Abstract
Objective:
This meta-analysis aimed to evaluate the efficiency and safety of tranexamic acid for reducing blood loss and transfusion requirements in patients undergoing total shoulder arthroplasty.
Methods:
A systematic search was performed in Embase (1980–2017.04, embase.com), Medline (1966–2017.04, medline.com), PubMed (1966–2017.04, pubmed.com), ScienceDirect (1985–2017.04, sciencedirect.com), and Web of Science (1950–2017.04, webofknowledge.com). Study which assessed the efficiency and safety of tranexamic acid in total shoulder arthroplasty was selected. Meta-analysis was performed using Stata 11.0 software.
Results:
In all, 484 patients from 2 randomized controlled trials (RCTs) and 2 non-RCTs were subjected to meta-analysis. The present meta-analysis demonstrated that there was less total blood loss (mean difference [MD] −172.16, 95% confidence interval [CI] −35.46 to −308.87, P = .01, d = 0.33) and transfusion rate (odds ratio 0.34, 95% CI 0.13 to 0.91, P = .03, d = 0.29) in tranexamic acid groups compared with the control groups. There were no significant differences in duration of surgery (MD 0.02, 95% CI −0.12 to 0.22, P = .89, d = 0.19), length of stay (MD −0.06, 95% CI −0.26 to 0.14, P = .56, d = 0.20), or incidence of adverse effects such as deep venous thrombosis (odds ratio 1.15, 95% CI 0.33 to 4.00, P = .83, d = 0.53).
Conclusion:
Clinical application of tranexamic acid seemed to result in significant reductions in total blood loss, hemoglobin decline and transfusion requirements following total shoulder arthroplasty. Moreover, no increased risk of the thrombotic events was identified. Due to the limited quality of the evidence currently available, higher quality RCTs are required.
Keywords: blood loss, meta-analysis, total shoulder arthroplasty, tranexamic acid
1. Introduction
Total shoulder arthroplasty has been considered as a successful treatment with pain relief and functional improvement for glenohumeral arthritis.[1] There was an estimated more than 60 thousands of total shoulder arthroplasties performed in 2011, which predicted an increasing trend and requirement for the next few years.[2] However, total shoulder arthroplasty was usually associated with substantial perioperative blood loss. Many methods have been used to manage blood loss including controlled permissive hypotension, administration of hemostatic agents, and autologous donation.[3–5] Blood transfusions were still required to treat anemia in many cases. It was reported that overall rate of transfusions after total shoulder arthroplasty ranged from 7.4% to 43%.[6–9] Allogenic blood transfusion would increase the risk of adverse events, such as virus infections, immunologically mediated diseases, and cardiovascular dysfunction, resulting in a financial burden and potentially life-threatening effects on patients.
Tranexamic acid is a synthetic analog of an amino acid whose biological activity inhibits plasminogen from dissolving clots, thereby decreasing the degradation of fibrin.[10,11] This property results in less blood loss and fewer wound hematoma. Recently, the use of tranexamic acid has become popularized in multiple surgical procedures for reducing perioperative blood loss and transfusion requirements. A number of randomized controlled trials (RCTs) and high-quality meta-analyses have assessed the efficiency and safety of tranexamic acid in multiple routines and reported that tranexamic acid was a cost-effective antifibrinolytic agent for blood management in total joint arthroplasty without increasing the risk of thrombotic events or other side effects.[12–14]
Currently, the both intravenous and topical application of tranexamic acid in total shoulder arthroplasty was seldom reported. Thus, there is a lack of scientific evidence regarding the hemostatic effect of tranexamic acid in total shoulder arthroplasty. Therefore, we perform a meta-analysis to evaluate the efficiency and safety of tranexamic acid for reducing blood loss and transfusion requirements in patients undergoing total shoulder arthroplasty.
2. Methods
2.1. Search strategy
We systemically search electronic databases including Embase (1980–2017.04, embase.com), Medline (1966–2017.04, medline.com), PubMed (1966–2017.04, pubmed.com), ScienceDirect (1985–2017.04, sciencedirect.com), and Web of Science (1950–2017.04, webofknowledge.com) for potential relevant articles. Complete search strategy for Embase was provided Table 1. Gray academic studies are also identified from the reference of included studies. No language was restricted. The following terms was considered as key words: “Total shoulder replacement or arthroplasty”, “tranexamic acid” and “blood loss.” The retrieval process is presented in Fig. 1. The study was approved by the ethics committee of The Second Affiliated Hospital of Dalian Medical University.
Table 1.
EMBASE search strategy.

Figure 1.
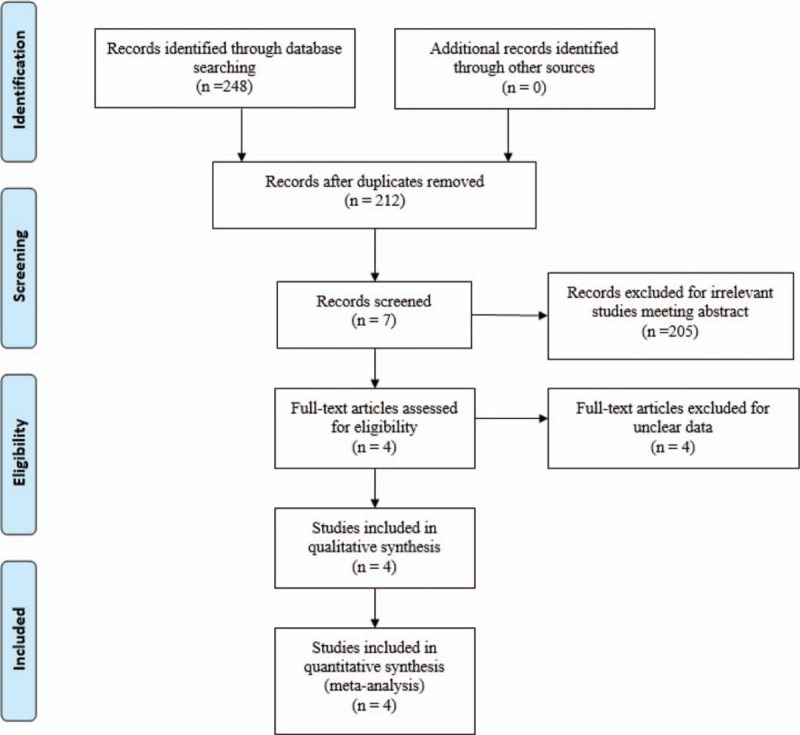
Search results and the selection procedure.
2.2. Inclusion and exclusion criteria
Studies were considered eligible if they met the following criteria: published clinical RCTs and non-RCTs; patients undergoing total shoulder arthroplasty, experiment group received intravenous or topical tranexamic acid for blood management, and control group received normal saline or none; the primary outcomes included total blood loss, postoperative hemoglobin level, transfusion rate, drainage volume. Secondary outcomes included duration of surgery, length of hospital stay, and postoperative adverse effects such as deep vein thrombosis (DVT) and pulmonary embolism (PE). Studies were excluded if they were current meta-analyses for incomplete data, case reports, conference abstracts, or review articles.
2.3. Selection criteria
Two authors independently reviewed all the abstracts of the potential studies identified by the above searches. After an initial decision, full text of the studies that potentially met the inclusion criteria were reviewed and final decision was made. A senior reviewer was consulted in case of disagreement regarding which studies to include.
2.4. Data extraction
A standard form for data extraction was printed. Two authors independently extracted the relevant data from the included articles. Details of incomplete data of included studies were obtained by consulting the corresponding author. Following data were extracted: first author names, published year, sample size, study design, comparable baseline, anesthesia methods, intervention procedures, and transfusion trigger. Other relevant data were also extracted from individual studies.
2.5. Quality assessment
Quality assessment of the included RCTs was done by 2 authors independently using the Cochrane Collaboration's tool. We conducted “risk of bias” table including the following key points: random sequence generation, allocation concealment, blinding, incomplete outcome data, free of selective reporting, and other bias. Each item was recorded by “Yes,” “No,” or “Unclear.” The Methodological Index for Non-Randomized Studies (MINORS) scale was used to assess non-RCTs with scores ranging from 0 to 24. A consensus was reached through a discussion.
The qualities of evidence of main outcomes in the present meta-analysis were evaluated using the Recommendations Assessment, Development, and Evaluation (GRADE) system including the following items: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Two authors independently scored all the items of the GRADE systems that may influence quality of evidence. Items that may raise the quality of evidence was recorded by 0, +1, and +2. Items that may lower the quality of evidence was recorded by 0, −1, and −2. A senior reviewer was consulted in case of disagreement. Finally, GRADE systems evaluated the overall results. The recommendation level of evidence is classified into the following categories: high, which means that further research is unlikely to change confidence in the effect estimate; moderate, which means that further research is likely to significantly change confidence in the effect estimate and may change the estimate; low, which means that further research is likely to significantly change confidence in the effect estimate and to change the estimate; and very low, which means that any effect estimate is uncertain.
2.6. Data analysis and statistical methods
All calculations were carried out by Stata 11.0 (The Cochrane Collaboration, Oxford, UK). We planned to pool the results using the random-effects model, which would better incorporate the clinical heterogeneity typical among small studies investigating the long-term efficiency and safety of tranexamic acid in total shoulder arthroplasty. We hypothesized that the different articles were estimating randomly different yet related intervention effects. By choosing the more conservative random-effects model, confidence intervals (CIs) for the average intervention effect would be wider. Publication bias was examined by Begg test and Egger test that showed statistical evidence. Size of the effect (Cohen d) for each outcome was calculated. Subgroup analysis was conducted to explore the origins of heterogeneity. The results of dichotomous outcomes were expressed as odds ratio (OR) with a 95% CI. For continuous various outcomes, mean difference (MD) with a 95% CI was applied for assessment.
3. Results
3.1. Search result
In the primary search, 248 articles were reviewed. Finally, 4 studies meet eligibility criteria of the present meta-analysis—2 of them were RCTs[15,16] and 2 were non-RCTs.[17,18] Overall, the 4 studies included 250 patients in the tranexamic acid group and 234 patients in the control group.
3.2. Study characteristics
The sample size of the included studies ranged from 77 to 194. All of them compared efficiency and safety of tranexamic acid for reducing perioperative blood loss in total shoulder arthroplasty. Experimental groups received intravenous or topical tranexamic acid, whereas control groups received normal saline or none. There was variation in dosage of tranexamic acid among included studies. Two studies applied general anesthesia[15,18] and others did not provide a detailed description. All studies[15–18] reported that surgical procedure was operated by the same team. Prophylactic anticoagulation therapy was performed as a routine in all included articles. All of them suggested the outcomes for at least 95% of the patients. The follow-up period ranged from 1 to 2 months.
3.3. Risk of bias assessment
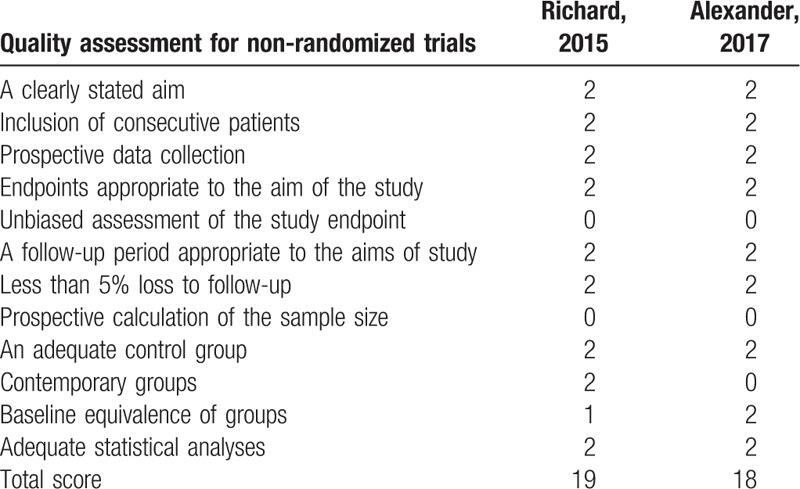
Demographic characteristics and the details about the included studies are summarized in Table 2. Quality assessment of the RCTs was based on the Cochrane Collaboration tool (Table 3). All RCTs[15,16] provided clear inclusion and exclusion criteria and suggested a methodology of randomization, all of which described that randomization algorithm was generated from computer. One RCT[15] stated allocate concealment was achieved by sealed envelope. Double blinding was provided in all RCTs. One of them had attempted to blind assessors.[15] Each risk of bias item was presented as the percentage across all included studies, which indicated the proportion of different levels of risk of bias for each item (Table 4). All RCTs demonstrated complete outcome data. None of them performed intent-to-treatment analysis, thus a potential risk for type II statistical error would exist. The MINORS scale was used to assess non-RCTs by assigning scores ranging from 0 to 24 (Table 5).
Table 2.
Cohort characteristics.
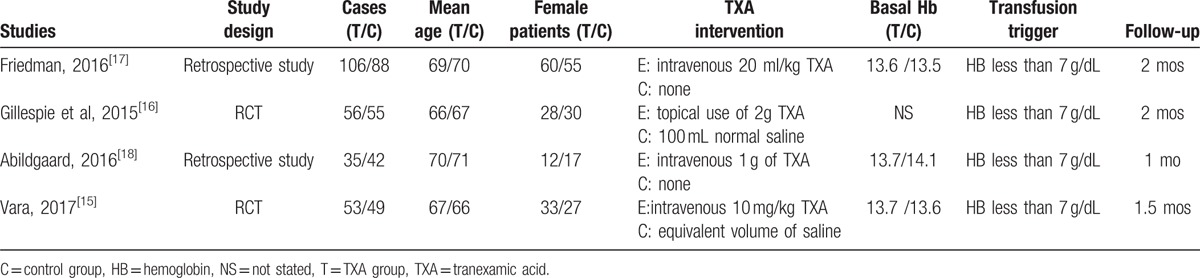
Table 3.
Methodological quality of the randomized controlled trials.
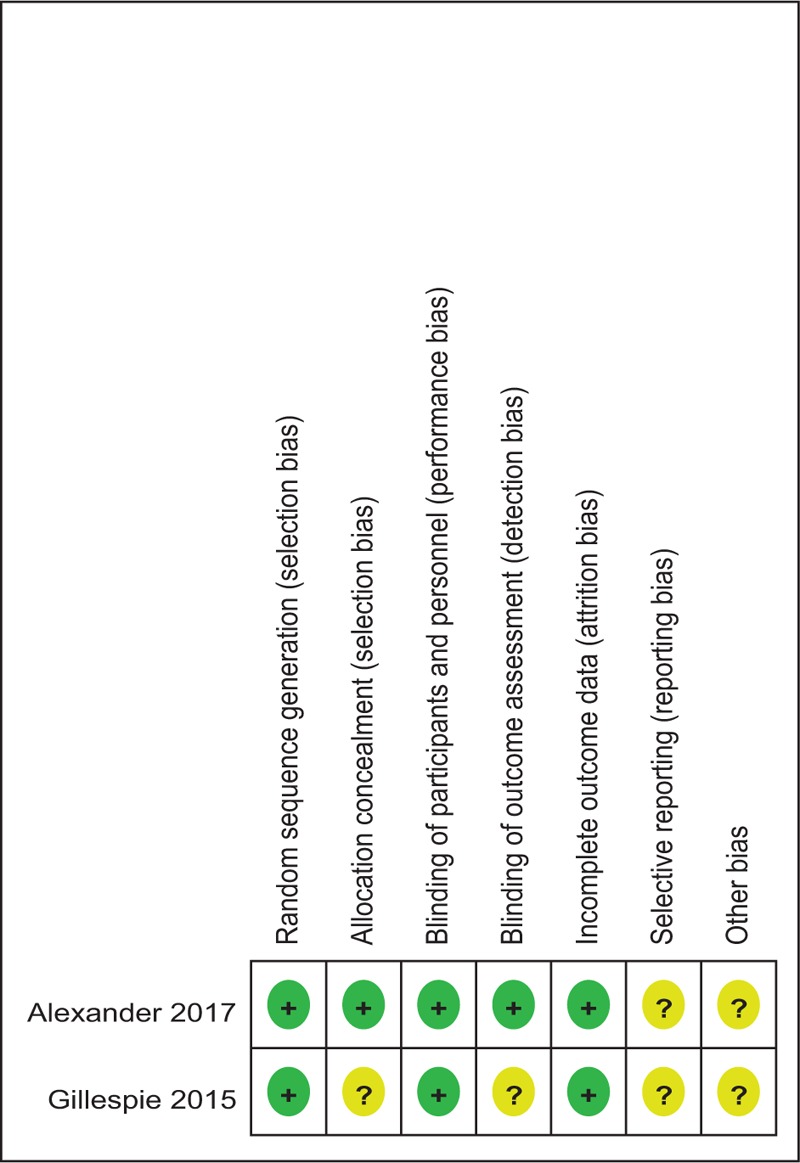
Table 4.
Risk of bias.
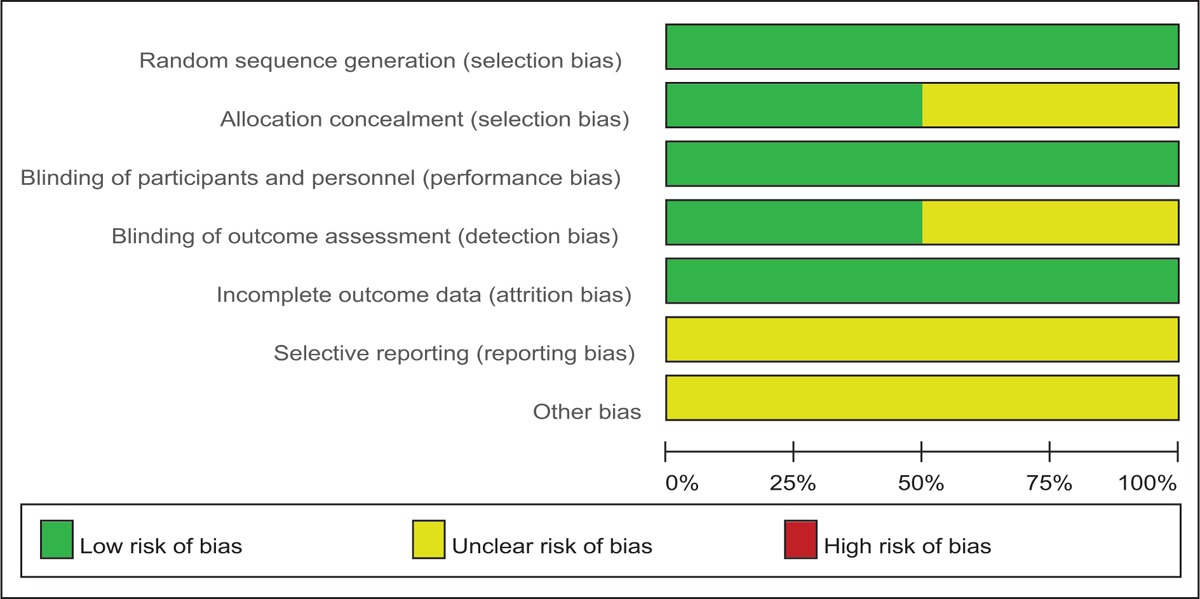
Table 5.
Methodological quality of the non-randomized controlled trials.
3.4. Outcomes for meta-analysis
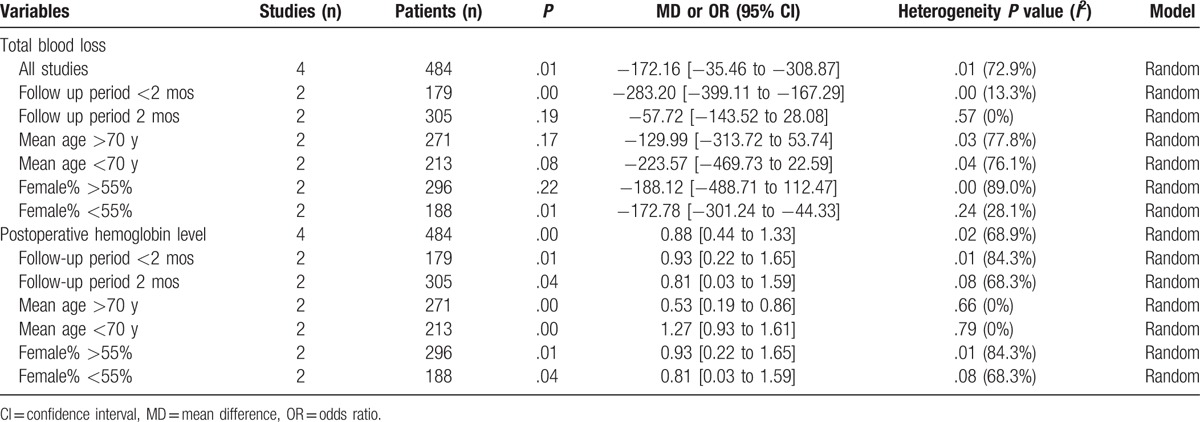
3.4.1. Total blood loss
Four studies[15–18] reported the total blood loss after total shoulder arthroplasty. A random-effects model was applied (χ2 = 11.09, df = 3, I2 = 72.9%, P = .01). We found that there was significant difference between the tranexamic acid groups and control groups regarding the total blood loss (MD −172.16, 95% CI −35.46 to −308.87, P = .01, d = 0.33; Fig. 2).
Figure 2.
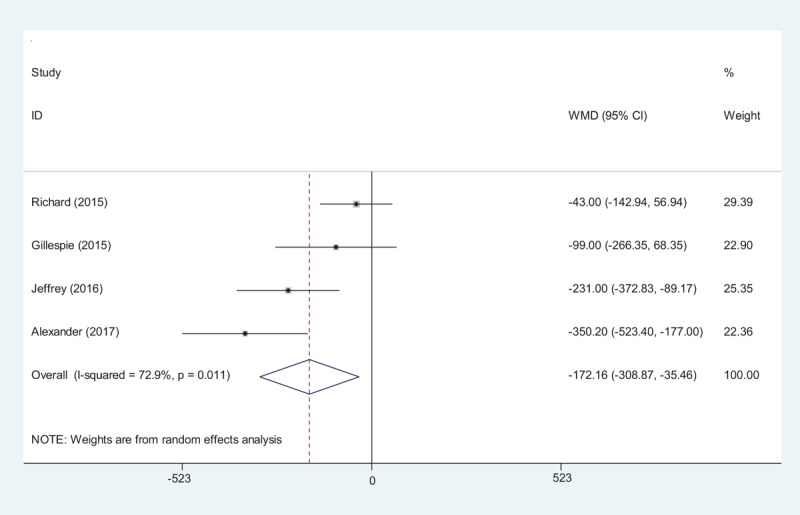
Forest plot diagram showing effect of tranexamic acid on total blood loss.
3.4.2. Postoperative hemoglobin level
Four studies[15–18] reported the postoperative hemoglobin level after total shoulder arthroplasty. A random-effects model was used (χ2 = 9.64, df = 3, I2 = 68.9%, P = .02). The result of meta-analysis showed that there was significant difference between the tranexamic acid groups and control groups regarding the postoperative hemoglobin level (MD 0.88, 95% CI 0.44–1.33, P = .00, d = 0.48; Fig. 3).
Figure 3.
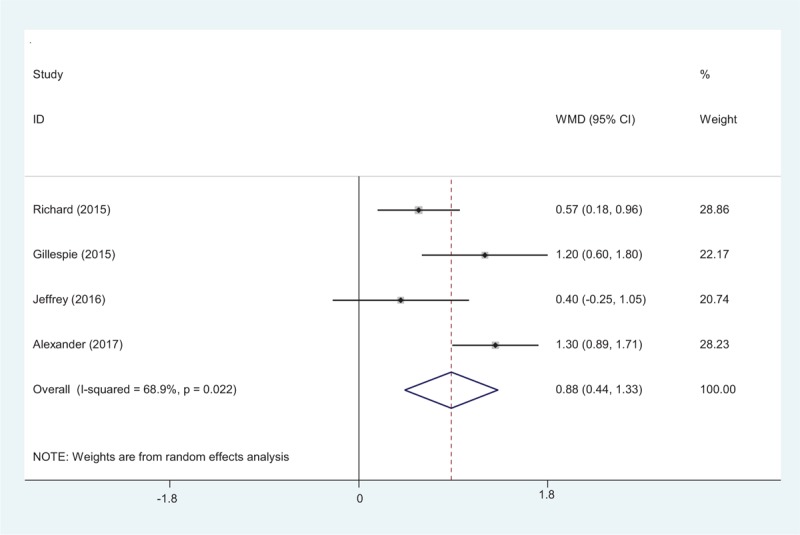
Forest plot diagram showing effect of tranexamic acid on hemoglobin level.
3.4.3. Transfusion requirements
Transfusion requirements after total shoulder arthroplasty were presented in 4 studies.[15–18] A random-effects model was used (χ2 = 3.13, df = 3, I2 = 4.0%, P = .373). The present meta-analysis showed that there was significant difference between the tranexamic acid and control groups in terms of transfusion requirements (OR 0.34, 95% CI 0.13–0.91, P = .03, d = 0.29; Fig. 4).
Figure 4.
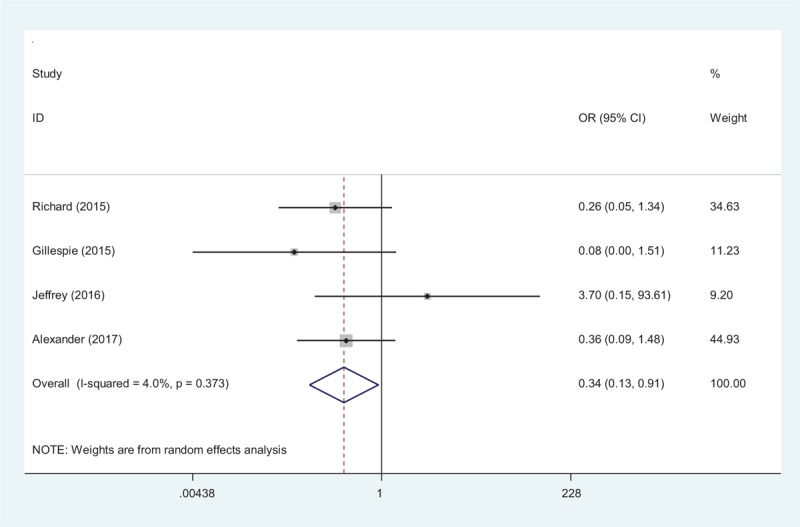
Forest plot diagram showing effect of tranexamic acid on transfusion rate.
3.4.4. Duration of surgery
The duration of surgery was reported in 3 studies.[15–17] A random-effects model was used (χ2 = 0.88, df = 2, I2 = 0.0%, P = .643). No significance difference was observed regarding duration of surgery between the 2 groups (MD = 0.02, 95% CI −0.12 to 0.22, P = .89, d = 0.19; Fig. 5).
Figure 5.
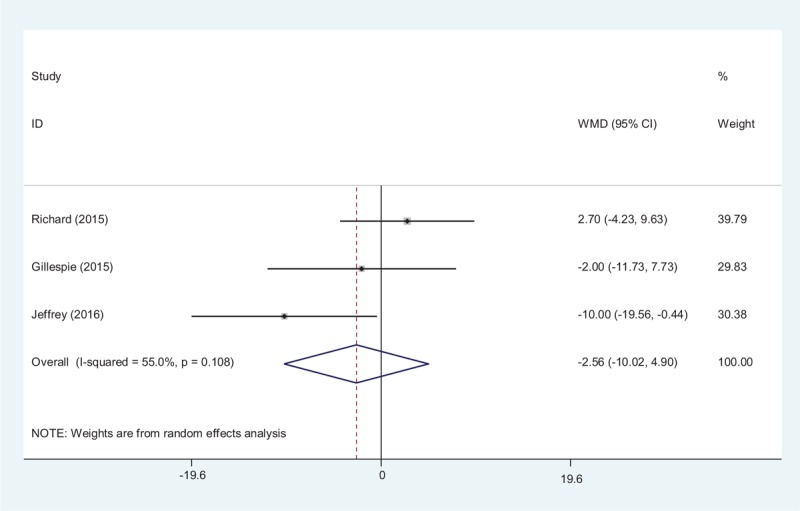
Forest plot diagram showing effect of tranexamic acid on duration of surgery.
3.4.5. Length of hospital stay
Three studies reported the lengths of the hospital stays for the groups.[15–17] A random-effects model was used (χ2 = 12.78, df = 2, I2 = 84.3%, P = .002). No significant difference in the length of hospital stay was observed between the 2 groups (MD −0.06, 95% CI −0.26 to 0.14, P = .56, d = 0.20; Fig. 6).
Figure 6.
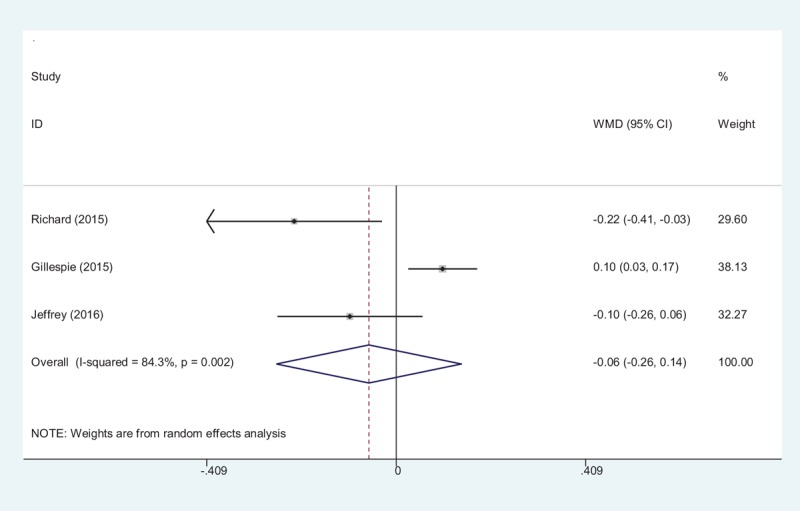
Forest plot diagram showing effect of tranexamic acid on the length of hospital stay.
3.4.6. Deep vein thrombosis
Four articles[15–18] reported the incidence of DVT after total shoulder arthroplasty. A random-effects model was used (χ2 = 0.64, df = 2, I2 = 0.0%, P = .726). No significant difference was found between the groups (OR 1.15, 95% CI 0.33–4.00, P = .83, d = 0.53; Fig. 7).
Figure 7.
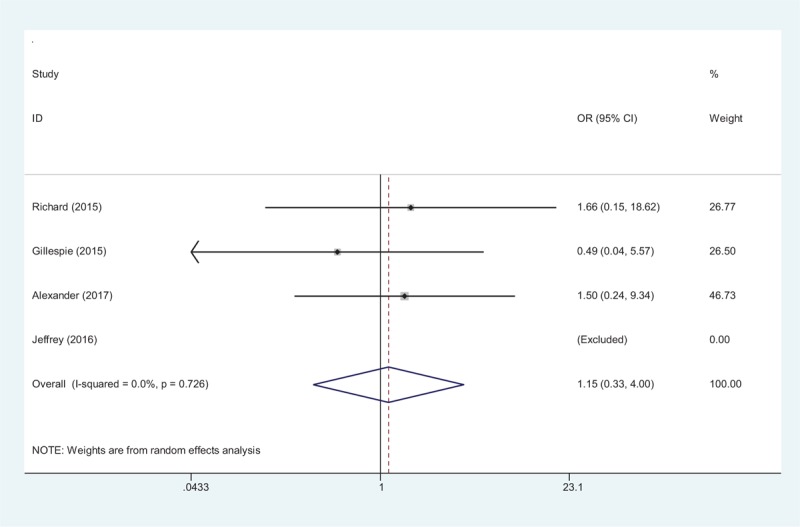
Forest plot diagram showing effect of tranexamic acid on the risk of DVT.
3.4.7. Publication bias and subgroup analysis
Publication bias was examined for transfusion rate by Begg test (P = .174) and Egger test (P = .531) that showed statistical evidence (Fig. 8). Low risk of publication bias was observed. However, we could not eliminate publication bias as a small number of articles were enrolled. Subgroup analysis is presented in Table 6. We have not found the source of heterogeneity regarding total blood loss. Age might be the source of heterogeneity for postoperative hemoglobin level.
Figure 8.
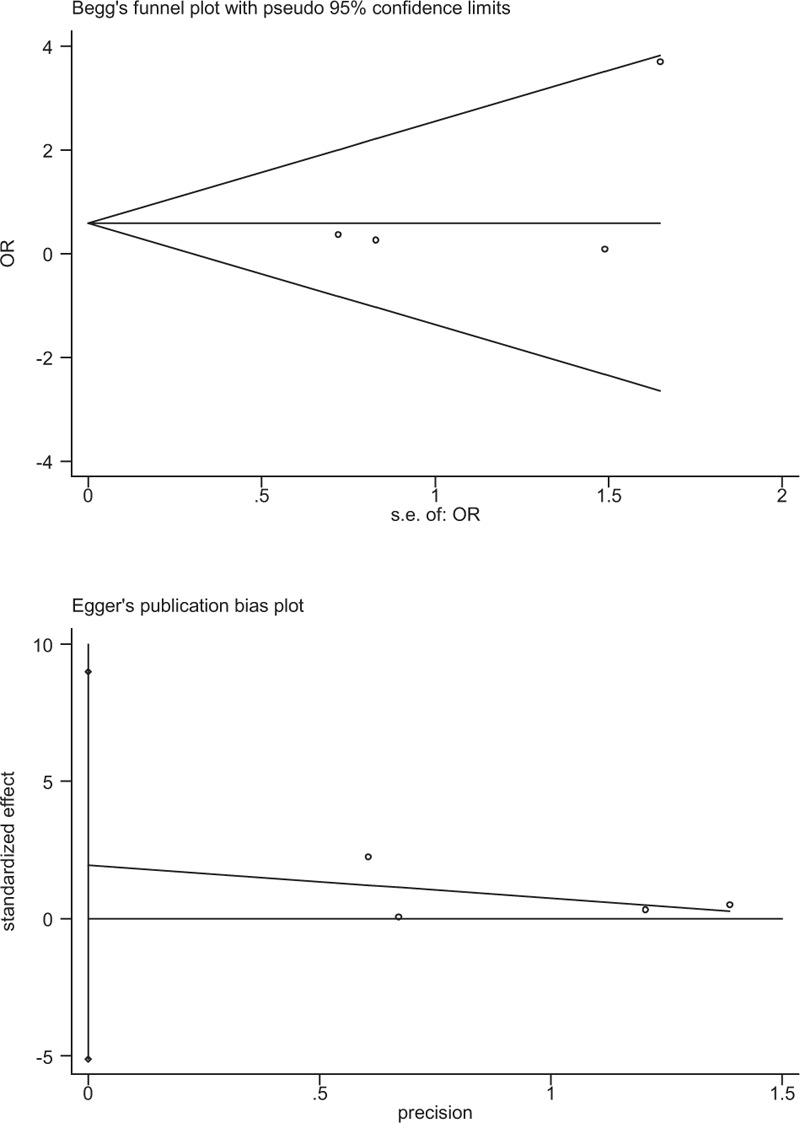
Plots of Begg test and Egger test for publication bias of transfusion rate.
Table 6.
Subgroup analysis for main outcomes.
4. Discussion
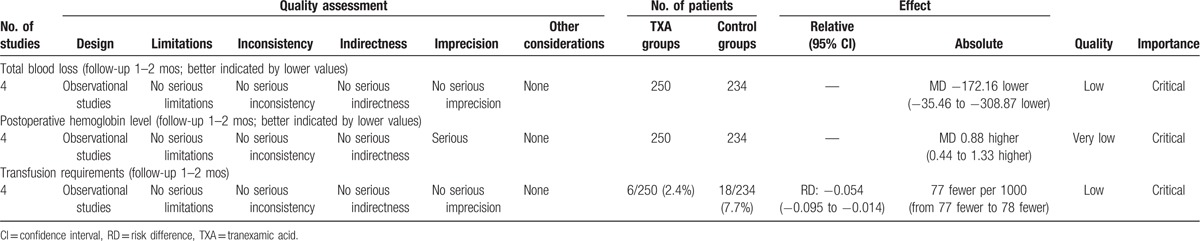
To the best of our knowledge, this study is the first systemic review and meta-analysis to evaluate the efficiency and safety of the tranexamic acid use in patients undergoing total shoulder arthroplasty. The most important finding of the present meta-analysis was that the application of tranexamic acid seemed to reduce total blood loss, hemoglobin decline, and transfusion requirements compared with controls. All outcomes in this meta-analysis were evaluated using the GRADE system. The evidence quality for each outcome was low to very low (Table 7), which means that any effect estimate is uncertain. This finding may lower the confidence in any recommendations.
Table 7.
The GRADE evidence quality for main outcome.
Tranexamic acid, which acts as antifibrinolytic agent, is famous for proven success in reducing perioperative and postoperative blood loss and is widely used in surgical procedure. Tranexamic acid can be applied by various routes including intravenous, intra-articular, oral, and intramuscular. Total shoulder arthroplasty is a major orthopedic surgery with a considerable perioperative blood loss and complications. Previous articles have reported improved outcomes in patients with total shoulder arthroplasty who have been administered this agent. Vara at al[15] reported that intravenous application of tranexamic acid for patients undergoing primary total shoulder arthroplasty was effective and safe. Anthony et al[19] demonstrated that allogenic blood transfusion was the most frequent complication after total shoulder arthroplasty. Optimal blood management could relieve pain and achieve preferable functional outcome. With more total shoulder arthroplasties being performed, there is a crucial need for blood management appropriately. Although administration of tranexamic acid in joint arthroplasty surgery has been studied widely and demonstrated significant clinical improvements in minimizing the blood loss, the application of tranexamic acid in the setting of total shoulder arthroplasties has been limited due to the seldom published studies. Currently, it is unknown for us whether tranexamic acid would be considered an excellent hemostatic efficiency in total shoulder arthroplasty. The present meta-analysis indicates that the application of tranexamic acid in total shoulder arthroplasty seems to significantly reduce total blood loss and hemoglobin decline.
With the ageing population, the occurrence of glenohumeral arthritis is increasing, and total shoulder arthroplasty is a popular treatment to improve motor function and relieve pain. It was reported that total shoulder arthroplasty without antifibrinolytics was associated with massive blood loss ranging from 650 to 1540 mL[6,19–21] within the first few postoperative days and allogenic blood transfusion was frequently performed to relieve anemia. However, potential adverse effects might occur, for instance, transmission of infection, possible hemolytic transfusion reactions, and so on.[22,23] Substantial high-quality RCTs and meta-analysis have been published to confirm that administration of tranexamic acid could diminish the need for allogeneic blood transfusions in patients undergoing total joint arthroplasty. Currently, the efficiency of tranexamic acid in reducing transfusion rate in total shoulder arthroplasty remains controversial. Meta-analysis is performed as major statistical method in the present study. It could strengthen statistical power and enlarge sample size by pooling results of published articles that could point out stronger evidence. In addition, no guidelines have been proposed to normalize the administration of tranexamic acid in total shoulder arthroplasty. Thus, there is a requirement for an evidence base to help orthopedists make clinical decisions. The present meta-analysis indicates that the application of tranexamic acid seems to be associated with a further significant reduction in the transfusion requirements.
Deep vein thrombosis has been considered as a common postoperative complication that may develop into PE and even result in death after major orthopedic surgery.[24] All patients received routine prophylactic antithrombotic therapy. Published articles have suggested a potential higher risk of DVT and PE when tranexamic acid was used. This result may be due to the tendency of tranexamic acid, which is an antifibrinolytic agent, to increase the risk of clotting. Although the intravenous application of tranexamic acid could be more likely to result in the formation of a thrombus because of the higher tranexamic acid level of blood concentration, there was no significant difference between both groups regarding the incidence of DVT in the present analysis. However, more high-quality RCTs are required to further assess the safety of tranexamic acid.
Several potential limitations of the study should be noted. Apart from the 2 RCTs, we also included non-RCTs, which lowered evidence grade, and the sample size was relatively small. Some important parameters, for instance, the functional outcome and drainage volume, were not fully introduced in the meta-analysis. We have tried to contact authors for incomplete data; however, there was no response and no other methods were used to obtain data, which can result in a source of bias in this review. Short-term follow-up may lead to the underestimation of complications. Publication bias is an inherent weakness that exists in all meta-analyses.
Despite the aforementioned limitations, this study was the first meta-analysis to evaluate the efficiency and safety of the application of tranexamic acid in patients undergoing total shoulder arthroplasty. Long-term of high-quality RCTs is needed to explore the dose–response effect and other adverse effects.
5. Conclusions
Clinical application of tranexamic acid seemed to result in significant reductions in total blood loss, hemoglobin decline, and transfusion requirements after total shoulder arthroplasty. Moreover, no increased risk of the thrombotic events was identified. Due to the limited quality of the evidence currently available, higher-quality RCTs are required.
Footnotes
Abbreviations: DVT = deep vein thrombosis, PE = pulmonary embolism, RCT = randomized controlled trial.
Authors’ contributions: S.-W.H. and L.Z. conceived of the design of the study. L.-D.M. and G.-Y.D. performed and collected the data and contributed to the design of the study. C.-X.S. and X.-G.S. finished the manuscript. All authors read and approved the final manuscript.
Funding: The study was funded by the Science and Technology Project of Liaoning province (no. 2012225021).
Conflicts of interest: The authors declare that they have no competing interests.
References
- [1].Izquierdo R, Voloshin I, Edwards S, et al. Treatment of glenohumeral osteoarthritis. J Am Acad Orthopaed Surg 2010;18:375–82. [DOI] [PubMed] [Google Scholar]
- [2].Jain NB, Yamaguchi K. The contribution of reverse shoulder arthroplasty to utilization of primary shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:1905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg 2014;96:1836–44. [DOI] [PubMed] [Google Scholar]
- [4].Cherian JJ, Kapadia BH, Banerjee S, et al. Surgical intra-operative blood management strategies for total hip arthroplasty. Surg Technol Int 2014;24:319–25. [PubMed] [Google Scholar]
- [5].Banerjee S, Issa K, Kapadia BH, et al. Intraoperative nonpharmacotherapeutic blood management strategies in total knee arthroplasty. J Knee Surg 2013;26:387–93. [DOI] [PubMed] [Google Scholar]
- [6].Kandil A, Griffin JW, Novicoff WM, et al. Blood transfusion after total shoulder arthroplasty: which patients are at high risk? Int J Shoulder Surg 2016;10:72–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jiang JJ, Toor AS, Shi LL, et al. Analysis of perioperative complications in patients after total shoulder arthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:1852–9. [DOI] [PubMed] [Google Scholar]
- [8].Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg 2013;22:233–9. [DOI] [PubMed] [Google Scholar]
- [9].Gruson KI, Accousti KJ, Parsons BO, et al. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg 2009;18:225–30. [DOI] [PubMed] [Google Scholar]
- [10].Varney SJ, Guest JF. The annual cost of blood transfusions in the UK. Transfusion Med 2003;13:205–18. [DOI] [PubMed] [Google Scholar]
- [11].Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood 2008;112:2617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 2014;349:g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oremus K, Sostaric S, Trkulja V, et al. Influence of tranexamic acid on postoperative autologous blood retransfusion in primary total hip and knee arthroplasty: a randomized controlled trial. Transfusion 2014;54:31–41. [DOI] [PubMed] [Google Scholar]
- [14].Li JF, Li H, Zhao H, et al. Combined use of intravenous and topical versus intravenous tranexamic acid in primary total knee and hip arthroplasty: a meta-analysis of randomised controlled trials. J Orthopaed Surg Res 2017;12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vara AD, Koueiter DM, Pinkas DE, et al. Intravenous tranexamic acid reduces total blood loss in reverse total shoulder arthroplasty: a prospective, double-blinded, randomized, controlled trial. J Shoulder Elbow Surg 2017;Feb 3. pii: S1058-2746(17)30016-2. doi: 10.1016/j.jse.2017.01.005. [DOI] [PubMed] [Google Scholar]
- [16].Gillespie R, Shishani Y, Joseph S, et al. Neer Award 2015: A randomized prospective evaluation on the effectiveness of tranexamic acid in reducing blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:1679–84. [DOI] [PubMed] [Google Scholar]
- [17].Friedman RJ, Gordon E, Butler RB, et al. Tranexamic acid decreases blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:614–8. [DOI] [PubMed] [Google Scholar]
- [18].Abildgaard JT, McLemore R, Hattrup SJ. Tranexamic acid decreases blood loss in total shoulder arthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:1643–8. [DOI] [PubMed] [Google Scholar]
- [19].Anthony CA, Westermann RW, Gao Y, et al. What are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? A review of 1922 cases. Clin Orthopaed Related Res 2015;473:2099–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ryan DJ, Yoshihara H, Yoneoka D, et al. Blood transfusion in primary total shoulder arthroplasty: incidence, trends, and risk factors in the United States from 2000 to 2009. J Shoulder Elbow Surg 2015;24:760–5. [DOI] [PubMed] [Google Scholar]
- [21].Chebli C. CORR Insights((R)): what are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? A review of 1922 cases. Clin Orthopaed Related Res 2015;473:2106–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sahu S, Hemlata, Verma A. Adverse events related to blood transfusion. Ind J Anaesth 2014;58:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferraris VA, Hochstetler M, Martin JT, et al. Blood transfusion and adverse surgical outcomes: the good and the bad. Surgery 2015;158:608–17. [DOI] [PubMed] [Google Scholar]
- [24].Saleh J, El-Othmani MM, Saleh KJ. Deep vein thrombosis and pulmonary embolism: considerations in orthopedic surgery. Orthoped Clin N Am 2017;48:127–35. [DOI] [PubMed] [Google Scholar]


