Abstract
The aim of this study was to explore the feasibility and clinical value of ultrasound combined with a warm bath test in assessing the differences in reactivity of toe microcirculation between healthy adults and patients with type 2 diabetes mellitus (T2DM).
A total of 56 T2DM patients were recruited as case group, whereas 50 healthy volunteers were enrolled as control group.
Fasting blood glucose, glycated hemoglobin, total cholesterol, triglyceride, low-density lipoprotein cholesterol in T2DM group were significantly higher than in control group. Under stationary condition, peak systolic velocity (PSV), end-diastolic velocity (EDV), and mean velocity (MV) were lower, but pulsatility index (PI) and resistance index (RI) were higher in patients with T2DM than in controls both in dorsalis pedis artery (DPA) and plantar digital artery (PDA). On response to the warm test, PSV, EDV, and MV increased and PI and RI decreased both in DPA and PDA in these 2 groups. Moreover, the change rate in PSV, EDV, MV, PI, and RI of PDA was significantly lower in T2DM group than in control group.
Color Doppler combined with a warm bath test may be used as a new method in evaluating the differences in reactivity of distal limb microvascular between healthy adults and patients with T2DM.
Keywords: color doppler ultrasonography, microcirculation, toe, type 2 diabetes mellitus, Warm bath test
1. Introduction
Type 2 diabetes mellitus (T2DM) markedly increases the risk of all forms of cardiovascular disease with prospective studies demonstrating a significantly increased incidence of morbidity and mortality. Endothelial dysfunction, an early indicator of diabetic vascular disease, is common in T2DM and independently predicts cardiovascular risk.[1–5] Identification of endothelial dysfunction in the microcirculation of the extremities in patients with T2DM is critical for predicting future cardiovascular complication and prompt treatment during the early stage of T2DM.[6,7] Both invasive and noninvasive methods have been generated for assessing microcirculation in T2DM. Being a dynamic structure, the human skin can be used as a microcirculation model for investigating the generalized endothelium function. Previous studies have revealed a correlation of vascular reactivity in different vascular beds over the body (e.g., coronary arteries, brachial artery, and skin microcirculation) of healthy people and patients. Color Doppler ultrasound is the most frequently used tool for monitoring the microvascular blood flow by far, as it is noninvasive, can determine dynamic changes, easy to operate, reproducible, and inexpensive. Based on that, the cutaneous microcirculation can be a “mirror” reflecting the global microvascular function. In the study, we observed the blood flow measures to explore the feasibility and clinical value of color Doppler combined with a warm bath test in assessing reactivity of toe microcirculation in healthy adults and patients with T2DM.
2. Methods
2.1. Subjects
This study has been approved by the Ethics Committee of The Second Affiliated Hospital & Yuying Children's Hospital of Wenzhou Medical University (2015–06–1J). According to the etiological classification and diagnostic criteria recommended by the American Diabetes Association,[8] 56 in-patients (29 males and 27 females, aged 32–58 years with a mean age of 48.14 ± 12.65 years), who were diagnosed with T2DM and admitted in the Department of Endocrinology at the Second Affiliated Hospital of Wenzhou Medical University, were enrolled as case group between June 2015 and May 2016. Fifty healthy volunteers (27 males and 23 females, aged 30–59 years, mean 47.43 ± 12.01 years) who were excluded T2DM by oral glucose tolerance test were enrolled during the same period as the control group. Exclusion criteria were: high blood pressure,[9] thyroid disease,[10] heart failure,[1] severe kidney disease, and vascular disease caused by other endocrine diseases[2,6,7]; deformity and stenosis in ascending aorta, aortic arch or thoracic aorta confirmed by chest CT scans in the latest 3 months (criteria of stenosis with rate of stenosis >50%)[11]; existence of ischemic gangrene in distal extremities[12]; intake of caffeine- and alcohol-containing products or medicine with vasodilator effects during the 24 hours before the test[13,14]; The peripheral arteries (including brachial artery, radial artery, ulnar artery, arteria cruralis, popliteal artery, anterior tibial artery and posterior tibial artery, among others) diseases, for example, deformity, athrosclerosis, among others, which could be identified through ultrasonography just before test.
2.2. Clinical manifestations
Information including age,[15] sex,[16] body mass index (BMI),[3] systolic blood pressure (SBP) and diastolic blood pressure (DBP),[4] ankle-brachial index (ABI), duration of T2DM, rest pain, intermittent claudication, and pulse of dorsalis pedis artery (DPA)[5,17–19] of all subjects were collected by an endocrinologist through detailed history and physical examination.
2.3. Laboratory investigations
Fasting blood glucose (FBG), glycated hemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), fibrinogen (Fib), high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) of all subjects were tested within 24 hours before ultrasonography.
2.4. Ultrasonic instruments and methods
Ultrasonography was conducted using Siemens Acuson Sequoia 512 Ultrasound Imaging System, which was equipped with an 15L8w-S transducer (frequency 8–14 MHz), the sampling gate vascular width about two-thirds of diameter, the wall filter between 50 and ∼100 Hz, whereby the angle between sound beam and blood flow was <60 degrees. The room temperature was set to 25°C. Subject in supine position had a rest for 10 minutes before ultrasonography. Then the right DPA and plantar digital artery (PDA) on fibular side of the right 1st toe were scanned carefully, peak systolic velocity (PSV), end-diastolic velocity (EDV), mean velocity (MV), pulsatility index (PI), and resistance index (RI) were measured through pulse Doppler. Then the right foot of each subject was immersed in 40°C warm water (product model ZF-YZ5091 foot tub, thermostat 40°C was set) for 5 minutes. The above operations were repeated 3 times wherein the PSV, EDV, MV, PI, and RI after warm bath test were measured at rest. The change rate of each parameter was calculated according to the following equation: change rate of parameter = (parameter after warm bath test − parameter before warm bath test)/parameter before warm bath test. Drugs with vasodilating effects were forbidden before ultrasound examination, but hypoglycemic agents were allowed.
2.5. Measures of blood flow parameters
PSV is the peak velocity during systolic period. EDV is the velocity at the end of diastolic period. Both PSV and EDV can be recognized easily on a Doppler spectrum. MV is calculated through time velocity integral and cardiac cycle, and it represents average velocity. PI is calculated through this formula: PI = (PSV − EDV)/MV. RI is calculated through this formula: RI = (PSV − EDV)/PSV. Both PI and RI are indexes, which can reflect the elasticity of the local artery where sampling position is and the resistance at the distal circulation. So PSV, EDV, MV, PI, and RI all can be acquired from Doppler spectrum directly. Furthermore, change rate of these parameters was calculated in our study through the formula mentioned in the above paragraph. Change rate represents changes of these parameters after warm bath. In another word, the change rate of these parameters reflects the changes of hemodynamics when the temperature of the tissue around arteries increases.
2.6. Statistical analysis
ABI and blood parameters were acquired separately by 2 independent blinded investigators. Kolmogorov-Smirnov test was carried to identify whether the data are normally distributed. All measurements were expressed as mean ± standard deviation. All the original data were statistically processed by using SPSS 18.0 software package. A comparison between groups was made using a t test, whereas the intragroup parameters were compared by paired t test. Sex ratio was compared by χ2. P value <.05 was considered as statistically significant.
3. Results
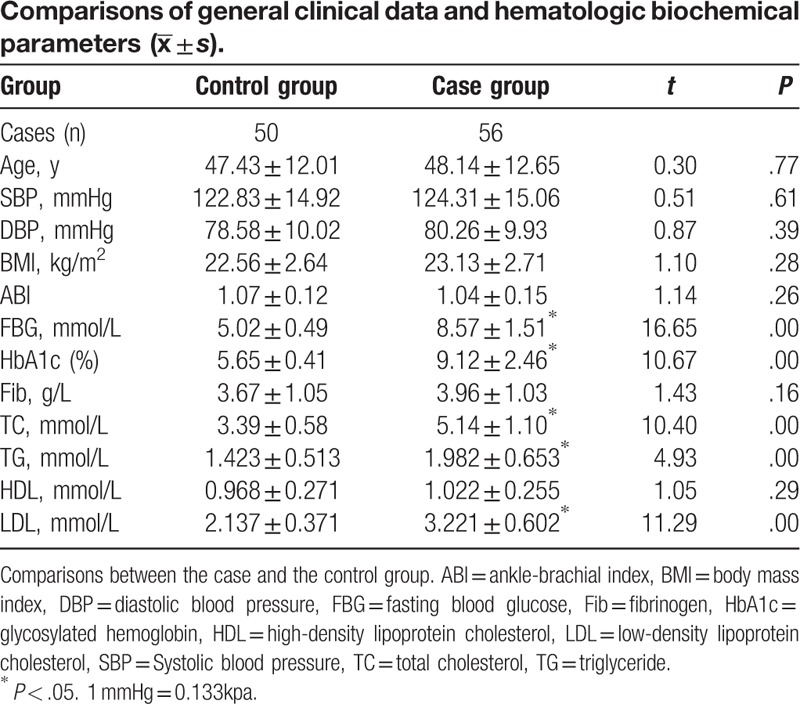
3.1. Comparisons of general clinical data and hematologic biochemical parameters
There were no differences between these 2 groups in sex ratio (P > .05). There were no differences between these 2 groups in age, SBP, DBP, ABI, BMI, HDL-c, and Fib at baseline. FBG, HbA1c, TC, TG, and LDL-c were significantly higher in T2DM group than control group (P < .05) (Table 1).
Table 1.
3.2. Comparison of blood flow parameters before and after warm bath test between case and control group
Doppler flow data of right DPA and PDA on fibular side of 1st toe were obtained in all subjects. Figures 1 and 2 presented the PDA Color Doppler results of a person from the control group, before and after water bath. Figures 3 and 4 showed the PDA Color Doppler results of a person from the T2DM group, before and after water bath (Figures 1–4).
Figure 1.

Ultrasonography of plantar digital artery of 1 subject from control group before warm bath test. Spectrum Doppler image of plantar digital artery on fibular side of 1st toe of 1 subject from control group was acquired through pulse wave Doppler before warm bath test. Blood flow in plantar digital artery on fibular side of 1st toe was displayed clearly in the middle of the upper portion of this image, and the sampling gate was put on the position where plantar digital artery was. Width of sampling gate was 1.0 mm, and the angle between blood flow and acoustic beam was 57 degree. Spectrum Doppler graphics of blood flow in plantar digital artery on fibular side of 1st toe was displayed in the lower portion of this image, and the spectrum Doppler graphics was traced during a cardiac cycle by hand. Then the indexes that we wanted were shown in this image. PSV was 43.9 cm/s, which was equal to Max; EDV was 4.1 cm/s, which was equal to Min; MV was 15.4 cm/s, which was equal to TAMx. PI was 2.59; RI was 0.91. EDV = end-diastolic velocity, PSV = peak systolic velocity, MV = mean velocity, PI = pulsatility index, RI = resistance index.
Figure 2.

Ultrasonography of plantar digital artery of one subject from control group after warm bath test. Spectrum Doppler image of plantar digital artery on fibular side of 1st toe of one subject from control group was acquired through pulse wave Doppler before warm bath test. Blood flow in plantar digital artery on fibular side of 1st toe was displayed clearly in the middle of the upper portion of this image, and the sampling gate was put on the positon where plantar digital artery was. Width of sampling gate was 1.0 mm, and the angle between blood flow and acoustic beam was 58 degree. Spectrum Doppler graphics of blood flow in plantar digital artery on fibular side of 1st toe was displayed in the lower portion of this image, and the spectrum Doppler graphics was traced during a cardiac cycle by hand. Then the indexes which we wanted were shown in this image. PSV was 62.5 cm/s, which was equal to Max; EDV was 12.0 cm/s, which was equal to Min; MV was 25.4 cm/s, which was equal to TAMx. PI was 1.99; RI was 0.81. EDV = end-diastolic velocity, PSV = peak systolic velocity, MV = mean velocity, PI = pulsatility index, RI = resistance index.
Figure 3.

Ultrasonography of plantar digital artery of one subject from case group after warm bath test. Spectrum Doppler image of plantar digital artery on fibular side of 1st toe of one subject from control group was acquired through pulse wave Doppler before warm bath test. Blood flow in plantar digital artery on fibular side of 1st toe was displayed clearly in the middle of the upper portion of this image, and the sampling gate was put on the position where plantar digital artery was. Width of sampling gate was 1.0 mm, and the angle between blood flow and acoustic beam was 54 degree. Spectrum Doppler graphics of blood flow in plantar digital artery on fibular side of 1st toe was displayed in the lower portion of this image, and the spectrum Doppler graphics was traced during a cardiac cycle by hand. Then the indexes which we wanted were shown in this image. PSV was 24.4 cm/s, which was equal to Max; EDV was 1.3 cm/s, which was equal to Min; MV was 6.8 cm/s, which was equal to TAMx. PI was 3.41; RI was 0.95. EDV = end-diastolic velocity, PSV = peak systolic velocity, MV = mean velocity, PI = pulsatility index, RI = resistance index.
Figure 4.

Ultrasonography of plantar digital artery of one subject from case group after warm bath test. Spectrum Doppler image of plantar digital artery on fibular side of 1st toe of one subject from control group was acquired through pulse wave Doppler before warm bath test. Blood flow in plantar digital artery on fibular side of 1st toe was displayed clearly in the middle of the upper portion of this image, and the sampling gate was put on the position where plantar digital artery was. Width of sampling gate was 1.0 mm, and the angle between blood flow and acoustic beam was 49 degree. Spectrum Doppler graphics of blood flow in plantar digital artery on fibular side of 1st toe was displayed in the lower portion of this image, and the spectrum Doppler graphics was traced during a cardiac cycle by hand. Then the indexes which we wanted were shown in this image. PSV was 33.4 cm/s, which was equal to Max; EDV was 4.4 cm/s, which was equal to Min; MV was 9.4 cm/s, which was equal to TAMx. PI was 3.08; RI was 0.87. EDV = end-diastolic velocity, PSV = peak systolic velocity, MV = mean velocity, PI = pulsatility index, RI = resistance index.
Under stationary condition, PSV, EDV, and MV were significantly lower, but PI and RI were significantly higher in patients with T2DM than in controls both in right DPA and PDA on fibular side of 1st toe (P all <.05) (Table 2).
Table 2.

After warm test, PSV, EDV, and MV were significantly lower, but PI and RI were significantly higher in patients with T2DM than in controls both in right DPA and PDA on fibular side of 1st toe (P all <.05). On response to the warm test, PSV, EDV, and MV were significantly increased and PI and RI were significantly decreased both in right DPA and PDA in these 2 groups (P all < .05). The change rate in PSV, EDV, MV, PI, and RI of PDA were significantly lower in T2DM group than in control group (P all <.05). No significant change rate was observed in PSV, EDV, MV, PI, and RI of right DPA between the 2 groups (Table 2).
4. Discussion
In the present study, we investigated for the first time the blood flow parameters change of microvascular capillaries by increasing the local temperature. As the results showed, PSV, EDV, and MV were significantly lower, but PI and RI were significantly higher in patients with T2DM than in healthy controls in DPA and PDA at baseline, which presented baseline changes of hemodynamics as a decrease of blood flow velocity and increase of resistance,[20] suggesting that microvascular impairment in distal limb might occur before ischemic symptoms and signs in patients with T2DM. After warm bath, the parameters of PSV, EDV, and MV increased sharply both in PDA and DPA, indicating that warm bath could obviously increase blood flow of these 2 arteries in subjects from whatever T2DM or healthy controls. Furthermore, we compared and found that change rate of these parameters was lower in T2DM group than in normal control group in these 2 arteries; however, only change rate in DPA reached significance. These results indicated that the reactivity of blood microvasculation in distal limb end was evidently slower in T2DM. In another word, the change rate of the values after warm water bath reflected the endothelium function. On the other side, there were no differences between case group and control group in ABI, which was seemed as a classical index to evaluate lower extremity arterial diseases in clinics. Microcirculatory hemodynamics in distal limb may change in spite of ABI is normal in patients with T2DM. During the warm water bath test, the bath time and water temperature for PDA and DPA were the same (external intervention). The difference between the PDA and DPA might be caused by the 2 reasons. First, PDA and DPA are different in artery categories and blood vessels structures. PDA is similar to end of human vascular bed. Second, T2DM has different stage of endothelium damage on PDA and DPA. Our study found that, after the warm water bath, T2DM group showed a statistically nonsignificant lower change in DPA, but a significant lower change in PDA. The differences proved that T2DM patients’ PDA had a significant decreased ability in response to warm stimulation (endothelium function), but the response in DPA did not change much. The findings support the theory that T2DM has an earlier and more obviously damage on microvascular endothelium function compared to small arteries. Moreover, it proved that the new method introduced by our study is feasible and practical.
The pathophysiology of these changes might be related to thrombosis, poor blood flow, increased peripheral resistance, and decreased elasticity, which are caused by endothelial dysfunction in T2DM.[21–24] Although the pathogenesis of endothelial dysfunction in diabetes is complex, the major contributing factors include dyslipoproteinemia,[10] oxidative stress, and inflammation.[1,2,6] When endothelial function is impaired, nitric oxide produced and secreted by endothelium decreases and oxygen-free radicals’ production increased.[25] Hyperglycemia and insulin resistance are risk factors too.[26,27] Clinical data in the present study also showed that FBG, HbA1c in T2DM group were significantly higher than those in control group, which leads to the proteins saccharified in the blood and advanced glycation end product accumulated gradually in the vessels.[28,29] Dyslipidemia is also an important risk factor to lower extremity vascular disease in T2DM,[30] which can promote blood clotting, inhibiting fibrinolysis, platelet aggregation, prostacyclin synthesis reduction, and endothelium dysfunction.[31]
Indirect measures in the peripheral circulation assess the vasodilatory responses of conduit and resistance arteries to stimuli that increase nitric oxide release.[3,9,12,16] In the brachial artery, shear stress is generated by hyperemia following an induced period of local ischemia, and flow-mediated dilatation, an endothelium-dependent function, is measured using high-resolution ultrasonography or even magnetic resonance imaging. An emerging noninvasive clinical tool to assess peripheral vasodilator response is digital peripheral arterial tonometry (PAT) (Endo-PAT, Itamar Medical).[19,32] Recently, a new method is to evaluate endothelial function of superficial vasculary with laser ultrasound combined cold pressor test. In the test, the temperature of index finger of right hand is monitored by laser ultrasound when left hand is immersed in mixture of iced water (0°C) for 3 minutes. But because special instruments are required and the test temperature is too low for some weak patients, this method is not practical and popular in clinic. Although the warm bath test is a repeated external operation that is safe, easy, and feasible. There are also some limitations in the study. First, the strong site-and time-dependence of the obtained signal and a great interindividual variability must be taken into account. Furthermore, the sample size in the study is not large and the results we got are still not so powerful and need confirmation.
In conclusion, ultrasound combined with the warm bath test may be used as a new method for detecting early changes in reactivity of toe microvascular in patients with T2DM. It has the feasibility and potential value in clinical practice.
Footnotes
Abbreviations: ABI = ankle-brachial index, BMI = body mass index, DBP = diastolic blood pressure, DPA = dorsalis pedis artery, EDV = end-diastolic velocity, FBG = fasting blood glucose, Fib = Fibrinogen, HbA1c = glycated hemoglobin, HDL-c = high density lipoprotein cholesterol, LDL-c = low density lipoprotein cholesterol, MV = mean velocity, PAT = peripheral arterial tonometry, PDA = plantar digital artery, PI = pulsatility index, PSV = peak systolic velocity, RI = resistance index, SBP = systolic blood pressure, T2DM = type 2 diabetes mellitus, TC = total cholesterol, TG = triglyceride.
CZ and YJ contributed equally to this study.
This study was supported by National Natural Science Foundation of China (NSFC) (No. 81670777 and No. 81161120550), Natural Science Foundation of Zhejiang Province, China (No. LQ12H07001), Wenzhou City Science & Technology Bureau, China (No. Y20150084, No. Y20150085, and No. Y20160495), and Health and Family Planning Commission of Zhejiang Province, China (No.2015KYA159 and No. 2016KYA143).
The authors report no conflicts of interest.
References
- [1].De Backer D, Creteur J, Dubois MJ, et al. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J 2004;147:91–9. [DOI] [PubMed] [Google Scholar]
- [2].Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007;30:1212–8. [DOI] [PubMed] [Google Scholar]
- [3].Resnick HE, Valsania P, Halter JB, et al. Differential effects of BMI on diabetes risk among black and white Americans. Diabetes Care 1998;21:1828–35. [DOI] [PubMed] [Google Scholar]
- [4].Estacio RO, Jeffers BW, Gifford N, et al. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000;23(Suppl 2):B54–64. [PubMed] [Google Scholar]
- [5].Kreitner KF, Kalden P, Neufang A, et al. Diabetes and peripheral arterial occlusive disease: prospective comparison of contrast-enhanced three-dimensional MR angiography with conventional digital subtraction angiography. Am J Roentgenol 2000;174:171–9. [DOI] [PubMed] [Google Scholar]
- [6].Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res 2004;95:560–7. [DOI] [PubMed] [Google Scholar]
- [7].Whitworth JA, Mangos GJ, Kelly JJ. Cushing, cortisol, and cardiovascular disease. Hypertension 2000;36:912–6. [DOI] [PubMed] [Google Scholar]
- [8].Resnick HE, Harris TB. New diagnostic criteria for diabetes mellitus. New criteria result in fewer cases in older adults. BMJ (Clinical research ed) 1999;318:531.author reply 533. [PMC free article] [PubMed] [Google Scholar]
- [9].Scuteri A, Nilsson PM, Tzourio C, et al. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J Hypertens 2011;29:1469–77. [DOI] [PubMed] [Google Scholar]
- [10].Glastras SJ, Craig ME, Verge CF, et al. The role of autoimmunity at diagnosis of type 1 diabetes in the development of thyroid and celiac disease and microvascular complications. Diabetes Care 2005;28:2170–5. [DOI] [PubMed] [Google Scholar]
- [11].van Velzen JE, Schuijf JD, de Graaf FR, et al. Diagnostic performance of non-invasive multidetector computed tomography coronary angiography to detect coronary artery disease using different endpoints: detection of significant stenosis vs. detection of atherosclerosis. Eur Heart J 2011;32:637–45. [DOI] [PubMed] [Google Scholar]
- [12].Schaper NC, Andros G, Apelqvist J, et al. Diagnosis and treatment of peripheral arterial disease in diabetic patients with a foot ulcer. A progress report of the International Working Group on the Diabetic Foot. Diabetes Metab Res Rev 2012;28(suppl 1):218–24. [DOI] [PubMed] [Google Scholar]
- [13].Tolentino NJ, Wierenga CE, Hall S, et al. Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol Clin Exp Res 2011;35:1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heinonen I, Kemppainen J, Kaskinoro K, et al. Bone blood flow and metabolism in humans: effect of muscular exercise and other physiological perturbations. J Bone Mineral Res 2013;28:1068–74. [DOI] [PubMed] [Google Scholar]
- [15].Parisi TJ, Mandrekar J, Dyck PJ, et al. Meralgia paresthetica: relation to obesity, advanced age, and diabetes mellitus. Neurology 2011;77:1538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Babiker FA, van Golde J, Vanagt WY, et al. Pacing postconditioning: impact of pacing algorithm, gender, and diabetes on its myocardial protective effects. J Cardiovasc Transl Res 2012;5:727–34. [DOI] [PubMed] [Google Scholar]
- [17].Kawano M, Omori Y, Katayama S, et al. A questionnaire for neurological symptoms in patients with diabetes--cross-sectional multicenter study in Saitama Prefecture, Japan. Diabetes Res Clin Pract 2001;54:41–7. [DOI] [PubMed] [Google Scholar]
- [18].Lozano FS, Gonzalez-Porras JR, March JR, et al. Diabetes mellitus and intermittent claudication: a cross-sectional study of 920 claudicants. Diabetol Metab Syndr 2014;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wongkongkam K, Thosingha O, Riegel B, et al. Factors influencing the presence of peripheral arterial disease among Thai patients with type 2 diabetes. Eur J Cardiovasc Nurs 2012;11:70–6. [DOI] [PubMed] [Google Scholar]
- [20].Zhang T, Xia LH, Bian YY, et al. Blood flow of the acral finger arterioles in patients with type 2 diabetes by quality Doppler profiles. Cell Biochem Biophys 2013;67:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chisolm GM, Irwin KC, Penn MS. Lipoprotein oxidation and lipoprotein-induced cell injury in diabetes. Diabetes 1992;41(suppl 2):61–6. [DOI] [PubMed] [Google Scholar]
- [22].Morishita R, Nakamura S, Nakamura Y, et al. Potential role of an endothelium-specific growth factor, hepatocyte growth factor, on endothelial damage in diabetes. Diabetes 1997;46:138–42. [DOI] [PubMed] [Google Scholar]
- [23].Futrakul N, Butthep P, Vongthavarawat V, et al. Early detection of endothelial injury and dysfunction in conjunction with correction of hemodynamic maladjustment can effectively restore renal function in type 2 diabetic nephropathy. Clin Hemorheol Microcirc 2006;34:373–81. [PubMed] [Google Scholar]
- [24].Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med 2012;2012:918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Veves A, Akbari CM, Primavera J, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes 1998;47:457–63. [DOI] [PubMed] [Google Scholar]
- [26].Steinbrenner H, Speckmann B, Pinto A, et al. High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr 2011;48:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Seidell JC. Obesity, insulin resistance and diabetes--a worldwide epidemic. Br J Nutr 2000;83(suppl 1):S5–8. [DOI] [PubMed] [Google Scholar]
- [28].Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care 1995;18:258–68. [DOI] [PubMed] [Google Scholar]
- [29].Esposito K, Giugliano D, Nappo F, et al. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 2004;110:214–9. [DOI] [PubMed] [Google Scholar]
- [30].Dixon JL, Shen S, Vuchetich JP, et al. Increased atherosclerosis in diabetic dyslipidemic swine: protection by atorvastatin involves decreased VLDL triglycerides but minimal effects on the lipoprotein profile. J Lipid Res 2002;43:1618–29. [DOI] [PubMed] [Google Scholar]
- [31].Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res 2004;63:582–92. [DOI] [PubMed] [Google Scholar]
- [32].Jayawardena R, Ranasinghe P, Galappatthy P, et al. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr 2012;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]


