Supplemental Digital Content is available in the text
Keywords: chronic hepatitis B, entecavir, hepatitis B virus, interferon, nucleoside analogues, telbivudine
Abstract
Little is known about the optimal treatment following the initial failure of interferon therapy and the potential different efficacy with de novo therapy with entecavir (ETV) or telbivudine (LDT) and following the interferon therapy failure.
ETV or LDT therapy following the interferon therapy failure was compared with that of de novo therapy with ETV or LDT in patients with chronic hepatitis B virus (HBV) infection. Treatment parameters included virological response, hepatitis B e antigen (HBeAg) seroconversion, and alanine aminotransferase (ALT) normalization.
Of 180 patients studied, 56 received de novo telbivudine monotherapy (LDT group); 45 received entecavir monotherapy (ETV group); 40 received LDT following interferon (interferon-telbivudine [IFN-LDT] group); and 39 received ETV following interferon (interferon-entecavir [IFN-ETV] group). At week 52, virological response occurred in significantly more patients in the IFN-ETV group than the ETV group (87.2% vs 57.8%, P = .003). At week 104, HBeAg seroconversion occurred in significantly more patients in the IFN-ETV group than the ETV group (44.4% vs 22.2%, P = .03). At week 52, virological response was achieved by significantly more patients in the IFN-LDT group than the LDT group (85.0% vs 64.3%, P = .02).
This study showed that switch to rescue therapy with ETV or LDT therapy after failure of interferon therapy resulted in more rapid virologic response than with de novo treatment with either ETV or LDT; rescue therapy with ETV resulted in a greater HBeAg seroconversion rate.
1. Introduction
Chronic hepatitis B virus (HBV) infection occurs worldwide and is associated with an increased risk of end-stage liver disease, cirrhosis, and hepatocellular carcinoma.[1] Although universal HBV immunization programs, implemented from birth, have been highly effective in reducing the incidence and prevalence of HBV infection in many endemic countries, these programs have had little impact on the outcome for patients with chronic HBV infection. Complete eradication of HBV infection is difficult to achieve because of the nature of HBV replication, as will persist and provides an intracellular HBV template for replication.[2]
Treatment options for patients with chronic HBV infection include interferon therapy, which is now licensed globally and is known to enhance host immunity to HBV and to have a modest antiviral effect.[1,3] However, the effectiveness of interferon treatment is limited, and it is not recommended for a long-term therapy due to its adverse effects.[1,3] Furthermore, treatment failure with interferon treatment or partial response have been previously reported.[4,5]
Alternative treatment options for patients with chronic HBV infection now include nucleos(t)ide analogues (NUCs), such as lamivudine, adefovir, telbivudine (LDT), entecavir (ETV), and tenofovir.[6] These newer NUCs act by inhibition of the reverse transcription of pre-genomic RNA with suppression of HBV replication.[6] Patients with chronic HBV infection who have experienced previous interferon treatment failure are often recommended to switch to “rescue” therapy with a NUC.[1] Lamivudine is an inexpensive NUC agent but has high rates of resistance to long-term therapy.[7] Adefovir is less efficacious and associated with high rates of treatment resistance.[8] Therefore, either lamivudine or adefovir may not be considered the most optimal treatment option for patients with previous interferon treatment failure.
ETV is an NUC that has potent HBV inhibition and is rarely associated with resistance; for this reason, ETV is a first-line drug recommended by the current treatment guidelines for chronic HBV infection.[9] Telbivudine is a potent inhibitor of HBV replication with a relatively high hepatitis B e antigen (HBeAg) conversion rate and with immunoregulatory effects.[9–11]
There is increasing awareness of the limitations of interferon therapy for patients with chronic HBV infection, and the advantages of the NUCs, such as ETV and LDT. However, there is no consensus regarding whether patients with chronic HBV infection with previous interferon treatment failure should switch to ETV or LDT. In addition, there have been few studies to determine whether patients with chronic HBV infection may have improved treatment response and outcome following treatment with ETV or LDT following previous interferon treatment failure.
In the present study, we included patients with chronic HBV infection with a history of interferon treatment failure who have then switched to ETV or LDT, and patients treated de novo with ETV or LDT, to compare the differences in virological response, HBeAg seroconversion rate, and biochemical response rate in the 2 treatment groups.
2. Methods
2.1. Patients studied
Patients with chronic HBV infection who were treated between January 2011 and December 2015 were included in the study. Patients were treated de novo with ETV or LDT or had interferon treatment with partial or no responses, who then switched to LDT or ETV treatment. Patients were included in the study consecutively, according to the protocol shown in the flow chart (Fig. 1).
Figure 1.
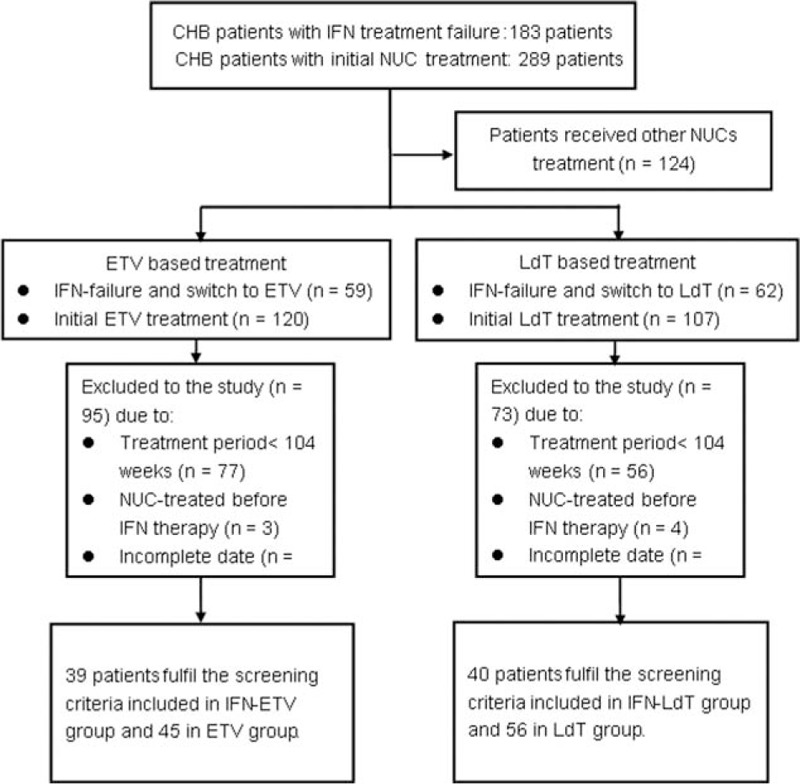
Flow chart of study.
Chronic HBV infection was diagnosed by the presence of hepatitis B surface antigen ≥6 months.[9] Exclusion criteria for the study included patients who were co-infected with hepatitis C virus, hepatitis D virus, and who had autoimmune liver disease and alcoholic liver disease.
Interferon treatment failure was defined as serum HBV DNA levels ≥104 copies/mL after 1 year of treatment.[3] Sequential or “rescue” treatment was defined as NUC treatment that was initiated after interferon treatment cessation due to interferon treatment failure.
2.2. Laboratory studies
All patients enrolled in the study were followed up regularly at Nanfang Hospital. Biochemical tests included serum HBV markers, with serum HBV DNA levels monitored every 24 weeks. Alanine aminotransferase (ALT) was tested biochemically (Olympus AU5400 High-Volume Chemistry Immuno Analyzer; Beckman Coulter, Inc.) with the upper limit of normal (ULN) at 40 IU/L. HBV markers were analyzed by enzyme immunoassay. Serum HBV DNA levels were measured by Daan real-time polymerase chain reaction with a linear range of 1000 IU/mL to 1 × 108 IU/mL according to the manufacturer's instructions.[12]
2.3. Treatment efficacy parameters
The primary efficacy parameter in this study was the proportion of patients with chronic HBV infection with virological response during NUC treatment with ETV or LDT, defined as HBV DNA <1000 IU/mL. Secondary efficacy parameters included the proportions of patients with ALT normalization, HBeAg seroconversion, and genotypic resistance during NUC treatment. Safety analysis was performed for all patients who enrolled in the study, and safety assessment included monitoring of adverse events and laboratory abnormalities.
ALT normalization was defined as an ALT level that decreased from abnormal levels at baseline to levels within the ULN. HBeAg seroconversion was defined as negative HBeAg with positive hepatitis B e antibody for HBeAg-positive patients at the start of NUC treatment.[1] Genotypic resistance was defined as the detection of mutations in the HBV genome that were known to confer resistance during antiviral treatment.[8]
2.4. Statistical analysis
Mean and standard deviation (SD) were used for continuous variables, and percentages were used for categorical variables. HBV DNA levels were expressed in logarithmic units (log10 copies/mL). The χ2 test and Student's t test were applied to determine whether the results were statistically different, when appropriate. The statistical significance of all tests was defined as P < .05 by 2-tailed tests. Data analysis was performed using SPSS for Windows, version 13.0.
2.5. Compliance with ethical requirements
The institutional review board of Nanfang Hospital, Southern Medical University, had approved the study. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for inclusion in the study.
3. Results
3.1. Demographic and baseline parameters
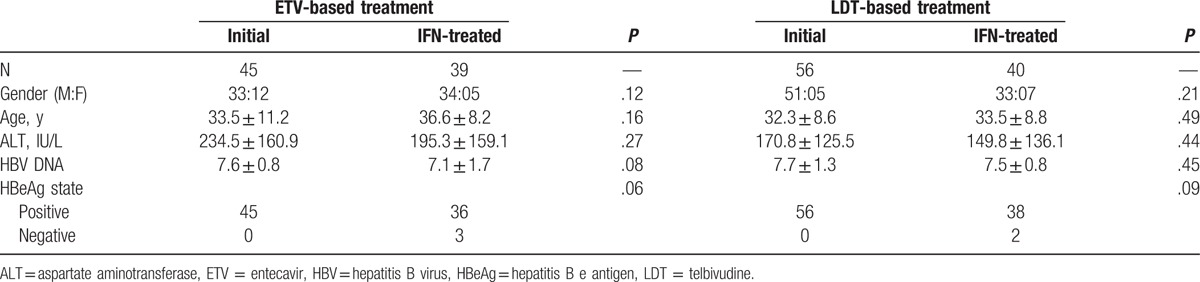
A total of 180 patients with chronic HBV infection were eligible to be included in the study and who were followed up at Nanfang Hospital. There were 101 patients treated with de novo NUC therapy, of whom 56 patients received telbivudine monotherapy (LDT group) and 45 received ETV monotherapy (ETV group). There were 79 patients who received sequential therapy with interferon and a NUC, 40 of these patients received interferon followed by LDT treatment (interferon-telbivudine [IFN-LDT] group) and 39 received interferon followed by ETV treatment (interferon-entecavir [IFN-ETV] group). Demographic data and baseline parameters were not significantly different in the 2 comparative groups, as shown in Table 1.
Table 1.
Demographic and baseline clinical characteristics of patients with chronic HBV infection included in the study.
3.2. Antiviral effectiveness in ETV-based treatment
In the ETV-based treatment groups, virological response was achieved in significantly more patients in the IFN-ETV group than the de novo ETV group with 87.2% (34/39) versus 57.8% (26/45) (P = .003) (Fig. 2A). At week 104, there was no difference between the HBV DNA level in the IFN-ETV group compared with the de novo ETV group, with 89.7% (35/39) patients with undetectable HBV DNA level in the IFN-ETV group and 84.4% (38/45) in the de novo ETV group (P = .47).
Figure 2.
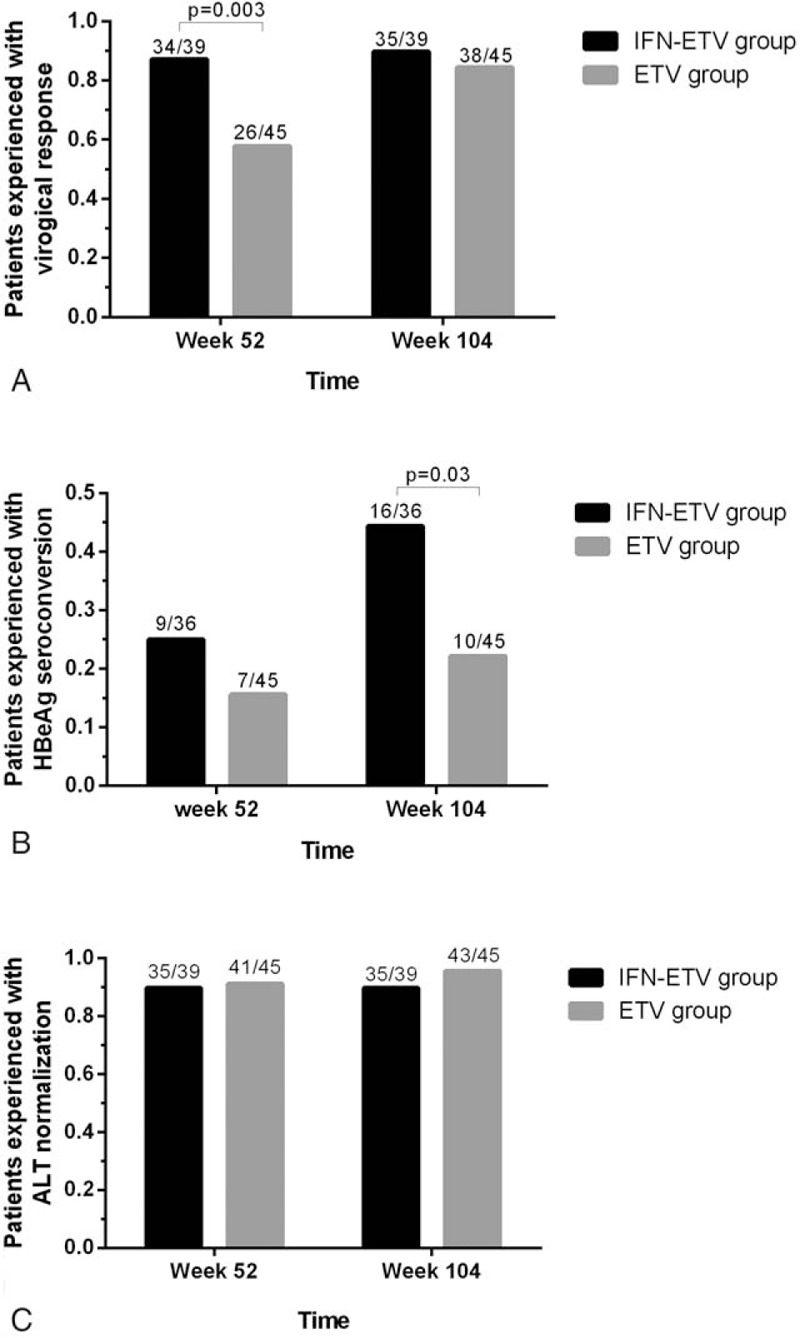
Antiviral efficacy in entecavir (ETV)-based treatment. (A) Comparison of the virological response in ETV-based treatment. Virologic response was achieved by significantly more patients of in the interferon-entecavir (IFN-ETV) group with 87.2% (34/39) versus 57.8% (26/45) in the ETV group (P = .003) at week 52. (B) Comparison of the HBeAg seroconversion in ETV-based treatment. Significantly more patients in the IFN-ETV group experienced HBeAg seroconversion compared with the ETV group at week 104 (44.4% vs 22.2%, P = .03). (C) Comparison the biochemical response in ETV-based treatment. Rates of alanine aminotransferase (ALT) normalization in the IFN-ETV group and the ETV group at week 52 and week 104 were 89.7% (35/39) versus 91.1% (41/45) and 89.7% (35/39) versus 95.6% (43/45).
In all HBeAg seropositive patients, a larger proportion of the patients in the interferon- ETV group experienced HBeAg seroconversion compared with the ETV group at week 104 (44.4% vs 22.2%, P = .03) (Fig. 2B). However, there was no difference observed in the rates of serum ALT normalization between the IFN-ETV group and the de novo ETV group at week 52 and week 104 with 89.7% (35/39) versus 91.1% (41/45) and 89.7% (35/39) versus 95.6% (43/45), as shown in Fig. 2C.
3.3. Antiviral effectiveness in LDT-based treatment
The proportion of patients who achieved a virological response at week 52 and week 104 with LDT treatment are shown in Fig. 3A. There were 85.0% (34/40) of patients in the IFN-LDT group who experienced virologic response, which was significantly greater than for the de novo LDT group with 64.3% (36/56) at week 52 (P = .02). There was an increase in the proportion of patients who achieved virologic response during treatment from week 52 to week 104 in both groups; during week 104, 35 out of 40 patients (87.5%) had undetectable HBV DNA levels in the IFN-LDT group and 44 out of 56 patients (78.6%) from the de novo LDT group. No significant differences were observed in virological response rate between the LDT group and the IFN-LDT group at week 104 (P = .26).
Figure 3.
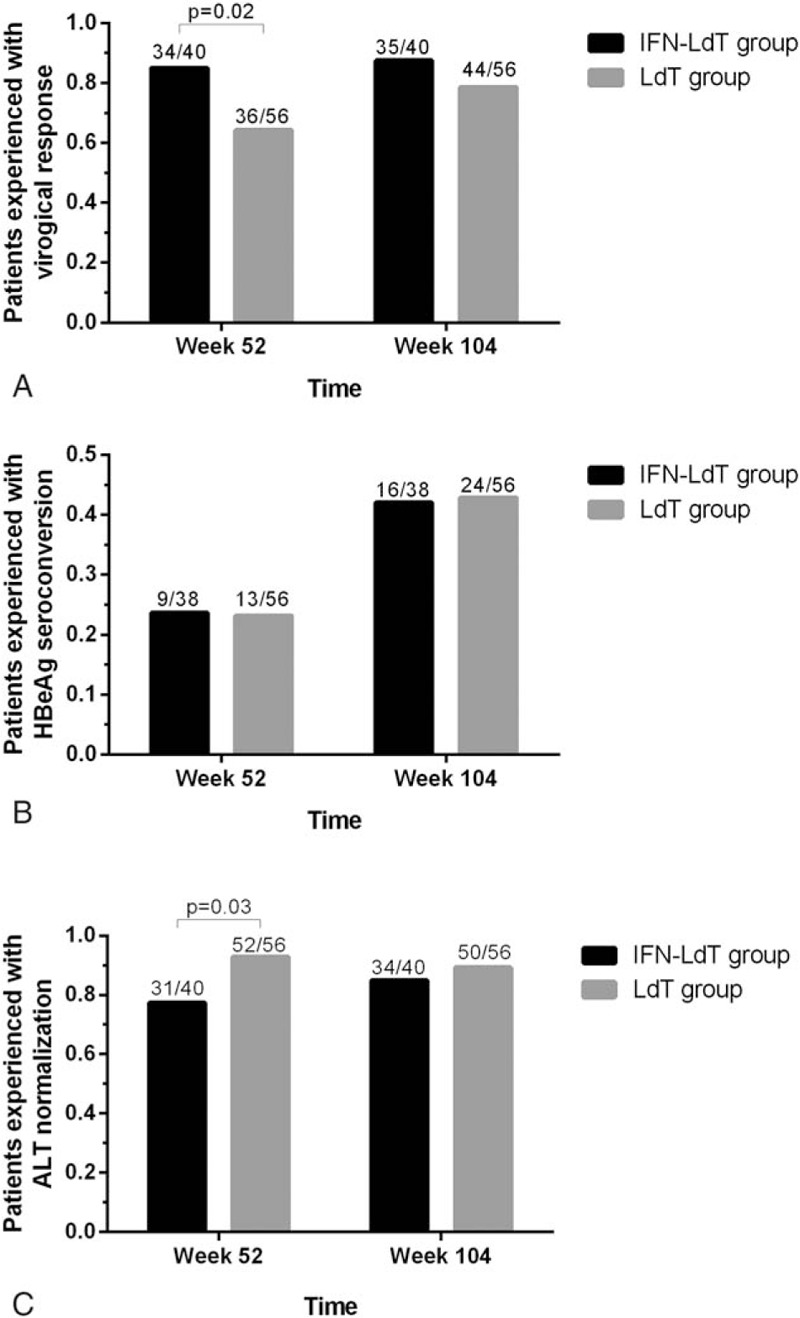
Antiviral efficacy in telbivudine (LDT)-based treatment. (A) Comparison the virological response in LDT-based treatment. At week 52, 85.0% (34/40) of patients in the interferon-telbivudine (IFN-LDT) group achieved a virological response, greater than the de novo LDT group with 64.3% (36/56) (P = .02). At week 104, 87.5% (35/40) of patients had a virological response from the IFN-LDT group and 78.6% (44/56) from LDT group (P = .26). (B) Comparison of the HBeAg seroconversion in LDT-based treatment. HBeAg seroconversion was achieved in 23.7% (9/38) at week 52 and 42.1% (16/38) at week 104 in the IFN-LDT group, with 23.2% (13/56) and 42.9% (24/56) in the de novo LDT group, respectively. (C) Comparison the biochemical response in LDT-based treatment 77.5% of patients in the IFN-LDT group achieved alanine aminotransferase (ALT) normalization versus 92.9% in the de novo LDT group at week 52 (P = .03).
HBeAg seroconversion (Fig. 3B) was compared in the 2 groups. Of the HBeAg-positive patients at baseline in the IFN-LDT group, HBeAg seroconversion was achieved in 23.7% (9/38) at week 52 and 42.1% (16/38) at week 104, respectively. Whereas in the de novo LDT group, HBeAg seroconversion was achieved in 23.2% (13/56) and 42.9% (24/56), respectively. No significant difference was observed in the HBeAg seroconversion rate at 104 weeks of treatment between the 2 groups.
The ALT normalization rate at week 52 of the IFN-LDT group was observed in 77.5% of the patients, while in the de novo LDT treatment group this was 92.9% (P = .03), as shown in Fig. 3C. However, the proportion of patient with biochemical response in the 2 groups showed no significant different at week 104.
3.4. Antiviral effectiveness in the IFN-ETV group compared with the IFN-LDT group
To evaluate which NUC, LDT or ETV, was the better choice for patients with chronic HBV infection after partial responses to interferon, the antiviral effectiveness of IFN-LDT group and IFN-ETV group were compared. The virological, serological, and biochemical responses are shown in Fig. 4. After 104 weeks of treatment, the proportion of patients with undetectable HBV DNA level, HBeAg seroconversion among HBeAg seropositive patients, and ALT normalization, was similar and no significant difference between the 2 groups.
Figure 4.
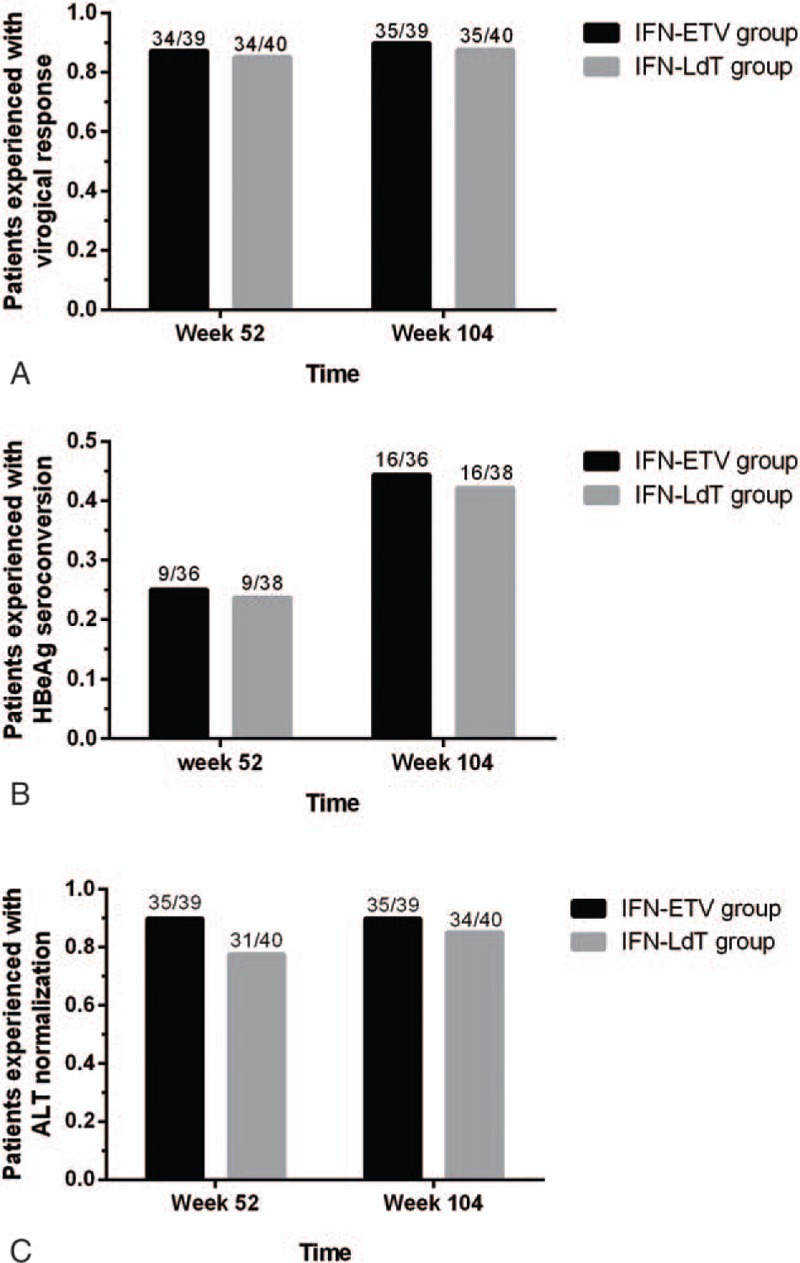
Comparison of the antiviral efficacy between the interferon-entecavir (IFN-ETV) group and the interferon-telbivudine (IFN-LDT) group. (A) Comparison of the virological responses in interferon-nucleos (t)ide analogue (IFN-NUC) treatment. At week 52, 85.0% (34/40) of patients in the IFN-LDT group experienced with virologic response, similar to the IFN-ETV group with 87.2% (34/39). At week 104, 35/40 of patients had virologic response from IFN-LDT group and 35/39 from the IFN-ETV group (P = .75). (B) Comparison the HBeAg seroconversion in IFN-NUC treatment. For IFN-LDT group, HBeAg seroconversion was achieved in respective 23.7% (9/38) at week 52 and 42.1% (16/38) at week 104, whereas 25.0% (9/36) and 44.4% (16/36) in IFN-ETV group. (C) Comparison the biochemical response in IFN-NUC treatment. There were 77.5% of patients in the IFN-LDT group experienced alanine aminotransferase (ALT) normalization versus 89.7% in the IFN-ETV group at week 52 (P = .14) and 85.0% versus 89.7% at week 104 (P = .53).
3.5. Analysis of baseline ALT level and HBV DNA level as predictors of antiviral efficacy at 104 weeks
To evaluate the baseline characteristics as predictors of clinical outcome at week 104, we compared the baseline characteristics between patients with or without virologic response. The ALT level was 200.39 ± 153.52 U/L and HBV DNA load was 7.38 ± 1.25 log10 IU/mL among patients who experienced virological response, significantly different from the other patients with baseline ALT levels of 117.31 ± 70.88 U/L (P = .006) and HBV DNA load of 8.13 ± 0.85 log10 IU/mL (P = .003).
A receiver operating characteristic (ROC) curve was used to determine the performance of the baseline ALT levels and HBV DNA load in the prediction of virological response at week 104 (Fig. 5). The area under the ROC (AUROC) was 0.66 (95% CI 0.57–0.76, P = .008) for baseline ALT levels and 0.69 (95% CI 0.58–0.79, P = .002) for baseline HBV DNA load.
Figure 5.
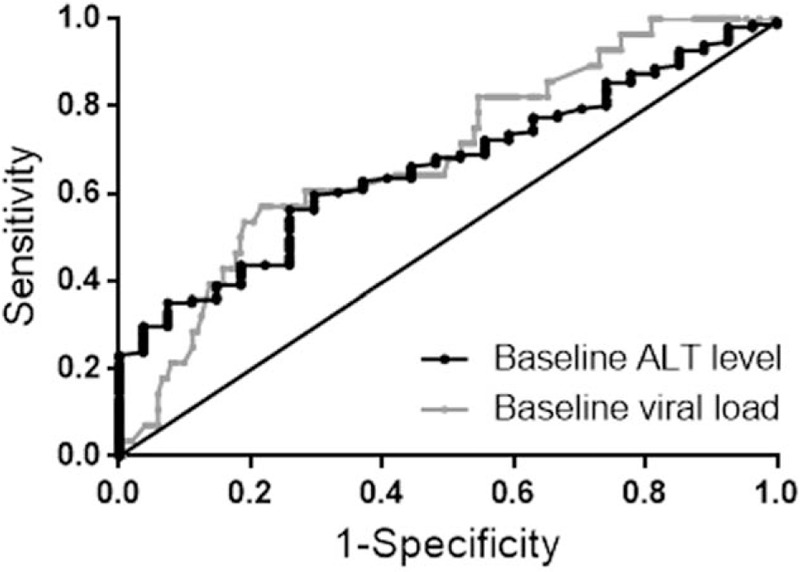
Receiver operating characteristic (ROC) curve to determine the performance of the baseline alanine aminotransferase (ALT) levels and hepatitis B virus (HBV) DNA load in predicting virological response at week 104. The area under the receiver operating characteristic (AUROC) curve was 0.66 (95% CI 0.57–0.76, P = .008) for baseline ALT levels and 0.69 (95% CI 0.58–0.79, P = .002) for baseline HBV DNA load. When using baseline ALT >132 U/L to predict virological response at week 104, the sensitivity was 56.3% and the specificity was 74.1%, the positive likelihood ratio (+LR) was 2.17 and the negative likelihood ratio (−LR) was 0.59. When using baseline HBV DNA <8.34 log10 IU/mL to predict virological response at week 104, the sensitivity, specificity, +LR and −LR were 57.1%, 78.3%, 2.63, and 0.55, respectively.
When using baseline ALT levels to predict virological response at week 104 with ALT >132 U/L, the sensitivity was 56.3%, and the specificity was 74.1%; the positive likelihood ratio (+LR) was 2.17, and the negative likelihood ratio (−LR) was 0.59. When using baseline HBV DNA load to predict virological response at week 104 with HBV DNA <8.34 log10 IU/mL, the sensitivity, specificity, +LR, and −LR were 57.1%, 78.3%, 2.63, and 0.55, respectively.
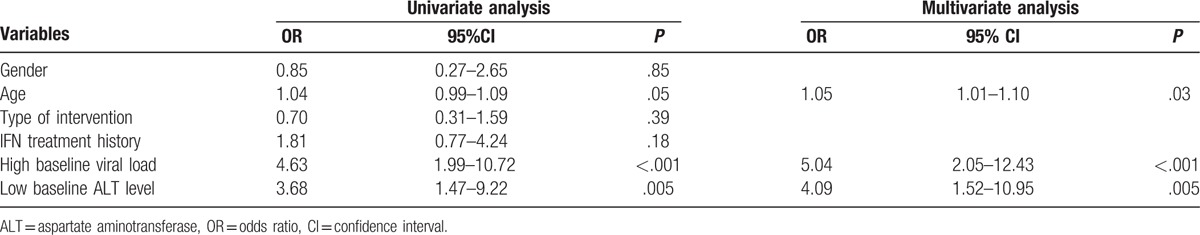
In addition to the analysis of baseline HBV DNA and ALT levels, we performed multivariate logistic regression analysis to identify the predictive value of virological response at week 104. We defined a high ALT level with ALT >132 U/L, and a high baseline HBV DNA load with HBV DNA >8.34 log10 IU/mL (Table 2). Results indicated that patient age, high baseline HBV DNA load, and low baseline ALT levels were risk factors to develop virological response at week 104 for patients included in this study. The odds ratio for patient age was 1.05 (95% CI 1.01–1.10, P = .03) and for high baseline HBV DNA viral load was 5.04 (95% CI 2.05–12.43, P < .001) and for a low baseline ALT level was 4.09 (95% CI 1.52–10.95, P = .005).
Table 2.
Multivariate logistic regression analysis to identify the predictive value of virological responses.
To verify the relationship between age and clinical outcomes, the average age was compared between patients with or without virologic response. The average age among patients who experienced virological response was 33.11 ± 9.10 years, significantly lower than patients without virological response with age 36.96 ± 10.44 years (P = .045), as shown in Supplementary Figure 1.
3.6. Drug safety and drug resistance
A total of 17 patients developed LDT resistance during the follow-up period. Five of these patients were from the IFN-LDT group and 12 patients were from the de novo LDT group (P = .26). When compared with the absence of cases in the ETV group, significantly increased creatine kinase (CK) levels were found in LDT group in 8 patients (8.33%). All patients in the ETV group were had no adverse reactions and treatment was well tolerated.
4. Discussion
Although interferon is a first-line drug for the treatment of patients with chronic HBV infection recommended by current guidelines, a considerable proportion of patients fail to achieve a sustained response.[1] Data from this study have shown that, at week 52, treatment with IFN-LDT resulted in a significantly greater virological response rate than from the de novo LDT group, and from IFN-ETV than from the de novo ETV group. In addition, patients treated IFN-ETV achieved a higher HBeAg seroconversion rate when compared with the de novo ETV. The results indicated that patients who switch to NUCs after partial response to interferon had a faster virological response than patients treated de novo with NUCs. The results of this study also indicate that when patients switch to ETV after partial response to interferon, they may show an increase in HBeAg seroconversion compared with patients who receive de novo ETV therapy. According to our knowledge, this is the first study report that clinical phenomenon. However, whether this clinical phenomenon happens because of the delay in the effect of interferon is still unknown. Patients from the IFN-LDT group with a greater virological response achieved a lower ALT normalization rate at week 52, which may imply that the delayed effect of interferon possibly did exist.
Previously, it has been suggested that combined therapy or a switch to NUC therapy for patients with chronic HBV infection should be considered when treatment response with interferon is poor.[13] However, at this time, there is no consensus to recommend which kind of NUC should be the optimal choice after the failure of interferon therapy. It has been previously reported that patients with chronic HBV infection had undergone HBeAg seroconversion after discontinuing interferon therapy.[14] Extending the interferon treatment course for patients who do not initially respond may improve the response rate, but the risk of adverse effects may also increase; there is also the economic factor to consider, as interferon therapy is expensive, especially in low-income countries. Data from our study imply that switching to NUC therapy is convenient and provides a higher virological response rate. However, whether the delayed effect of interferon therapy will improve response rate to NUCs in patients with chronic HBV infection has not been reported previously. This present study has shown that patients with chronic HBV infection who failed interferon therapy, a switch to LDT or ETV could be effective in suppressing HBV replication and in achieving ALT normalization and HBeAg seroconversion.
However, in this study, there was no statistically significant difference found in virological response, HBeAg seroconversion rates, and biochemical responses between the 2 groups of patients. At week 104, 12.5% patients (5/40) from the IFN-LDT group developed drug resistance compared with the absence of drug resistance in the IFN-ETV group. ETV may be a better NUC treatment choice than LDT for patients with chronic HBV infection with partial response to interferon. ETV is a potent NUC with a high barrier to the development of resistance, which was confirmed by this study, as no drug resistance was found in the ETV group.[15] However, 4 patients in the IFN-ETV group did not achieve virological treatment response at 104 weeks, but none of them had detectable resistance mutations. The adherence should be taken into consideration.[16] Although the difference in drug resistance between the IFN-LDT group and the de novo LDT group did not reach significance, it is worth considering that there was a nearly 2-fold difference in the proportion of patients who experienced drug resistance in the IFN-LDT group (12.5%) and the de novo LDT group (21.4%).
Previous studies have reported that baseline high ALT level and low HBV DNA viral load is a strong predictor for better antiviral efficacy at week 104 in patients with chronic HBV infection with LDT treatment.[17] This study confirmed that a relatively low ALT level at baseline and a high HBV DNA viral load at baseline were factors associated with the development of a virological response for patients with chronic HBV infection. In addition, this study confirmed that a relatively low ALT level and high HBV DNA viral load were factors that could be applied in clinical practice for patients who switch to NUC treatment after partial response to interferon. Previous studies have confirmed that age is a risk factor for poor response in patients who received interferon treatment.[17] This study also found that older age was a factor in reducing virological treatment response when switching to NUC treatment following interferon treatment.
Combination therapy with interferon and LDT has not been recommended by current treatment guidelines due to the neuromuscular toxicity of LDT.[18] Therefore, it is important to monitor patients closely for musculoskeletal symptoms and signs when switching to LDT following interferon therapy. Although increased CK levels were initially observed in the IFN-LDT patient group, the CK levels normalized on follow-up. In this preliminary study, patients with previous interferon treatment who switched to LDT tolerated the treatment well.
This study has several limitations. This study was small and preliminary in nature, conducted in a single center and without patient randomization. Patients included in the study were recruited from the outpatient department during a defined period of time. The data from the study was collected and analyzed by the authors of the study, which may have introduced interpretation bias. For these reasons, further studies would be recommended with larger, controlled, prospective, randomized patient groups and in multiple centers. Because the number of patients included in the current study was limited, a larger number of patients with chronic HBV infection are needed to explore the difference in drug resistance between patients who switch to NUC after partial response to interferon and patients with de novo NUC treatment.
5. Conclusion
This is the first study, as far as we know, has shown that for patients with chronic HBV infection, switch to rescue therapy with ETV or LDT therapy after failure of interferon therapy, resulted in more rapid virological response when compared with de novo treatment with either ETV or LDT, and rescue therapy with ETV resulted in a greater rate of HBeAg seroconversion. However, the findings of this preliminary study should be applied with caution and should be supported by further, larger, randomized and controlled clinical studies.
Supplementary Material
Footnotes
Abbreviations: +LR = positive likelihood ratio, −LR = negative likelihood ratio, ALT = alanine aminotransferase, AUROC = area under the ROC, CK = creatine kinase, ETV = entecavir, HBeAg = hepatitis B e antigen, HBV = hepatitis B virus, LDT = telbivudine, NUCs = nucleos(t)ide analogues, ROC = receiver operating characteristic, ULN = the upper limit of normal.
SC and JC contributed equally to this work.
All authors declare that they have no financial or personal relationships with other people or organizations that could inappropriately influence this work.
Supplemental Digital Content is available for this article.
References
- [1].Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pollicino T, Saitta C, Raimondo G. Hepatocellular carcinoma: the point of view of the hepatitis B virus. Carcinogenesis 2011;32:1122–32. [DOI] [PubMed] [Google Scholar]
- [3].European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012;57:399–420. [DOI] [PubMed] [Google Scholar]
- [4].Dogan UB, Golge N, Akin MS. The comparison of the efficacy of pegylated interferon alpha-2a and alpha-2b in chronic hepatitis B patients. Eur J Gastroenterol Hepatol 2013;25:1312–6. [DOI] [PubMed] [Google Scholar]
- [5].Karabay O, Tuna N, Esen S. PEG-HBV Study Group. Comparative efficacy of pegylated interferons alpha-2a and 2b in the treatment of HBeAg-negative chronic hepatitis B infection. Eur J Gastroenterol Hepatol 2012;24:1296–301. [DOI] [PubMed] [Google Scholar]
- [6].Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology 2009;50:661–2. [DOI] [PubMed] [Google Scholar]
- [7].Cai S, Yu T, Jiang Y, et al. Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week result. Clin Exp Med 2015;16:429–36. [DOI] [PubMed] [Google Scholar]
- [8].Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liaw Y, Kao J, Piratvisuth T, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 2012;6:531–61. [DOI] [PubMed] [Google Scholar]
- [10].Shi TD, Zhang JM, Wang XF, et al. Effects of antiviral therapy with Telbivudine on peripheral iNKT cells in HBeAg(+) chronic hepatitis B patients. Clin Exp Med 2012;12:105–13. [DOI] [PubMed] [Google Scholar]
- [11].Ma SW, Huang X, Li YY, et al. High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J Hepatol 2012;56:775–81. [DOI] [PubMed] [Google Scholar]
- [12].Cai SH, Lv FF, Zhang YH, et al. Dynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHB. BMC Infect Dis 2014;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wan M, Weng X. Experts advice on treatment of chronic hepatitis B with interferon (update 2010). Chin J Infect Dis 2010;28:193–200. [Google Scholar]
- [14].Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–95. [DOI] [PubMed] [Google Scholar]
- [15].Wu X, Cai S, Li Z, et al. Potential effects of telbivudine and entecavir on renal function: a systematic review and meta-analysis. Virol J 2016;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Peng J, Yin J, Cai S, et al. Factors associated with adherence to nucleos(t)ide analogs in chronic hepatitis B patients: results from a 1-year follow-up study. Patient Prefer Adherence 2015;9:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liaw YF, Gane E, Leung N, et al. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology 2009;136:486–95. [DOI] [PubMed] [Google Scholar]
- [18].Marcellin P, Wursthorn K, Wedemeyer H, et al. Telbivudine plus pegylated interferon alfa-2a in a randomized study in chronic hepatitis B is associated with an unexpected high rate of peripheral neuropathy. J Hepatol 2015;62:41–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


