Abstract
Background:
Current publications present contradictory findings regarding interleukin-8 (IL-8) levels in patients with chronic periodontitis (CP). This systematic review compile evidences of the IL8 mRNA and protein levels in gingival tissue, saliva, and gingival crevicular fluid (GCF) investigated in patients with CP. Moreover, 2 meta-analyses were made focusing on the IL-8 levels in GCF and saliva of patients with or without CP.
Methods:
Electronic searches of the PubMed, Web of Science, and Scopus databases were conducted for publications up to February 2016 that investigated the levels of IL-8 detected in individuals with CP compared with health individuals. A total of 31 publications were included in the systematic review. For meta-analyses, the strength of association was calculated by pooled odds ratios with 95% confidence intervals using RevMan 5.1 software. Heterogeneity was examined using Higgins I-squared, tau-squared, and χ2 tests.
Results:
In biopsies of gingival tissue of CP patients, all studies found higher IL8 mRNA levels, and the majority of studies showed higher IL-8 protein levels in this tissue of individuals with moderate to severe CP. Four studies investigating the IL-8 levels in saliva showed inconclusive results. In spite of some studies seemed to indicate higher levels of IL-8 in GCF of CP patients, the meta-analysis results showed significantly lower IL-8 levels (pg/μL) in GCF of CP patients in comparison with periodontally healthy subjects.
Conclusions:
We concluded that IL8 gene expression and IL-8 protein levels were higher in gingival tissues of CP patients when compared to periodontally health individuals. Meta-analysis of studies that measured IL-8 (pg/uL) in GCF found lower levels in CP patients. There are conflicting evidences regarding IL-8 levels in saliva.
Keywords: gingival, gingival crevicular fluid, interleukin-8, periodontal diseases, saliva
1. Introduction
The manifestation of periodontal diseases results from the interaction of host defense mechanisms, microbial agents, environmental and genetic factors. In patients with periodontitis, more frequent occurrence and higher quantities of red-complex bacteria were found. Bacterial metabolites and related molecules trigger the expression of proinflammatory cytokines, which have been associated with the immunopathology of periodontitis.[1]
Inflammation is driven and maintained by cytokines, including chemokines, which are continuously produced by immune cells.[2] Chemokines are a large family of chemotactic cytokines that stimulate the recruitment of inflammatory cells. They are produced by a number of cell types in the periodontium, such as fibroblasts, endothelial cells, macrophages, lymphocytes, and mast cells.[3]
The interplay between microbial species within subgingival biofilms and the adjacent periodontal tissues is often marked by an increase in gingival inflammation and the release of biologically active substances, such as cytokines and chemokines, into the gingival crevicular fluid (GCF).[4] However, in studies analyzing cytokine levels in the periodontium, the results obtained by analyzing the GCF do not always agree with the results from the gingival tissue derived from patients with the same diagnosis. Studies based on GFC analysis also exhibit contradictory findings regarding cytokine levels in patients with and without chronic periodontitis (CP).[5–9] Moreover, some publications have recently addressed the potential diagnostic properties of saliva, since it can be used to help diagnose oral diseases and systemic conditions.[10,11] Importantly, inflammatory cytokines detected in whole saliva do not originate from major salivary glands secretions; instead, the GCF is the probable source of these cytokines.[12]
The analysis of oral fluids (GCF and saliva) can be used to detect both clinical health and diseased status through the examination of the levels of biological markers.[13] The levels of various cytokines within GCF samples collected from periodontal sites can be quantified by enzyme-linked immunosorbent assay (ELISA).[14–18] The number of studies analyzing biomarker levels in saliva has also steadily increased with the development of more advanced methodologies.[19] Moreover, gingival tissues have been utilized to assess the transcriptional level (mRNA) of cytokines,[20–23] as well as to localize specific proteins by in situ hybridization[24,25] or immunohistochemistry.[24–28]
Interleukin-8 (IL-8) is an important chemokine of interest in periodontal diseases. IL-8 is a potent chemoattractant cytokine and activator of neutrophils in inflammatory regions which is released from endothelial cells, gingival fibroblasts, neutrophils, monocytes, and phagocytes in the gingival crevice.[29,30] The unique coordinated expression of IL-8 facilitates the transit of neutrophils from the highly vascularized gingival tissue to the gingival crevice.[25,31]
In spite of the importance of IL-8 chemokine in the panel of CP, a variety of studies have shown contradictory findings regarding IL-8 levels in the GCF and saliva of patients with CP. While some studies demonstrate higher levels of IL-8 in the GCF of patients with CP,[32,33] others show the opposite result[5,34]. Therefore, the aim of this study was to develop a systematic review of the available data regarding IL-8 mRNA and protein levels in gingival tissue, saliva, and GCF in patients with CP. In addition, 2 meta-analyses focusing on IL-8 concentrations in GCF and saliva of patients with or without CP were performed.
2. Material and methods
2.1. Focused question
The focused question was “Do individuals with chronic periodontal disease have different IL-8 levels compared with healthy patients?” Because this question focused on IL-8 levels in the GCF and saliva of subjects, 2 meta-analyses were performed.
2.2. Eligibility criteria
We selected original research articles according to the following inclusion criteria: human clinical studies; cytokine profile in the GCF, saliva, and gingival tissue of patients with and without CP; articles published in the English language. Original research articles that failed to follow 1 or more of the 3 criteria described before were excluded from the systematic review. Furthermore, experimental studies, letters to the editor, historical reviews, and unpublished articles were excluded.
2.3. Literature search
Electronic searches of the PubMed, Web of Science, and Scopus databases were conducted for publications up to February 2016 that investigated the levels of IL-8 in GCF, saliva, and gingival tissue from individuals with chronic periodontal disease compared with healthy individuals. No date filters were applied. Relevant papers were identified through database searches using some combination of the following terms: “IL-8” and “Periodontal Disease”; “IL-8” and “Periodontitis”; “Interleukin-8” and “Periodontal Disease”; “Interleukin-8” and “Periodontitis.” A literature search was conducted using the EndNote Program X7 version (Thomson Reuters, New York, NY) in order to eliminate duplicate references. For searching, we utilized general terms such as periodontal disease (PD), but for the analyses we restricted the studies to CP. Including PD studies, it could decrease the chances of losing studies that had investigated CP, even if they had also investigated aggressive periodontitis (AgP).
Two investigators (LSF and RN) performed the initial search for assessment of titles and abstracts independently, and the results were checked for the agreement. The full text of the articles judged to be relevant based on title and abstract were then independently read and assessed based on the selection criteria. For conflicting evaluations, an agreement was reached following a discussion including a third investigator (RMSC).
2.4. Data extraction
Three investigators (LSF, SCP, and RN) independently reviewed all studies and extracted the data using a standardized form. Among the studies, we classified 3 types of studies according to the biological sample collected for analysis.
2.5. Meta-analysis of the IL-8 GCF levels regarding CP
In order to perform the meta-analysis, it was necessary to select studies that presented numerical values of mean and standard deviation of IL-8 levels in the GCF of patients by ELISA.[5,8,32–36] The meta-analysis was performed by combining the results of different studies (Table 1 ), providing a numerical estimate of the overall effect of interest. It is noteworthy that within the GCF studies we found 2 different units used for measuring the IL-8 levels: pg/site and pg/μL. For this reason, papers for this meta-analysis were divided according to the unit of IL-8 measure (Fig. 1). Six studies presented their results in pg/μL[5,32–36] and 3 studies presented their results in pg/site.[8,34,36] It is worth mentioning that Jin et al[34] and Tsai et al[36] presented their results as both pg/μL and pg/site.
Table 1.
Characteristics of the studies included systematic review.
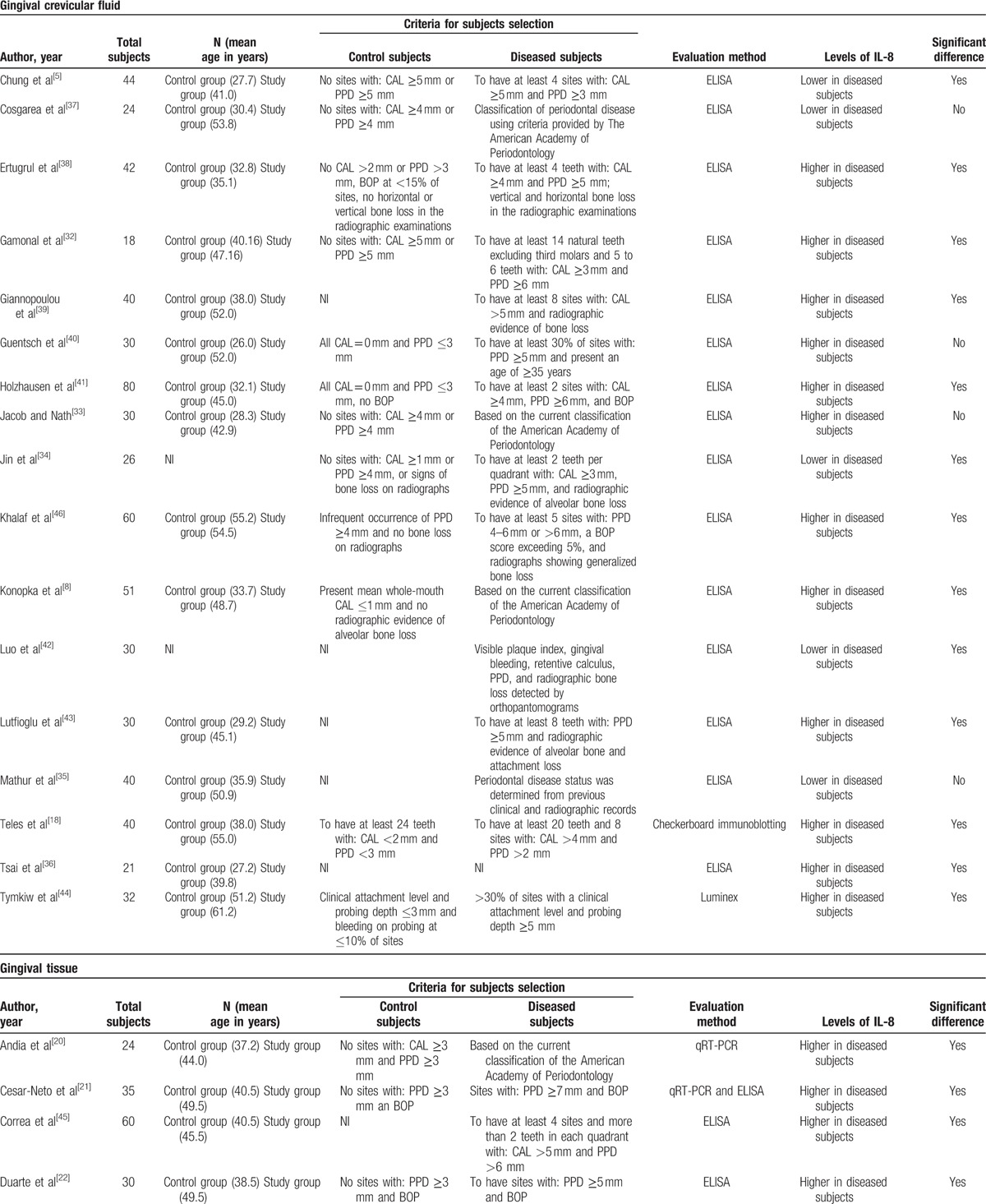
Figure 1.
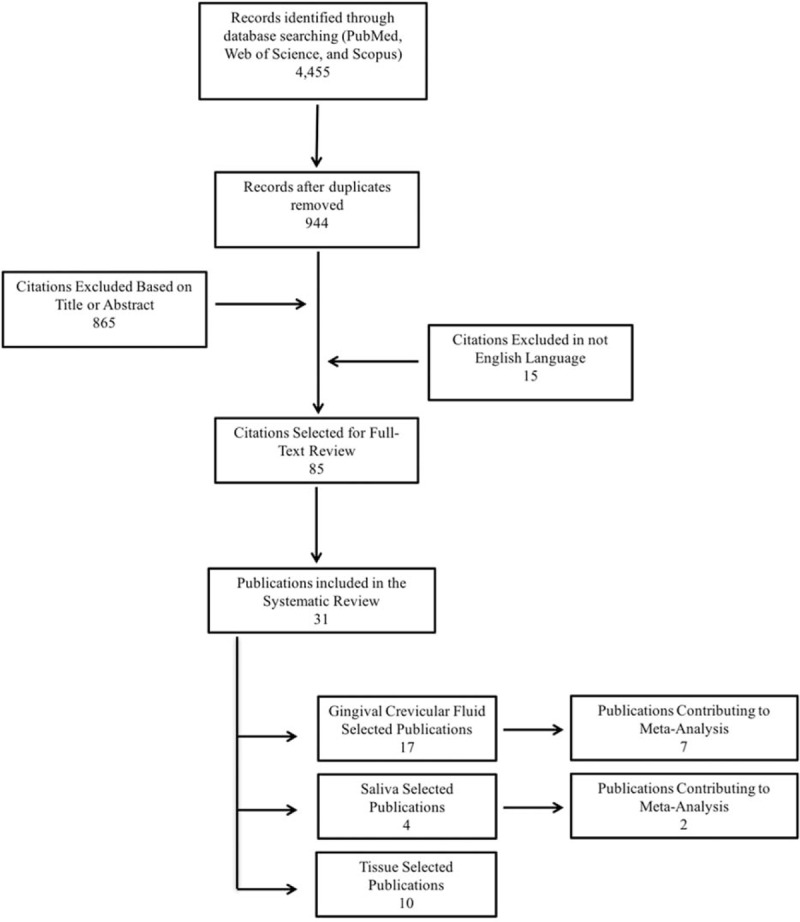
Meta-analysis forest plots of IL-8 levels in the GCF of patients with and without chronic periodontitis. The forest plot is a graphical depiction of the individual results that contributed to meta-analyses. The study results contributing to the meta-analyses were dived into groups according to the unit of measure used in each study. (A) Picograms/μL unit; (B) picograms/site unit. GCF = gingival crevicular fluid, IL-8 = interleukin-8.
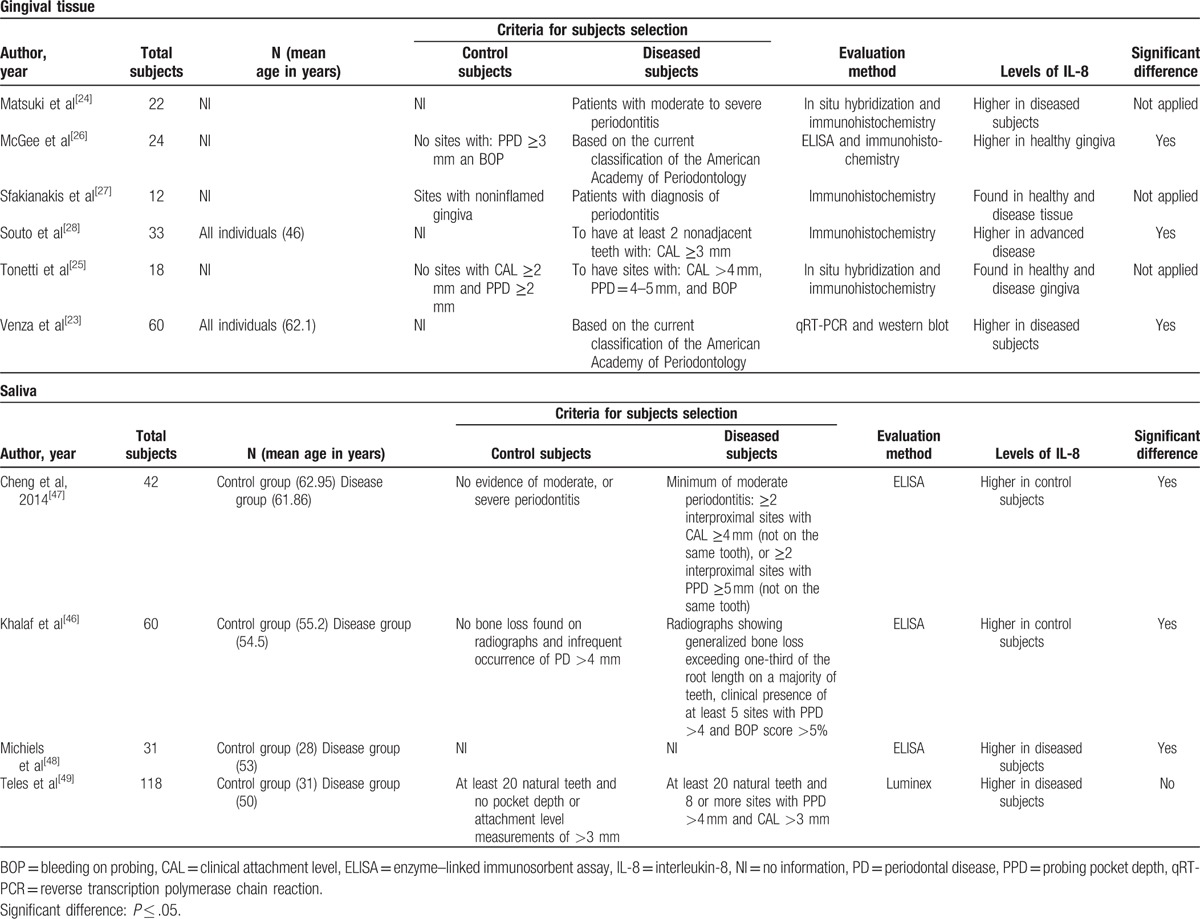
Table 1 (Continued).
Characteristics of the studies included systematic review.
The variance of IL-8 levels was estimated using a 95% confidence interval (CI). The pooled effect was considered significant if 2-sided P-values < .05 were reached. Statistical software (Review Manager (RevMan) Version 5.1, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011) was used to pool the data and produce the forest plots. Forest plots for each meta-analysis present the raw data (i.e., means, standard deviations (SD), and sample sizes), point estimates (displayed as blocks) and CIs (displayed as lines) for the chosen effect, as well as heterogeneity statistics (χ2 and I2), total number of participants per group, overall average effect (mean difference and Z-statistics), and percent weight assigned to each study. Chi-square (χ2) and inconsistency index (I2) tests were used to assess the heterogeneity of the studies included in this meta-analysis. The heterogeneity of the trials will be significantly indicated if I2 >25% and P < .1, instead of P < .05, as that test has a low power. The random-effects model (Der Simonian-Laird method) was applied.
3. Results
The electronic search generated 4455 hits, which represented 944 unique citations. A total of 85 publications were obtained as full-text copies and 54 of these publications were later excluded on the basis of a priori criteria (Fig. 2). The remaining 31 publications were divided according to the biological sample collected for analysis: 17 studies used GCF, 4 studies used saliva, and 10 studies used gingival tissue.
Figure 2.
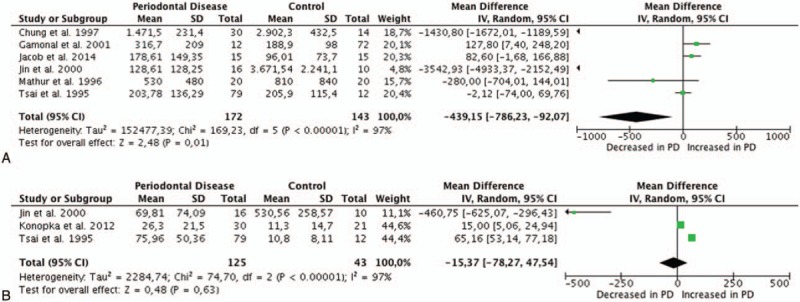
Flowchart outlining the search and the results obtained at different stages of this study.
3.1. Findings of the IL-8 levels in GCF and meta-analysis
A total of 17 studies evaluated IL-8 profiles in the GCF of patients with and without CP. According to Table 1 , only 2 studies did not use the ELISA method for cytokine detection.[18,44] The number of subjects sampled ranged from 18 to 80 patients. Twelve studies reported higher IL-8 levels, and 5 presented lower IL-8 levels in the GCF of the diseased group in comparison to healthy individuals. This elevated number of publications and contradictions among studies motivated us to perform the meta-analysis for assessing the IL-8 levels in the GCF of patients with and without CP. The heterogeneity test showed a statistically significant high heterogeneity among studies which measured IL-8 levels in pg/μL (I2 = 97%; P = < .0001), as well as for studies using pg/site analysis (I2 = 97%; P < .0001).
In studies using pg/μL, the random effect model showed an estimated mean difference of –439.15 pg/μL (95% confidence interval [CI]: –786.23 to –92.07; Fig. 1A). However, studies using pg/site, the meta-analysis revealed a nonsignificant mean difference of –15.37 pg/site (95% CI: –78.27 to 47.54; Fig. 1B). These findings indicate that patients with CP demonstrated significant lower levels of IL-8 in comparison to healthy individuals when IL-8 levels were measured in pg/μL, while no significant results were observed when levels were measured in pg/site.
3.2. Findings of the IL-8 levels in gingival tissue
Table 1 shows 10 studies that assessed the IL-8 mRNA or protein levels in gingival tissue. All 4 studies investigating the IL8 mRNA levels (IL8 gene expression) using qRT-PCR demonstrated higher levels of IL8 mRNA in the gingival tissue of individuals with CP in comparison to healthy individuals.[20–23] In order to investigate IL-8 protein levels in the gingival tissues of individuals with and without CP, the studies utilized different methodologies, including ELISA (3 studies),[21,26,45] western blot (1 study),[23] and immunohistochemistry (5 studies)[24–28] (Table 1 ). Two of the ELISA studies[22,45] and the western blot[23] study showed higher IL-8 protein levels in the gingival tissue of patients with CP. These results agreed with those examining protein levels in GCF via ELISA, without considering the meta-analysis, as mentioned above. However, the results of the immunohistochemistry studies were inconsistent. Two of these studies[24,28] reported higher levels of IL-8 in individuals with periodontal disease and only 1[27] study found higher levels of IL-8 in healthy tissues. The remaining 2 studies only evaluated the IL-8 presence in healthy or diseased gingival tissue, without comparing the IL-8 levels between individuals with and without CP.[25,26]
3.3. Findings of the IL-8 levels in saliva
According to Table 1 , the electronic search generated 4 studies assessing cytokine profile (including IL-8 levels) in the saliva of patients with and without CP.[46–49] Only 1 study used the Luminex evaluation method[49] and the other 3 used the ELISA method.[46–49] The number of subjects sampled ranged from 31 to 118 patients. In regard to the assessed parameters, probing pocket depth was evaluated by 3 studies, whereas clinical attachment level was evaluated by 2 studies and bleeding on probing by 1. Two studies presented higher[46,47] while the other 2 studies[46,47] detected lower IL-8 protein levels in the diseased group. Interestingly, significant differences were found only in 1 study, which reported higher levels of IL-8 in control subjects; however, this study did not report the numerical data (mean/standard deviation) of IL-8 protein levels.[46] Only 2 other studies reported the numerical data (mean/standard deviation) for IL-8 protein levels.[47,48]
Using the heterogeneity test, we observed no statistically significant heterogeneity among the studies (I2 = 0%; P = 1). The fixed effect model showed a nonsignificant mean difference of 0.01 pg/μL (95% CI: –0.14 to 0.16) among them (Fig. 3).
Figure 3.
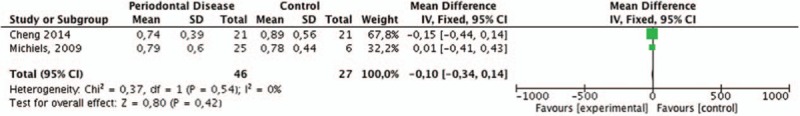
Meta-analysis forest plots of IL-8 levels (picograms/μL) in the saliva of patients with and without chronic periodontitis. IL-8 = interleukin-8.
4. Discussion
This study investigated IL-8 mRNA and protein levels in GCF, saliva, and gingival tissues from patients with CP compared with periodontally healthy individuals. In general, the meta-analysis results demonstrated significantly lower levels of IL-8 in the GCF of patients with CP when levels were measured in pg/μL. On the other hand, the majority of studies that investigated gingival tissues found higher levels of IL8 gene expression and IL-8 protein levels in individuals with CP. For IL-8 protein levels in saliva, the results were inconclusive.
As the systematic review revealed an elevated number of publications with contradictory results of the IL-8 levels in GCF, a meta-analysis was developed to assess the IL-8 levels in the GCF of patients with and without CP. Some of the studies presented in Table 1 were eligible to be included in the meta-analysis. Interestingly, the meta-analysis of studies that utilized pg/μL as the unit of IL-8 measurement showed that the GCF of patients with CP presented significantly lower IL-8 levels than the GCF of healthy control subjects (Fig. 1A). Zhang et al[50] demonstrated that both gingival and oral epithelial cells infected with P. gingivalis produced IL-8, and after infection these cells continued to express IL8 mRNA, although the accumulation of the secreted protein could not be detected. The authors suggested that IL-8 could be degraded locally by P. gingivalis proteinases.[50] This finding might explain the results of the present meta-analysis, but further research should confirm or negate this hypothesis.
The units used to measure IL-8 levels in GCF are also important and may contribute to some of the discrepancies between studies. Chung et al[5] suggested that the function and biological activity of different mediators should influence the unit of measurement. In the case of IL-8 and other cytokines, in which functional activity depends on binding and interaction with cell surface receptors, these mediators should be more appropriately reported as GCF concentrations. The majority of case–control studies which were eligible for this meta-analysis fell into this category (i.e., they presented the data as pg/μL). Interestingly, a significant result was obtained after meta-analysis only when studies that measured IL-8 in pg/μL were included. Therefore, it is evident that, for the purposes of statistical analysis, it is necessary to group together only studies that used the same units to measure IL-8 levels. There is currently no agreement between among researchers concerning the methodology for measuring IL-8 concentrations in GCF.[5,35] In the literature, 3 methods of measurement to quantify IL-8 have been reported: total activity per timed sample (amount or units per 30-second sample); protein concentration (amount or units per unit of GCF volume); or total activity (amount per sample or site). Lamster et al[51] suggested that the total cytokine amount in GCF might be more representative of the disease status than the evaluation of protein concentration.[51] In addition, Chapple et al[52] emphasized that volumes are variable regardless of inflammatory status. Therefore, they proposed utilizing the total marker activity per 30 seconds in GCF samples, rather than the concentration of the marker, because it might provide a better correlation with health or disease status.[52] To measure gingival fluid, the use of micropipettes, ninhydrin staining, and fluorescein is complicated and time-consuming, but an electric measuring device, the “Periotron,” is efficient and can digitally demonstrate the amount of gingival fluid.[53] Therefore, the use of a Periotron provides a confident measurement of GCF volume, which is required for calculating the protein concentration present in the GCF. This procedure eliminates the influence of gingival inflammatory status when measuring protein levels in GCF. Consequently, the best choice for quantifying a protein in the GCF seems to be using the Periotron and pg/μL as a unit of measurement.[5]
The IL-8 protein was also found in saliva; however, the studies examining salivary IL-8 levels showed were variable and inconclusive (Table 1 ), as demonstrated by the meta-analysis presented in Fig. 3. Only Khalaf et al[46] reported significantly higher IL-8 levels in saliva from periodontally healthy individuals in comparison with those affected by CP.[46] Interestingly, this result is in agreement with the meta-analysis in GCF, which showed higher levels of IL-8 in the GCF of control subjects. The final dilution provided by saliva depends on the percentage of sites exhibiting severe periodontitis (deep pocket sites) in a subject.[54] Therefore, more severe periodontal disease, exemplified by a higher mean percentage of sites with pocket depth, might be associated with higher IL-8 levels.[11,49] The lack of association between the levels of salivary biomarkers and periodontal disease could be explained by differences in the methods of saliva collection (stimulated or unstimulated), processing (speed and time of centrifugation), storage (time, temperature, and presence/absence of protease inhibitors), or the methodology used for biomarker quantification (ELISA vs Luminex).[49] In addition, an extensive dilution of the GCF containing these cytokines in saliva[49] could have relevant impact on different results in studies focusing saliva and periodontitis. Furthermore, the presence of putative inhibitors, such as mucin-like proteins or other large molecules and enzymes, could also interfere with the IL-8 levels in saliva.[55]
The IL8 mRNA levels in gingival tissue were found to be higher in individuals with CP in comparison to healthy individuals in 3 studies.[20,21,23] This transcriptional IL8 measurement agrees with the translational IL-8 measurement, as demonstrated in 7 of 10 studies utilizing ELISA, western blot, and immunohistochemistry.[22,24–28,45] These studies demonstrated higher IL-8 concentrations in gingival tissue from individuals with moderate to severe CP. Immunohistochemistry of periodontitis tissue specimens showed maximal IL-8 detection in deeper layers of the pocket epithelium, in close spatial relationship with the inflammatory infiltrate and associated with polymorphonuclear leukocyte (PMN) infiltration. This indicates an involvement of IL-8 in the induction and development of periodontitis.[25] Conversely, lower IL-8 levels and numbers of PMN were also detected in healthy gingival specimens, mainly in the coronal third of the junctional epithelium. This suggests a role of this chemokine in the constant migration of neutrophils through the gingival tissues and in the establishment of equilibrium between the continuous bacterial challenge and the host defense.[25,27] Therefore, IL-8 could play a multifunctional role in the pathogenesis of periodontal disease.[27]
The number of meta-analysis studies has increased in recent years, reflecting the interest of researchers in finding consistent information regarding different issues. In this context, the present meta-analysis and systematic review contributes to the area because of the methodological care used, since it was developed according to the QUOROM statement.[56] Reliable results depend on the use of rigor in eligibly reports in meta-analyses and systematic reviews. The main limitations of the present study were differences regarding the criteria used to classify an individual as periodontally diseased or healthy. This may have contributed to the high heterogeneity observed by the statistical analysis employed in the meta-analysis. The challenge for future meta-analysis studies is to find reports that are as similar as possible with regard to the clinical parameters used for selecting patients, the unit of cytokine measurement, and the utilized sampling.
Furthermore, the absence of numerical data (mean/standard deviation) in reports investigating the IL8 gene expression and IL-8 protein levels in gingival tissue precludes the inclusion of these studies in a meta-analysis. For IL-8 protein levels in saliva, only 2 studies reported the numerical data (mean/standard deviation) necessary to perform the meta-analysis. Moreover, only 1 study presented the criteria used by the authors to classify an individual as periodontally diseased or healthy.
In conclusion, this systematic review showed higher IL8 gene expression and IL-8 protein levels in gingival tissues from individuals with CP compared with periodontally healthy patients. There are conflicting evidences regarding IL-8 levels in saliva. Moreover, although the results of the studies were highly heterogeneous, many studies reported higher IL-8 levels in the GCF of patients with CP as compared to healthy controls; however, a meta-analysis of studies that measured cytokine levels using pg/uL found lower levels of IL-8 in CP patients.
Footnotes
Abbreviations: AgP = aggressive periodontitis, Chi2 = chi-squared, CI = confidence interval, CP = chronic periodontitis, ELISA = enzyme-linked immunosorbent assay, GCF = gingival crevicular fluid, I2 = inconsistency index, IL-8 = interleukin-8, mRNA = messenger RNA, ORs = odds ratios, PD = periodontal disease, PMN = polymorphonuclear leukocyte, SD = standard deviation.
The authors have no conflicts of interest to disclose.
References
- [1].Ferreira SB, Jr, Trombone AP, Repeke CE, et al. An interleukin-1beta (IL-1beta) single-nucleotide polymorphism at position 3954 and red complex periodontopathogens independently and additively modulate the levels of IL-1beta in diseased periodontal tissues. Infect Immun 2008;76:3725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andrukhov O, Ulm C, Reischl H, et al. Serum cytokine levels in periodontitis patients in relation to the bacterial load. J Periodontol 2011;82:885–92. [DOI] [PubMed] [Google Scholar]
- [3].Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol 2008;79(8 suppl):1585–91. [DOI] [PubMed] [Google Scholar]
- [4].Gursoy UK, Kononen E, Uitto VJ. Stimulation of epithelial cell matrix metalloproteinase (MMP-2, -9, -13) and interleukin-8 secretion by fusobacteria. Oral Microbiol Immunol 2008;23:432–4. [DOI] [PubMed] [Google Scholar]
- [5].Chung RM, Grbic JT, Lamster IB. Interleukin-8 and beta-glucuronidase in gingival crevicular fluid. J Clin Periodontol 1997;24:146–52. [DOI] [PubMed] [Google Scholar]
- [6].Gamonal J, Acevedo A, Bascones A, et al. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol 2000;71:1535–45. [DOI] [PubMed] [Google Scholar]
- [7].Goutoudi P, Diza E, Arvanitidou M. Effect of periodontal therapy on crevicular fluid interleukin-6 and interleukin-8 levels in chronic periodontitis. Int J Dent 2012;2012:362905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Konopka L, Pietrzak A, Brzezinska-Blaszczyk E. Effect of scaling and root planing on interleukin-1beta, interleukin-8 and MMP-8 levels in gingival crevicular fluid from chronic periodontitis patients. J Periodontal Res 2012;47:681–8. [DOI] [PubMed] [Google Scholar]
- [9].Teles RP, Sakellari D, Konstantinidis A, et al. Application of the checkerboard immunoblotting technique to the quantification of host biomarkers in gingival crevicular fluid. J Periodontol 2009;80:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hu S, Loo JA, Wong DT. Human saliva proteome analysis and disease biomarker discovery. Expert Rev Proteomics 2007;4:531–8. [DOI] [PubMed] [Google Scholar]
- [11].Miller CS, King CP, Jr, Langub MC, et al. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J Am Dent Assoc 2006;137:322–9. [DOI] [PubMed] [Google Scholar]
- [12].Ruhl S, Hamberger S, Betz R, et al. Salivary proteins and cytokines in drug-induced gingival overgrowth. J Dent Res 2004;83:322–6. [DOI] [PubMed] [Google Scholar]
- [13].Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 2010;8:481–90. [DOI] [PubMed] [Google Scholar]
- [14].Gorska R, Gregorek H, Kowalski J, et al. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol 2003;30:1046–52. [DOI] [PubMed] [Google Scholar]
- [15].Duarte PM, da Rocha M, Sampaio E, et al. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: a pilot study. J Periodontol 2010;81:1056–63. [DOI] [PubMed] [Google Scholar]
- [16].Yamazaki K, Honda T, Oda T, et al. Effect of periodontal treatment on the C-reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. J Periodontal Res 2005;40:53–8. [DOI] [PubMed] [Google Scholar]
- [17].Chen YW, Umeda M, Nagasawa T, et al. Periodontitis may increase the risk of peripheral arterial disease. Eur J Vasc Endovasc Surg 2008;35:153–8. [DOI] [PubMed] [Google Scholar]
- [18].Teles R, Sakellari D, Teles F, et al. Relationships among gingival crevicular fluid biomarkers, clinical parameters of periodontal disease, and the subgingival microbiota. J Periodontol 2010;81:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Salazar MG, Jehmlich N, Murr A, et al. Identification of periodontitis associated changes in the proteome of whole human saliva by mass spectrometric analysis. J Clin Periodontol 2013;40:825–32. [DOI] [PubMed] [Google Scholar]
- [20].Andia DC, de Oliveira NF, Letra AM, et al. Interleukin-8 gene promoter polymorphism (rs4073) may contribute to chronic periodontitis. J Periodontol 2011;82:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cesar-Neto JB, Duarte PM, de Oliveira MC, et al. Smoking modulates interleukin-6:interleukin-10 and RANKL:osteoprotegerin ratios in the periodontal tissues. J Periodontal Res 2007;42:184–91. [DOI] [PubMed] [Google Scholar]
- [22].Duarte PM, de Oliveira MC, Tambeli CH, et al. Overexpression of interleukin-1beta and interleukin-6 may play an important role in periodontal breakdown in type 2 diabetic patients. J Periodontal Res 2007;42:377–81. [DOI] [PubMed] [Google Scholar]
- [23].Venza I, Visalli M, Cucinotta M, et al. Proinflammatory gene expression at chronic periodontitis and peri-implantitis sites in patients with or without type 2 diabetes. J Periodontol 2010;81:99–108. [DOI] [PubMed] [Google Scholar]
- [24].Matsuki Y, Yamamoto T, Hara K. Detection of inflammatory cytokine messenger RNA (mRNA)-expressing cells in human inflamed gingiva by combined in situ hybridization and immunohistochemistry. Immunology 1992;76:42–7. [PMC free article] [PubMed] [Google Scholar]
- [25].Tonetti MS, Imboden MA, Gerber L, et al. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun 1994;62:4005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McGee JM, Tucci MA, Edmundson TP, et al. The relationship between concentrations of proinflammatory cytokines within gingiva and the adjacent sulcular depth. J Periodontol 1998;69:865–71. [DOI] [PubMed] [Google Scholar]
- [27].Sfakianakis A, Barr CE, Kreutzer DL. Localization of the chemokine interleukin-8 and interleukin-8 receptors in human gingiva and cultured gingival keratinocytes. J Periodontal Res 2002;37:154–60. [DOI] [PubMed] [Google Scholar]
- [28].Souto GR, Queiroz CM, Jr, Costa FO, et al. Relationship between chemokines and dendritic cells in human chronic periodontitis. J Periodontol 2014;85:1416–23. [DOI] [PubMed] [Google Scholar]
- [29].Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res 1993;28(6 pt 2):500–10. [DOI] [PubMed] [Google Scholar]
- [30].Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol 1993;64(5 suppl):456–60. [PubMed] [Google Scholar]
- [31].Tonetti MS, Imboden MA, Lang NP. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol 1998;69:1139–47. [DOI] [PubMed] [Google Scholar]
- [32].Gamonal J, Acevedo A, Bascones A, et al. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and RANTES chemokines in adult periodontitis. J Periodontal Res 2001;36:194–203. [DOI] [PubMed] [Google Scholar]
- [33].Jacob PS, Nath S. Evaluation of interleukin-1beta and 8 in gutka chewers with periodontitis among a rural Indian population. J Periodontal Implant Sci 2014;44:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jin L, Soder B, Corbet EF. Interleukin-8 and granulocyte elastase in gingival crevicular fluid in relation to periodontopathogens in untreated adult periodontitis. J Periodontol 2000;71:929–39. [DOI] [PubMed] [Google Scholar]
- [35].Mathur A, Michalowicz B, Castillo M, et al. Interleukin-1 alpha, interleukin-8 and interferon-alpha levels in gingival crevicular fluid. J Periodontal Res 1996;31:489–95. [DOI] [PubMed] [Google Scholar]
- [36].Tsai CC, Ho YP, Chen CC. Levels of interleukin-1 beta and interleukin-8 in gingival crevicular fluids in adult periodontitis. J Periodontol 1995;66:852–9. [DOI] [PubMed] [Google Scholar]
- [37].Cosgarea R, Dannewitz B, Sculean A, et al. Bacterial and inflammatory behavior of implants in the early healing phase of chronic periodontitis. Quintessence Int 2012;43:491–501. [PubMed] [Google Scholar]
- [38].Ertugrul AS, Sahin H, Dikilitas A, et al. Comparison of CCL28, interleukin-8, interleukin-1beta and tumor necrosis factor-alpha in subjects with gingivitis, chronic periodontitis and generalized aggressive periodontitis. J Periodontal Res 2013;48:44–51. [DOI] [PubMed] [Google Scholar]
- [39].Giannopoulou C, Kamma JJ, Mombelli A. Effect of inflammation, smoking and stress on gingival crevicular fluid cytokine level. J Clin Periodontol 2003;30:145–53. [DOI] [PubMed] [Google Scholar]
- [40].Guentsch A, Pfister W, Cachovan G, et al. Oral prophylaxis and its effects on halitosis-associated and inflammatory parameters in patients with chronic periodontitis. Int J Dent Hyg 2014;12:199–207. [DOI] [PubMed] [Google Scholar]
- [41].Holzhausen M, Cortelli JR, Araujo da Silva V, et al. Protease-activated receptor-2 (PAR(2)) in human periodontitis. J Dental Res 2010;89:948–53. [DOI] [PubMed] [Google Scholar]
- [42].Luo L, Xie P, Gong P, et al. Expression of HMGB1 and HMGN2 in gingival tissues, GCF and PICF of periodontitis patients and peri-implantitis. Archives Oral Biol 2011;56:1106–11. [DOI] [PubMed] [Google Scholar]
- [43].Lutfioglu M, Aydogdu A, Sakallioglu EE, et al. Gingival crevicular fluid interleukin-8 and lipoxin A levels of smokers and nonsmokers with different periodontal status: a cross-sectional study. J Periodontal Res 2015;51:471–80. [DOI] [PubMed] [Google Scholar]
- [44].Tymkiw KD, Thunell DH, Johnson GK, et al. Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. J Clin Periodontol 2011;38:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Correa JD, Madeira MF, Resende RG, et al. Association between polymorphisms in interleukin-17A and -17F genes and chronic periodontal disease. Mediators Inflamm 2012;2012:846052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Khalaf H, Lonn J, Bengtsson T. Cytokines and chemokines are differentially expressed in patients with periodontitis: possible role for TGF-beta1 as a marker for disease progression. Cytokine 2014;67:29–35. [DOI] [PubMed] [Google Scholar]
- [47].Lisa Cheng YS, Jordan L, Gorugantula LM, et al. Salivary interleukin-6 and -8 in patients with oral cancer and patients with chronic oral inflammatory diseases. J Periodontol 2014;85:956–65. [DOI] [PubMed] [Google Scholar]
- [48].Michiels K, Schutyser E, Conings R, et al. Carcinoma cell-derived chemokines and their presence in oral fluid. Eur J Oral Sci 2009;117:362–8. [DOI] [PubMed] [Google Scholar]
- [49].Teles RP, Likhari V, Socransky SS, et al. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J Periodontal Res 2009;44:411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang J, Dong H, Kashket S, et al. IL-8 degradation by Porphyromonas gingivalis proteases. Microb Pathog 1999;26:275–80. [DOI] [PubMed] [Google Scholar]
- [51].Lamster IB, Oshrain RL, Gordon JM. Enzyme activity in human gingival crevicular fluid: considerations in data reporting based on analysis of individual crevicular sites. J Clin Periodontol 1986;13:799–804. [DOI] [PubMed] [Google Scholar]
- [52].Chapple IL, Matthews JB, Thorpe GH, et al. A new ultrasensitive chemiluminescent assay for the site-specific quantification of alkaline phosphatase in gingival crevicular fluid. J Periodontal Res 1993;28:266–73. [DOI] [PubMed] [Google Scholar]
- [53].Tsuchida K, Hara K. Clinical significance of gingival fluid measurement by “Periotron”. J Periodontol 1981;52:697–700. [DOI] [PubMed] [Google Scholar]
- [54].Goodson JM. Gingival crevice fluid flow. Periodontology 20002003;31:43–54. [DOI] [PubMed] [Google Scholar]
- [55].Wozniak KL, Arribas A, Leigh JE, et al. Inhibitory effects of whole and parotid saliva on immunomodulators. Oral Microbiol Immunol 2002;17:100–7. [DOI] [PubMed] [Google Scholar]
- [56].Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896–900. [DOI] [PubMed] [Google Scholar]


