; 11/19; 58% for vitamin D, not significant; Fig. 3B). However, a considerable fraction of patients with normal BMD and no previous steroids nevertheless received replacement therapy (2/10 for calcium, 4/10 for vitamin D).
Bisphosphonate treatment was most frequently applied to patients with osteoporosis: 7 out of 51 osteoporosis patients (14%) received bisphosphonates at the time of the chart review. In addition, 11 out of 143 patients (8%) with osteopenia but no patient with normal BMD received bisphosphonate treatment at this time. For 13 patients bisphosphonate treatment had been started but discontinued and 15 out of 51 (29%) of patients with osteoporosis, 19 out of 143 with osteopenia (13%), and 1 out of 84 patients with normal BMD (1%) had ever received bisphosphonates. The reasons why treatment was discontinued were not evaluated.
3.4. Multiple DXA scans and improvement of BMD
Among the 259 IBD patients with DXA screening 129 (50%) received 1 DXA scan; 72 (28%) were tested twice, 26 (10%) 3 times, and 18 (7%) and 14 (5%) 4 or more times, respectively (Figure S1). Overall, for all patients with multiple DXA scans we note a slight improvement over time in T scores for the hip and the spine in a linear regression analysis (spine: slope: .06/year, CI: .0099–.12, P = .02; hip: slope: .04/year, CI: −.0002–.081, P = .051, not shown). However, in the subgroup of patients with calcium or vitamin D supplementation T scores for spine improved significantly (calcium: slope: .1/year, CI: .036–.17, P = .004; vitamin D: slope: .091/year, CI: .026–.16, P = .007, vitamin D and calcium: slope .089/year, CI: .028–.15, P = .005; Fig. 4A and B). In contrast, for patients without calcium and vitamin D supplementation spinal T scores did not increase significantly over time. Similar results were obtained for T scores of the hip (not shown). No significant improvement in serial DXA scans of 8 patients with bisphosphonate treatment was noted, but the low number of patients in this subgroup limits our conclusions.
Figure 4.
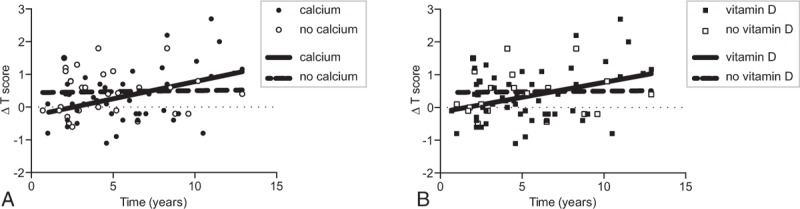
Improvement of dual-energy x-ray absorptiometry (DXA) results of the lumbar spine upon treatment with vitamin D or calcium. (A) Changes in T scores of the spine over time with and without calcium treatment (R2 = .17, P = .004, linear regression analysis). For comparison patients without calcium treatment are shown. (B) Changes in T scores with and without vitamin D treatment (R2 = .13, P = .007).
Treatment with any tumor necrosis factor inhibitor (Infliximab, Adalimumab, or Certolizumab) was not associated with improvements in T scores for hip or spine in contrast to 2 previous studies.[45,46]
4. Discussion
In our study, we address clinical practice of osteoporosis screening and treatment. We found widely differing screening rates among different IBD referral centers. Furthermore, osteoporosis treatment frequently did not follow recommendations, most profoundly in centers with low screening rates. Our data thus indicate low awareness of osteoporosis as an important medical problem for IBD patients. However, the subset of patients with calcium or vitamin D medication significantly improved their BMD over the course of treatment, in turn indicating, that appropriate evaluation for and treatment of osteopenia/osteoporosis may ultimately translate into clinical benefit for patients with IBD.
In 253 patients tested with DXA scans, rates of osteopenia and osteoporosis were 57% and 20%, respectively. These numbers are well in agreement with previous studies, reporting osteopenia and osteoporosis rates of 34% to 78% and 13% to 42%, respectively.[20,22,47–52] Although the rate of low BMD of a given cohort will depend on patient and disease characteristics, our data confirm osteoporosis and osteopenia as prevalent problems in IBD patients. Our study also confirms the important role of steroid treatment for osteoporosis in IBD patients (Table 4).
Screening rates for osteoporosis varied remarkably among the 6 study centers, ranging from 11% to 62%. All patients were treated by gastroenterologists specialized in IBD care, either in large academic centers or in large private practices. Nevertheless, the study center remained a strong and significant predictor for osteoporosis screening in univariate and multivariate analyses. Clinical variables such as steroid usage, disease duration, and presence of perianal disease were further predicting factors. Interestingly, even though the rates of DXA scans strongly differed among centers, the fraction of positive DXA scans (ie, with a diagnosis of osteoporosis or osteopenia) did not differ significantly (Fig. 2). Our analysis cannot formally distinguish between over usage and under usage of DXA scans. However, our data suggest that either the policy of a given center, awareness of osteoporosis, and/or availability of DXA scans strongly influenced clinical management of IBD patients regarding bone health.
Current guidelines agree that DXA scan should be recommended in individuals with significant steroid use longer than 3 or 6 months or recurrent and/or persistently active disease. However, compliance to most guidelines cannot be formally tested since no formal threshold for disease duration or “persistent” or “continuous” disease activity is defined by European Crohn and Colitis Organization or British Society of Gastroenterology guidelines.[25,26,28,29,33] Guidelines of AGA and ACG provide objective criteria[32] but none of these are specific to IBD patients. In 1 study, screening criteria mentioned by AGA and ACG guidelines did not predict low BMD in subsequent DXA scans.[53]
Steroid usage is mentioned by all guidelines but details of recommendations differ. However, during our chart review extracting steroid dosage over time proved to be time consuming (up to 1 hour per patient) and frequently an area under the curve could not be reconstructed with confidence. Considering complex patient histories or treatment in different clinical settings, we suspect that these limitations are not specific to our study and any screening recommendation based on duration or frequency of past steroid usage will be hard to implement in a rigorous manner.
Awareness of osteoporosis was directly tested in 1 previous study demonstrating low familiarity of physicians of AGA with guidelines regarding osteoporosis screening and treatment in IBD patients.[54] According to another prospective study, increasing physician's awareness of osteoporosis guidelines can in turn improve screening rates in IBD patients[55]: guidelines of ACG were sent to members, prompting additional DXA scans as well as increased familiarity in osteoporosis treatment, potentially preventing fractures in IBD patients.
Taken together, heterogeneity in clinical practice (as indicated by our study) might also reflect ambiguity and diversity in current guidelines. Overutilization and under usage of DXA scans will clearly limit cost-efficiency of osteoporosis screening and guidelines easily applicable in clinical practice would be desirable.
Treatment rates regarding calcium and vitamin D generally followed screening rates. A diagnosis of reduced BMD increased the likelihood of treatment with vitamin D and/or calcium. However, 27% and 36% of patients with osteoporosis and osteopenia, respectively, did not receive treatment. Our data thus reveal partial noncompliance with osteoporosis treatment guidelines in Switzerland. Although the vast majority of patients (almost 92%) with a DXA scan diagnostic for osteoporosis had received vitamin D and calcium in the past, only 55% of patients received calcium and 65% received vitamin D at the time of our chart review. Low treatment rates were also described in previous studies[53,56] (treatment rates for calcium and/or vitamin D of 59%–63.5% in IBD patients with low BMD).
Treatment with calcium and vitamin D is likely beneficial for IBD patients with low BMD and an increase in T scores in sequential DXA scans was noted in patients with calcium and vitamin D treatment. Similar effects were described in a previous randomized, placebo controlled study with 60 IBD patients.[57]
In our study, only 29% of all patients with osteoporosis were ever treated with bisphosphonates and for only 14% of patients this drug was part of the current treatment regiment, pointing to a relevant underutilization of this very efficient osteoporosis medication.[34–36] Treatment with bisphosphonates can reduce the incidence of spine and hip fractures by 33% to 50% over 3 years in osteoporotic postmenopausal women.[32,35,36] Underuse of bisphosphonates is unlikely due to financial constraints since these costs will be reimbursed by the universal Swiss public health insurance system. Besides bisphosphonates other powerful osteoporosis treatments are available including calcitonin, estrogens, PTH analogues, and denosumab.[35] However, prescription of these modern therapies was noted in only 3 out of 169 patients with low BMD.
Strengths of our study include the high level of patient data available. Furthermore, to the best of our knowledge, our study is the first comparing clinical practice regarding screening and treatment rates among individual centers. Our study has several limitations:
-
(i)
The retrospective study design.
-
(ii)
Our study population was recruited in secondary and tertiary referral centers and might not be representative for the Swiss population affected by IBD.
-
(iii)
Our analysis was limited to results of DXA scans and fractures were not considered.
-
(iv)
We did not analyze biochemical markers for systemic inflammation[21,58] or intestinal inflammation such as calprotectin, bone turnover,[47,50,58] or genetic markers for osteoporosis[59] as done in some previous studies.
-
(v)
We did not systematically assess comorbidities of our patients besides IBD; however, only 10.7% of all patients and 11% of patients with DXA scans were older than 65 years and the influence of comorbidities might be limited.
5. Conclusion
Our analysis identified inconsistent usage of osteoporosis screening and underuse of osteoporosis treatment with calcium, vitamin D, and bisphosphonates in IBD patients. Screening and treatment rates strongly differed among centers and opportunities for improving treatment remain in many centers. Treatment with calcium and vitamin D improved BMD in DXA scans. Better physician awareness regarding osteoporosis might thus improve bone health of IBD patients.
Acknowledgments
The authors thank Swiss National Science Foundation to BM (Grant No. 32473B_156525) and the Swiss IBD Cohort (Grant No. 3347CO-108792) for the support.
Supplementary Material
Footnotes
Abbreviations: ACG = American College of Gastroenterology, AGA = American Gastroenterological Association, BMD = bone mineral density, CD = Crohn disease, CI = confidence interval, DXA = dual-energy x-ray absorptiometry, IBD = inflammatory bowel disease, PTH = parathyroid hormone, SIBDCS = Swiss IBD cohort study.
BM, SRV, and GR designed the study; SS performed the chart review of IBD patients; BM and JBR did the statistical analysis; BM and SS wrote the paper; JBR, DF, LB, MS, JZ, TK, TG, NF, SRV, and GR reviewed and edited the paper for important intellectual content. All authors have seen and approved the final version of the manuscript.
This work was supported by research grants from the Swiss National Science Foundation to BM (Grant No. 32473B_156525), and the Swiss IBD Cohort (Grant No. 3347CO-108792).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Rothfuss KS, Stange EF, Herrlinger KR. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol 2006;12:4819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med 2010;42:97–114. [DOI] [PubMed] [Google Scholar]
- [3].Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol 2011;7:235–41. [PMC free article] [PubMed] [Google Scholar]
- [4].Bernstein CN, Blanchard JF, Leslie W, et al. The incidence of fracture among patients with inflammatory bowel disease. A population-based cohort study. Ann Intern Med 2000;133:795–9. [DOI] [PubMed] [Google Scholar]
- [5].Card T, West J, Hubbard R, et al. Hip fractures in patients with inflammatory bowel disease and their relationship to corticosteroid use: a population based cohort study. Gut 2004;53:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vestergaard P. Prevalence and pathogenesis of osteoporosis in patients with inflammatory bowel disease. Minerva Med 2004;95:469–80. [PubMed] [Google Scholar]
- [7].Bernstein CN, Seeger LL, Sayre JW, et al. Decreased bone density in inflammatory bowel disease is related to corticosteroid use and not disease diagnosis. J Bone Miner Res 1995;10:250–6. [DOI] [PubMed] [Google Scholar]
- [8].de Jong DJ, Corstens FH, Mannaerts L, et al. Corticosteroid-induced osteoporosis: does it occur in patients with Crohn's disease? Am J Gastroenterol 2002;97:2011–5. [DOI] [PubMed] [Google Scholar]
- [9].Azzopardi N, Ellul P. Risk factors for osteoporosis in Crohn's disease: infliximab, corticosteroids, body mass index, and age of onset. Inflamm Bowel Dis 2013;19:1173–8. [DOI] [PubMed] [Google Scholar]
- [10].Frei P, Fried M, Hungerbuhler V, et al. Analysis of risk factors for low bone mineral density in inflammatory bowel disease. Digestion 2006;73:40–6. [DOI] [PubMed] [Google Scholar]
- [11].Targownik LE, Bernstein CN, Nugent Z, et al. Inflammatory bowel disease has a small effect on bone mineral density and risk for osteoporosis. Clin Gastroenterol Hepatol 2013;11:278–85. [DOI] [PubMed] [Google Scholar]
- [12].Schulte C, Dignass AU, Mann K, et al. Bone loss in patients with inflammatory bowel disease is less than expected: a follow-up study. Scand J Gastroenterol 1999;34:696–702. [DOI] [PubMed] [Google Scholar]
- [13].Leslie WD, Miller N, Rogala L, et al. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol 2008;103:1451–9. [DOI] [PubMed] [Google Scholar]
- [14].Compston JE, Judd D, Crawley EO, et al. Osteoporosis in patients with inflammatory bowel disease. Gut 1987;28:410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Targownik LE, Bernstein CN, Leslie WD. Risk factors and management of osteoporosis in inflammatory bowel disease. Curr Opin Gastroenterol 2014;30:168–74. [DOI] [PubMed] [Google Scholar]
- [16].van Hogezand RA, Hamdy NA. Skeletal morbidity in inflammatory bowel disease. Scand J Gastroenterol Suppl 2006;243:59–64. [DOI] [PubMed] [Google Scholar]
- [17].Rodriguez-Bores L, Barahona-Garrido J, Yamamoto-Furusho JK. Basic and clinical aspects of osteoporosis in inflammatory bowel disease. World J Gastroenterol 2007;13:6156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ali T, Lam D, Bronze MS, et al. Osteoporosis in inflammatory bowel disease. Am J Med 2009;122:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Katz S, Weinerman S. Osteoporosis and gastrointestinal disease. Gastroenterol Hepatol 2010;6:506–17. [PMC free article] [PubMed] [Google Scholar]
- [20].Miznerova E, Hlavaty T, Koller T, et al. The prevalence and risk factors for osteoporosis in patients with inflammatory bowel disease. Bratisl Lek Listy 2013;114:439–45. [DOI] [PubMed] [Google Scholar]
- [21].Jahnsen J, Falch JA, Mowinckel P, et al. Bone mineral density in patients with inflammatory bowel disease: a population-based prospective two-year follow-up study. Scand J Gastroenterol 2004;39:145–53. [DOI] [PubMed] [Google Scholar]
- [22].Schule S, Rossel JB, Frey D, et al. Prediction of low bone mineral density in patients with inflammatory bowel diseases. United European Gastroenterol J 2016;4:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 1993;341:72–5. [DOI] [PubMed] [Google Scholar]
- [25].Scott EM, Gaywood I, Scott BB. Guidelines for osteoporosis in coeliac disease and inflammatory bowel disease. British Society of Gastroenterology. Gut 2000;46(Suppl 1):i1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lewis NR, Scott BB. Guidelines for osteoporosis in inflammatory bowel disease and coeliac disease. 2007. http://www.bsg.org.uk/pdf_word_docs/ost_coe_ibd.pdf. Accessed May 21, 2017. [Google Scholar]
- [27].Lichtenstein GR, Sands BE, Pazianas M. Prevention and treatment of osteoporosis in inflammatory bowel disease. Inflamm Bowel Dis 2006;12:797–813. [DOI] [PubMed] [Google Scholar]
- [28].Van Assche G, Dignass A, Bokemeyer B, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 3: special situations. J Crohns Colitis 2013;7:1–33. [DOI] [PubMed] [Google Scholar]
- [29].Van Assche G, Dignass A, Reinisch W, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: special situations. J Crohns Colitis 2010;4:63–101. [DOI] [PubMed] [Google Scholar]
- [30].Kornbluth A, Sachar DB. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 2010;105:501–23. quiz 524. [DOI] [PubMed] [Google Scholar]
- [31].Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn's disease in adults. Am J Gastroenterol 2009;104:465–83. quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- [32].Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology 2003;124:795–841. [DOI] [PubMed] [Google Scholar]
- [33].Guidelines for osteoporosis in inflammatory bowel disease and coeliac disease. http://www.bsg.org.uk/pdf_word_docs/ost_coe_ibd.pdf. Accessed May 21, 2017. [Google Scholar]
- [34].Melek J, Sakuraba A. Efficacy and safety of medical therapy for low bone mineral density in patients with inflammatory bowel disease: a meta-analysis and systematic review. Clin Gastroenterol Hepatol 2014;12:32.e5–44.e5. [DOI] [PubMed] [Google Scholar]
- [35].Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention, treatment of osteoporosis. Osteoporos Int 2014;25:2359–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cranney A, Wells G, Willan A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev 2002;23:508–16. [DOI] [PubMed] [Google Scholar]
- [37].Compston J, Bowring C, Cooper A, et al. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas 2013;75:392–6. [DOI] [PubMed] [Google Scholar]
- [38].Hodgson SF, Watts NB, Bilezikian JP, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract 2003;9:544–64. [DOI] [PubMed] [Google Scholar]
- [39].Pereira RM, Carvalho JF, Paula AP, et al. Guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis. Rev Bras Reumatol 2012;52:580–93. [PubMed] [Google Scholar]
- [40].Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 2010;62:1515–26. [DOI] [PubMed] [Google Scholar]
- [41].Warriner AH, Saag KG. Prevention and treatment of bone changes associated with exposure to glucocorticoids. Curr Osteoporos Rep 2013;11:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Targownik LE, Bernstein CN, Leslie WD. Inflammatory bowel disease and the risk of osteoporosis and fracture. Maturitas 2013;76:315–9. [DOI] [PubMed] [Google Scholar]
- [43].Schousboe JT, Shepherd JA, Bilezikian JP, et al. Executive Summary of the 2013 ISCD Position Development Conference on Bone Densitometry JCD 2013:455–67. [DOI] [PubMed] [Google Scholar]
- [44].Rousson V. Statistique Appliquée Aux Sciences De La Vie. Paris: Springer; 2013. [Google Scholar]
- [45].Bernstein M, Irwin S, Greenberg GR. Maintenance infliximab treatment is associated with improved bone mineral density in Crohn's disease. Am J Gastroenterol 2005;100:2031–5. [DOI] [PubMed] [Google Scholar]
- [46].Miheller P, Muzes G, Racz K, et al. Changes of OPG and RANKL concentrations in Crohn's disease after infliximab therapy. Inflamm Bowel Dis 2007;13:1379–84. [DOI] [PubMed] [Google Scholar]
- [47].Bjarnason I, Macpherson A, Mackintosh C, et al. Reduced bone density in patients with inflammatory bowel disease. Gut 1997;40:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pollak RD, Karmeli F, Eliakim R, et al. Femoral neck osteopenia in patients with inflammatory bowel disease. Am J Gastroenterol 1998;93:1483–90. [DOI] [PubMed] [Google Scholar]
- [49].Von Tirpitz C, Pischulti G, Klaus J, et al. [Pathological bone density in chronic inflammatory bowel diseases – prevalence and risk factors]. Zeitschr Gastroenterol 1999;37:5–12. [PubMed] [Google Scholar]
- [50].Ardizzone S, Bollani S, Bettica P, et al. Altered bone metabolism in inflammatory bowel disease: there is a difference between Crohn's disease and ulcerative colitis. J Intern Med 2000;247:63–70. [DOI] [PubMed] [Google Scholar]
- [51].Ismail MH, Al-Elq AH, Al-Jarodi ME, et al. Frequency of low bone mineral density in Saudi patients with inflammatory bowel disease. Saudi J Gastroenterol 2012;18:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dumitrescu G, Mihai C, Dranga M, et al. Bone mineral density in patients with inflammatory bowel disease from north-eastern Romania. Rev Med Chir Soc Med Nat Iasi 2013;117:23–8. [PubMed] [Google Scholar]
- [53].Etzel JP, Larson MF, Anawalt BD, et al. Assessment and management of low bone density in inflammatory bowel disease and performance of professional society guidelines. Inflamm Bowel Dis 2011;17:2122–9. [DOI] [PubMed] [Google Scholar]
- [54].Wagnon JH, Leiman DA, Ayers GD, et al. Survey of gastroenterologists’ awareness and implementation of AGA guidelines on osteoporosis in inflammatory bowel disease patients: are the guidelines being used and what are the barriers to their use? Inflamm Bowel Dis 2009;15:1082–9. [DOI] [PubMed] [Google Scholar]
- [55].Kane S, Reddy D. Guidelines do help change behavior in the management of osteoporosis by gastroenterologists. Am J Gastroenterol 2006;101:1841–4. [DOI] [PubMed] [Google Scholar]
- [56].von Tirpitz C, Steder-Neukamm U, Glas K, et al. [Osteoporosis in inflammatory bowel disease – results of a survey among members of the German Crohn's and Ulcerative Colitis Association]. Zeitschr Gastroenterol 2003;41:1145–50. [DOI] [PubMed] [Google Scholar]
- [57].Abitbol V, Mary JY, Roux C, et al. Osteoporosis in inflammatory bowel disease: effect of calcium and vitamin D with or without fluoride. Aliment Pharmacol Ther 2002;16:919–27. [DOI] [PubMed] [Google Scholar]
- [58].van Hogezand RA, Banffer D, Zwinderman AH, et al. Ileum resection is the most predictive factor for osteoporosis in patients with Crohn's disease. Osteoporos Int 2006;17:535–42. [DOI] [PubMed] [Google Scholar]
- [59].Noble CL, McCullough J, Ho W, et al. Low body mass not vitamin D receptor polymorphisms predict osteoporosis in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2008;27:588–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


