Supplemental Digital Content is available in the text
Keywords: adults, meta-analysis, migraine, serotonin norepinephrine reuptake inhibitor, selective serotonin reuptake inhibitors, tricycle antidepressants
Abstract
Background:
Migraine, ranked as the 7th-highest specific cause of disability worldwide, has caused an enormous burden on the economy and society. Tricyclic antidepressant (TCA) is one of the most commonly drugs for migraine prevention. However, evidence about the efficacy and tolerability of TCAs in the prophylaxis of migraine in adults is somewhat confusing.
Methods:
A computerized literature search of the PubMed, Embase, Cochrane, and Web of Science databases from inception to July 2016 was conducted. We reviewed all randomized controlled trials that assigned adults with a clinical diagnosis of migraine to TCAs or other treatments (placebo or other antidepressants). Reduction in migraine frequency or index and response rates to treatment were defined as the efficacy outcomes. Rates of dropout due to adverse effects were defined as the tolerability outcomes.
Results:
In total 12 trials consisting of 1006 participants were identified: 9 trials compared TCAs with placebo, and the other 3 compared amitriptyline with selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs). A significant advantage of TCAs compared with placebo in the prevention of migraine in adults was observed (standardized mean difference [SMD] = −.75; 95% confidence interval [CI] = −1.05 to −.46; P < .00001). Participants receiving TCAs were more likely to experience an ≥50% reduction in their headache burden than those receiving placebo (risk ratio [RR] =1.40; 95% CI = 0.89–2.20; P = .14). In addition, the efficacy between amitriptyline and SSRIs or SNRIs did not differ for migraine prevention in adults (SMD = −.01; 95% CI = −0.31 to 0.28; P = .94) based on the available limited trials. However, TCAs were less well tolerated than placebo (RR = 1.73; 95% CI = 1.00–2.99; P = .05) and SSRI or SNRI (RR = 2.85; 95% CI = 0.97–8.41; P = .06) on account of adverse events.
Conclusions:
This research reveals that TCAs were more effective than placebo, but no more than SSRI or SNRI in ameliorating the headache burden in adults with migraine. However, TCAs appeared to be less tolerated than placebo and SSRIs or SNRIs for some side effects.
1. Introduction
Migraine is a common and chronic primary headache form ranked as the seventh highest specific cause of disability worldwide.[1] The prevalence of migraine headaches in North America, Europe, and Asia had been found to be 8.4% to 18%,[2,3] causing a significant burden on the economy as well as society worldwide. The pathogenesis of migraine has not yet been fully understood, and it seems that abnormal serotonin (5-HT) and its receptors[4] might implicate in such processes. Evidence suggests that a low 5-HT levels facilitate the activation of the trigeminovascular nociceptive pathway.[5]
Most antidepressants aim at increasing the extracellular level of serotonin neurotransmission by inhibiting its reuptake into the presynaptic cell, and their potential benefits in the prophylaxis of migraine have been extensively evaluated. Preventive treatment aims at eliminating headache burden without intolerable harms, reducing chances of acute therapy, and improving the quality of patients’ life. Tricyclic antidepressants (TCAs) were the first agents shown to be effective in the prophylaxis of headaches in 1964[6] and have been one of the most commonly drugs for migraine prevention.[7] At the same time, selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) are also mentioned as possible preventive treatments for migraine in clinical guidelines.[8]
In a previous systematic review of TCAs for migraine,[9] TCAs were found to be more effective than placebo and SSRIs in achieving 50% reduction in migraine burden, whereas TCAs appeared to be less well tolerated. However, this analysis and another meta-analysis[10] included studies recruiting patients with comorbidity of migraine and tension-type headache, which was impossible to extract separate data on patients with migraine. Of course, as migraine usual combining with other type of headaches, mixed headache such as comorbidity of migraine and tension-type headache are worthy to study and need to be included in our analysis. But as previous studies have found that antidepressants were effective in preventing other headaches, recruiting patients with mixed headache may lead to an overestimate of the efficacy of TCAs in the prophylaxis of migraine and weakening the credibility of results. To resolve such concerns and sufficiently understand the magnitude of beneficial effects and adverse effects of TCAs in migraine, we conducted a meta-analysis that assess the efficacy and tolerability of TCAs in reducing the headache burden among adults with migraine only. And we also conducted several subgroup analyses and sensitivity analyses to further confirm our results.
2. Methods
2.1. Data sources and search strategy
PubMed, Embase, Cochrane, and Web of Science databases from inception to July 2016 were searched following the search strategies (Supplemental Table). No language restrictions were applied in this investigation. To avoid omitting relevant trials, conference abstracts and reference lists of all identified related publications were also searched.
2.2. Selection criteria
Studies were identified based on the following criteria: randomized controlled trials (RCTs) involving adults (≥18 years) with a primary diagnosis of migraine, described in the Ad Hoc Committee on the Classification of Headache[11] or the International Headache Society.[12–14] If there were no such criteria specified in the study, it had to be based on the distinctive features of migraine; RCTs comparing TCAs with placebo or comparing amitriptyline with other antidepressants; treatment duration lasting for ≥4 weeks; and complete efficacy outcome was reported.
The exclusion criteria were as follows: reviews, animal trials, or duplicate secondary analyses; combination therapy, such as TCAs combined with psychotherapy; trials including patients with a secondary headache, such as vestibular migraine or menstrual migraine; and outcome data were unavailable or incomplete.
2.3. Outcome measures
For efficacy analyses, both continuous and dichotomous measures for headache burden outcomes were accessed in this study. As migraine frequency and migraine index are the most common measures of migraine severity, we defined continuous outcome as the difference of posttreatment data in migraine frequency or index between groups. When a trial reported both migraine frequency and index, the migraine frequency was preferred, as it was recommended in the International Headache Society outcome recommendations.[15] Dichotomous outcome of headache burden was the proportion of patients who responded to treatment, which was defined as ≥50% reduction in migraine frequency or index from the baseline to endpoint.
For acceptability outcome, it was assessed by the proportion of patients who prematurely terminated the study for any reason. For tolerability outcome, it was represented by the proportion of patients who dropout for adverse effects of treatment.
2.4. Data extraction and risk of bias assessment
Two reviewers independently screened the titles and abstracts of each literature, verified all potentially suitable trials by the inclusion and exclusion criteria described above. Data abstracted from the RCTs included the study designs, participants’ characteristics, and outcomes (response and withdrawals). For data that could not be directly extracted, we tried to search other studies citing the RCT to obtain the data. We assessed the studies’ methodological quality using the “Risk of bias” tool developed by the Cochrane Collaboration.[16] Any disagreements were solved via discussion or following arbitration by a third reviewer if necessary.
2.5. Statistical analysis
We performed all analyses using RevMan5.3 software (Cochrane Information Management System). As the different measurement of headache burden in the studies, we expected that data sets on efficacy would be considerable heterogeneous. We calculated standardized mean differences (SMDs) along with 95% confidence intervals (CIs) for continuous outcomes. For dichotomous outcomes, we calculated risk ratios (RRs) along with 95% CIs. We carried out this meta-analysis on the full intention-to-treat (ITT) population when possible. Heterogeneity was evaluated with the χ2 -based Q test and I2 statistic.[16] We decided to use a random-effects model to do analysis, as there was expected diversity in the TCA medications and the measurement of headache burden. Owing to some trials did not provide baseline data of treatment, we analyzed the posttreatment data, which rely on allocation to achieve between-group balance, rather than the group mean change scores to calculate the effects of treatments.
We performed various subgroup analyses to examine whether effects evaluation would be influenced by the types of tricycle antidepressants (amitriptyline vs clomipramine vs opipramol), sample size (<50 vs >50) and the measurement of headache burden (migraine frequency vs migraine index). Moreover, a sensitivity analysis, excluding the crossover trials, was performed to reanalyze the overall effect size of tricycle antidepressants. Inverted funnel plots were used to examine the potential publication bias. All tests were 2-sided, and statistical significance was defined as P < .05 unless otherwise stated.
3. Results
3.1. Search findings
Overall, 3002 potential articles through the initial database search were identified (458 from PubMed, 642 from Embase, 1328 from Cochrane, and 574 from Web of Science). After screening of titles and abstracts by 2 reviewers independently, 43 full text articles were retrieved for eligibility. Finally, 12 trials[17–28] met the inclusion criteria were identified for further data extraction (Fig. 1). Nine trials compared TCAs with placebo,[20–28] whereas other 3 trials compared amitriptyline with SSRIs or SNRIs[17–19].
Figure 1.
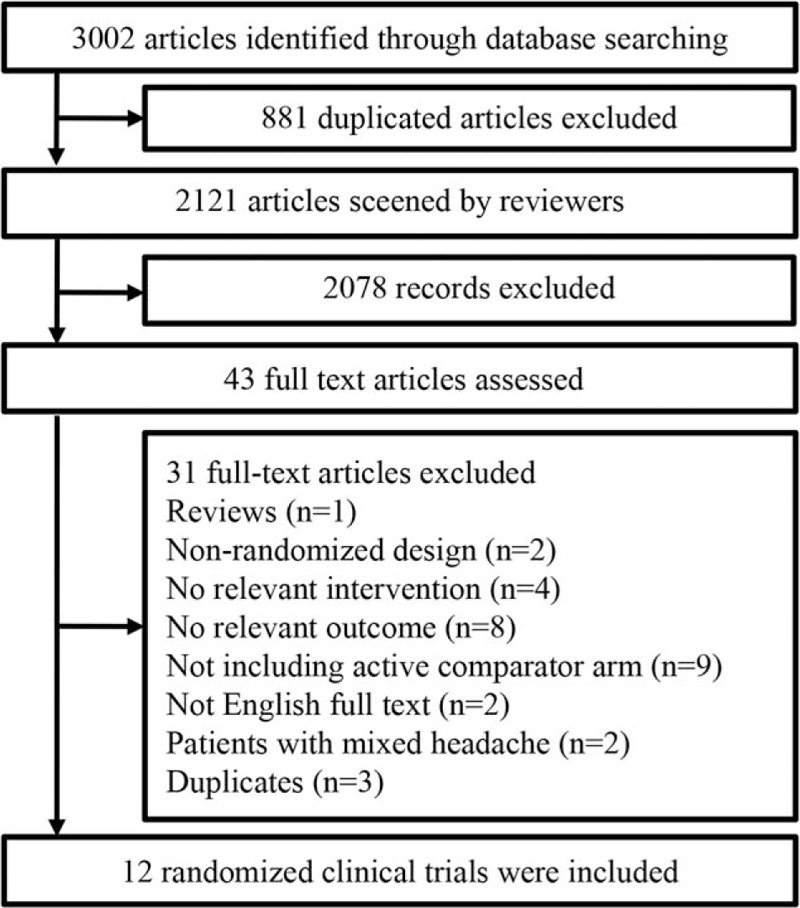
Flowchart of study selection.
3.2. Characteristics of included studies
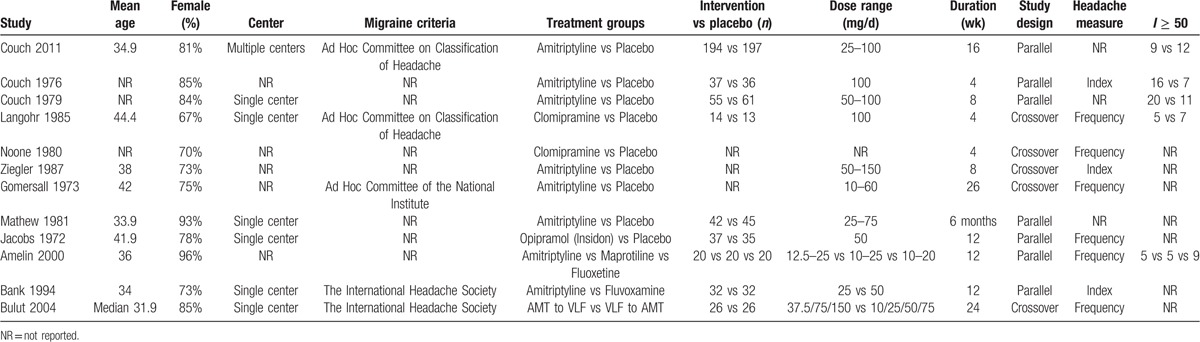
Table 1 summarizes patients’ characteristics and design features of each literature. A total of 1006 participants were enrolled in this analysis, with more women than men (82% vs 18%). Treatment duration of study averaged 11 weeks (range from 4 to 26 weeks) with a mean sample size of 84 participants (range from 10 to 391). The age of the patients ranged from 33.9 to 44.4 years old. Seven trials used parallel designs and 5 used crossover designs. As for the measurement of outcomes, 6 trials reported headache frequency whereas 4 reported headache index.
Table 1.
Demographic and clinical characteristics of included randomized controlled trials.
3.3. Efficacy outcomes
3.3.1. Tricyclics versus placebo
The overall pooled effect size from 7 trials presenting continuous outcomes showed a significant advantage of TCAs over placebo, with a SMD of −.75 (95% CI = −1.05 to −.46; P < .00001) and low heterogeneity (I2 = 30%; P = .20) (Fig. 2). Treatment response rates were available in 4 trials. Participators receiving TCAs therapy were more likely to experience an ≥50% reduction in their headache burden than those receiving placebo therapy, with a RR of 1.40 (95% CI = 0.89–2.20; P = .14) and moderate heterogeneity (I2 = 29%; P = .24) (Supplemental Figure 1). Sensitivity analyses excluding trials with crossover designs also confirmed the positive effects of TCAs for the prophylaxis of migraine in adults (SMD= −.91; 95% CI = −1.36 to −0.46; P < .0001) (Supplemental Figure 2).
Figure 2.
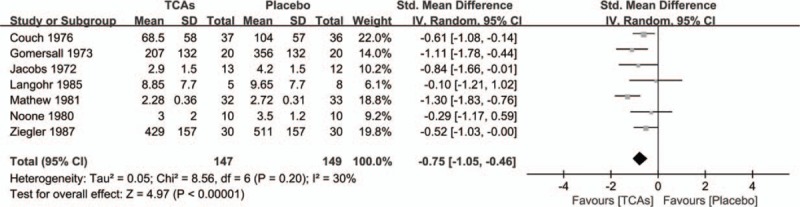
Effect of tricyclic antidepressants in the prevention of migraine compared with placebo.
In this meta-analysis, all antidepressants included in our study (amitriptyline, clomipramine, opipramol) had a significant advantage over placebo (Fig. 3A). Meanwhile, it seemed that longer duration of treatment was associated with greater effects for amitriptyline; patients in the first month (SMD = −.53, 95% CI = −0.97 to −.10; P = .02) of treatment had less improvement than those treated for 6 months (SMD = −.77, 95% CI = −1.34 to −0.20; P = .008) (Fig. 3B). In the groups with a sample size over 50, TCAs showed a statistically significant efficacy compared with the placebo group (SMD = −.94, 95% CI = −1.61 to −0.27; P = .006). This difference also persisted in trials with groups fewer than 50 patients (SMD = −.64, 95% CI = −0.96 to −0.31; P = .0001) (Fig. 3C). In addition, no relationship between types of measurement (Headache frequency vs Headache index) and outcomes was observed (Fig. 3D).
Figure 3.
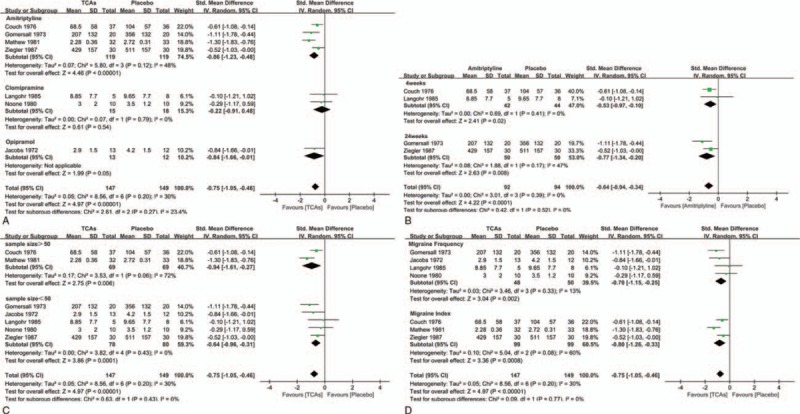
(A) Subgroup analysis of continuous outcomes compared with placebo based on the type of tricyclic antidepressants. (B). Subgroup analysis of continuous outcomes compared with placebo based on the treatment duration. (C). Subgroup analysis of continuous outcomes compared with placebo based on the sample size. (D) Subgroup analysis of continuous outcomes compared with placebo based on the type of measurement.
For tolerability outcomes, moderately higher rates of withdrawals due to adverse events had been found in groups treated with TCAs (RR = 1.73; 95% CI =1.00–2.99; P = .05) (Fig. 4B). However, there was no statistical difference in the number of withdrawals for any reason between TCAs and control groups (RR = .90; 95% CI = 0.76–1.06; P = .21) (Fig. 4A).
Figure 4.
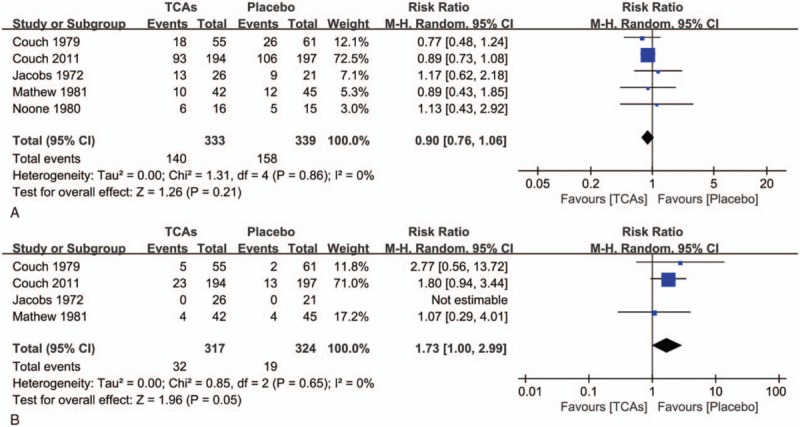
(A) Withdrawals for any reason between tricyclic antidepressants and control groups. (B) Withdrawals for adverse events between tricyclic antidepressants and control groups.
3.4. Amitriptyline versus other antidepressants (SSRIs or SNRIs)
As amitriptyline is a standard drug in migraine prevention, other TCAs are excluded in our analysis to investigate the comparative efficacy between TCAs and other antidepressants. Unfortunately, we did not find studies comparing amitriptyline with other antidepressants except for SSRIs and SNRIs for preventing migraine in adults. In a limited number of trials the efficacy between amitriptyline and SSRIs (SMD = .16; 95% CI = −0.32 to 0.63; P = .52) or SNRIs (SMD = −.13; 95% CI = −0.51 to 0.25; P = .51) did not demonstrate differences for migraine prevention in adults (SMD = −.01; 95% CI = −0.31 to 0.28; P = .94), with no heterogeneity presented (I2 = 0%; P = .38) (Fig. 5). Meanwhile, no significant difference in response rates between SSRIs and amitriptyline was found based on the only one available study (RR = 1.08; 95% CI = 0.41–2.83; P = .87) (Supplemental Figure 3).
Figure 5.
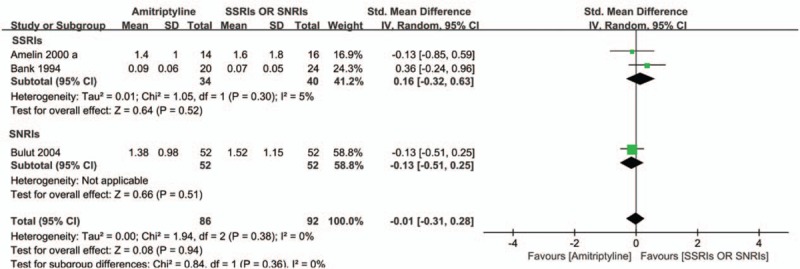
Comparison of effectiveness of t amitriptyline with SSRIs or SNRIs for migraine prevention.
Our analysis suggests that patients receiving amitriptyline were more likely to withdraw from treatment due to adverse effects than those treated with SSRIs or SNRIs (SMD = 2.85; 95% CI = 0.97–8.41; P = .06) with low heterogeneity (I2 = 0%; P = .54) (Fig. 6). However, these results need to be further confirmed because of the limited number of directly comparative efficacy trials that have been conducted.
Figure 6.
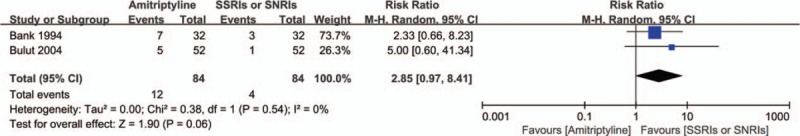
Withdrawals for adverse events between amitriptyline and SSRIs or SNRIs.
3.4.1. Quality assessment and publication bias
Although quality varied among the trials, limitations in reporting of designs were one major concern (Supplemental Figures 4A and 4B). Most studies were at unclear risk with respect to the methods of randomization and allocation concealment. Besides, information on blinding of participants, investigators, and outcome assessment was described in less than half of the trials. In addition, the inverted funnel plots of these trials appeared to be approximately symmetrical (Supplemental Figures 5A and 5B).
4. Discussion
Our meta-analysis justified that there was objective evidence for TCAs in reducing migraine burden in adults with migraine. Over all, we identified 12 trials comparing TCAs with placebo, and amitriptyline with other antidepressants (SSRIs, SNRIs) for preventing migraine headache in adults. We found that TCAs significantly reduced migraine burden and increased response rates in decreasing headache burden for adults with migraine when compared with placebo. Subgroup analyses based on treatment duration, sample size, types of TCAs, and measurements revealed the same pattern of results; and sensitivity analyses excluding trials with crossover designs also revealed a relatively consistent positive effects for TCAs in preventing migraine in adults. We also observed moderate higher rate of withdrawals due to the side effects of TCAs than placebo. Among patients receiving TCAs, they may experience adverse reactions including dry mouth, drowsiness, weight gain, dizziness, nausea, and gastrointestinal upset. However, as TCAs have a definite therapeutic effect on migraine and its side effects can be tolerated in clinic, it could be encouraged to be wildly used to prevent migraine in adults.
In additiony, patients with 6 months of TCAs treatment had more improvement in headache burden than those treated for only 1 month. That is to say, the benefit of TCAs for migraine seems to increase with longer treatment duration. Of course, this analysis should be viewed as exploratory rather than definitive. But this finding could remind us that clinicians should encourage their patients to take tricyclics for several months before deciding to try another prophylactic agent. This also suggests that migraine researches of longer duration are urgently needed.
In this study, we found that there were only 3 studies compared amitriptyline with other antidepressants. The pooled analyses suggested that there were no significantly difference between amitriptyline and SSRIs or SNRIs in terms of migraine frequency and response rates. Meanwhile, amitriptyline appeared to be less tolerated than SSRIs or SNRIs for some side effects. Notably, when we excluded other TCAs except amitriptyline, a standard drug in migraine prevention, we found that TCAs were not be more effective than SSRIs in achieving 50% reduction in migraine burden, which was different from the result of a previous systematic review.[9] Interestingly, though, there is a phenomenon that SSRIs or SNRIs are less popular than amitriptyline in preventing migraine in clinical conditions. As the efficacy of TCAs seems to increase with longer duration in preventing migraine, a highly possibility may be that TCAs are superior to SSRIs or SNRIs on reducing migraine burden with long-term treatment. And because of the more predictable benefits of TCAs in headache prevention, patients show a more tolerant to its side effects and prefer to choose it as a primary drug in migraine. In addition, studies compared amitriptyline with SSRIs in our study lasted only 3 months, which could not reflect the real effects of amitriptyline. So, given such a relatively limited treatment duration and sample size in our analysis, the conclusions that amitriptyline are not superior to SSRIs or SNRIs might be interpreted cautiously and such researches of longer duration are also needed.
Overall, after eliminating trails that discussing about mixed headache, outcomes were not significantly different from the previous results.[9] But we indeed found that the magnitude of beneficial effects of TCAs in migraine was smaller than previously recognized.[9] It means that including studies recruiting patients with mixed headache does lead to overestimating the efficacy of TCAs on migraine. Therefore, to obtain an accurate result, excluding trails on mixed headache is very needed now and in the future. In addition, as comorbidity of migraine and other headaches is very common in clinical, mixed headache is worthy of further investigation.
The strengths of our meta-analysis included the rigorous methodology, standard data extraction procedures and abundant data for analysis. However, several limitations also should be addressed here. First, the majority of the included trials had methodological and/or reporting shortcomings. Less than half of the trials reported the blindness of participants and observers. Information on allocation concealment and randomization was described in only a few studies. Some of these trials were likely to be underpowered, and had missed the intention to treat analysis, which is particularly worrisome since trial experienced up to 50% dropouts. The power of the reliability of outcomes in this meta-analysis could be impaired, and the effect of treatments was likely to be overestimated. Secondly, decreasing the need of symptomatic/analgesic medication could help patients to prevent “rebound” or drug overuse headaches, and has been recognized as an important goal of prophylactic therapy in migraine. Although most studies included patient diaries on the use of symptomatic/analgesic medication during or before the study period, only 2 placebo-controlled studies reported it as an outcome. Third, quality of individual's life, a global measure capable of making useful comparisons between adverse events of drugs,[8] could be affected by the pain associated with migraine both in social and occupational roles. However, we did not find any mention of days off work, data on cost-effectiveness or the quality of life.
5. Conclusions
Our present investigation reveals further evidence supporting the use of TCAs to ameliorate the headache burden in adult patients with migraine. Meanwhile, there were no significant differences between amitriptyline and SSRIs or SNRIs in the prophylaxis of migraine, while it needs to be further confirmed because of the limited treatment duration and methodological shortcomings. Up to now, there is lacking of data regarding sex differences in response to antidepressants, since no trials had controlled for sex, which could have critical implications for pharmacotherapy. Therefore, more RCTs in well-defined sample size, adequate doses and duration of treatment, and controlled sex responses are needed to strengthen our conclusions.
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, ITT = intention-to-treat, RCTs = randomized controlled trials, RRs = risk ratios, SSRIs = selective serotonin reuptake inhibitors, 5-HT = serotonin, SNRIs = serotonin norepinephrine reuptake inhibitors, SMDs = standardized mean differences, TCAs = tricyclic antidepressants.
This study was supported by the National Key Clinical Specialties Construction Program of China and natural science foundation project of Chongqing science and technology commission (ID: cstc 2016jcy jA0423).
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rasmussen BK. Epidemiology of headache. Cephalalgia 2001;21:774–7. [DOI] [PubMed] [Google Scholar]
- [3].Wang SJ. Epidemiology of migraine and other types of headache in Asia. Curr Neurol Neurosci Rep 2003;3:104–8. [DOI] [PubMed] [Google Scholar]
- [4].Segelcke D, Messlinger K. Putative role of 5-HT2B receptors in migraine pathophysiology. Cephalalgia 2017;37:365–71. [DOI] [PubMed] [Google Scholar]
- [5].Hamel E. Serotonin and migraine: biology and clinical implications. Cephalalgia 2007;27:1293–300. [DOI] [PubMed] [Google Scholar]
- [6].Lance JW, Curran DA. Treatment of chronic tension headache. Lancet 1964;1:1236–9. [DOI] [PubMed] [Google Scholar]
- [7].Punay NC, Couch JR. Antidepressants in the treatment of migraine headache. Curr Pain Headache Rep 2003;7:51–4. [DOI] [PubMed] [Google Scholar]
- [8].Banzi R, Cusi C, Randazzo C, et al. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database Syst Rev 2015;4: Art. No.: CD002919. DOI: 10.1002/14651858.CD002919.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jackson JL, Shimeall W, Sessums L, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ 2010;341:c5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jackson JL, Cogbill E, Santana-Davila R, et al. A Comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PloS One 2015;10:e0130733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ad hoc Committee on the Classification of Headache of the National Institute of Neurological Diseases and Blindness. Classification of headache. JAMA 1962;179:717–8. [Google Scholar]
- [12].Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8(Suppl. (7)):1–96. [PubMed] [Google Scholar]
- [13].Headache Classification Subcommittee of the International Headache S. The International Classification of Headache Disorders: 2nd edition. Ceph2lalgia. 2004; 24(Suppl. 1):9–160. [DOI] [PubMed] [Google Scholar]
- [14].Headache Classification Committee of the International Headache S. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. [DOI] [PubMed] [Google Scholar]
- [15].Silberstein S, Tfelt-Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia 2008;28:484–95. [DOI] [PubMed] [Google Scholar]
- [16].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. London, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- [17].Amelin AV, Skoromets AA, Korenko LA, et al. Tumelevich BCh, Gonchar MA. A comparative efficiency of amitriptyline, fluoxetine and maprotiline in prevention of migraine in attack-free period [Sravnitel’naia effektivnost’ amitriptilina, fluoksetina i maprotilina pri lechenii migreni v mezhpristupnom periode]. Zhurnal Nevrologii i Psikhiatrii Imeni SS Korsakova 2000;100:20–3. [PubMed] [Google Scholar]
- [18].Bank J. A comparative study of amitriptyline and fluvoxamine in migraine prophylaxis. Headache 1994;34:476–8. [DOI] [PubMed] [Google Scholar]
- [19].Bulut S, Berilgen MS, Baran A, et al. Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: randomized, double-blind, crossover study. Clin Neurol Neurosurg 2004;107:44–8. [DOI] [PubMed] [Google Scholar]
- [20].Couch JR. Amitriptyline Versus Placebo Study G. Amitriptyline in the prophylactic treatment of migraine and chronic daily headache. Headache 2011;51:33–51. [DOI] [PubMed] [Google Scholar]
- [21].Couch JR, Hassanein RS. Migraine and depression: effect of amitriptyline prophylaxis. Trans Am Neurol Assoc 1976;101:234–7. [PubMed] [Google Scholar]
- [22].Couch JR, Hassanein RS. Amitriptyline in migraine prophylaxis. Arch Neurol 1979;36:695–9. [DOI] [PubMed] [Google Scholar]
- [23].Gomersall JD, Stuart A. Amitriptyline in migraine prophylaxis. Changes in pattern of attacks during a controlled clinical trial. J Neurol Neurosurg Psychiatry 1973;36:684–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jacobs H. A trial of opipramol in the treatment of migraine. J Neurol Neurosurg Psychiatry 1972;35:500–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Langohr HD, Gerber WD, Koletzki E, et al. Clomipramine and metoprolol in migraine prophylaxis—a double-blind crossover study. Headache 1985;25:107–13. [DOI] [PubMed] [Google Scholar]
- [26].Mathew NT. Prophylaxis of migraine and mixed headache. A randomized controlled study. Headache 1981;21:105–9. [DOI] [PubMed] [Google Scholar]
- [27].Noone JF. Clomipramine in the prevention of migraine. J Int Med Res 1980;8(Suppl. 3):49–52. [PubMed] [Google Scholar]
- [28].Ziegler DK, Hurwitz A, Hassanein RS, et al. Migraine prophylaxis. A comparison of propranolol and amitriptyline. Arch Neurol 1987;44:486–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


