Abstract
Rationale:
Perivascular epithelial cell tumors (PEComas) of the pancreas are rare mesenchymal tumors and, to our knowledge, only 20 cases have been reported to date.
Patient concerns:
We report a 43-year-old female who presented with upper abdominal pain for 1 year. She underwent an exploratory laparotomy at a local hospital, which failed to resect the tumor. Five months later, she came to the Chinese National Cancer Center for surgery. Preoperative imaging revealed an 11.5-cm-sized mass located in the head of the pancreas. At the microscopic level, the tumor was composed of epithelioid and spindle cells possessing clear to focally granular eosinophilic cytoplasm, which grew in a nested and alveolar pattern around blood vessels. The tumor cells showed immunoreactivity for human melanoma black 45 (HMB-45), but did not express epithelial or endocrine markers.
Diagnoses:
Pancreatic PEComa.
Interventions:
Pancreaticoduodenectomy, partial hepatectomy, and vascular replacement were performed. After the surgery, the patient received 4 cycles of chemotherapy.
Outcomes:
The patient is free of recurrence and metastasis 1.5 years after surgical resection.
Lessons:
PEComa should be recognized as a preoperative differential diagnosis of pancreatic tumors. For treatment, removal of the tumor should be attempted, and in the case of tumors with malignant tendencies, the addition of chemotherapy should be considered.
Keywords: epithelioid cells, human melanoma black 45 (HMB-45), pancreatic neoplasm, pancreaticoduodenectomy, perivascular epithelioid cell tumor (PEComa)
1. Introduction
Perivascular epithelial cell tumors (PEComas) are a category of mesenchymal tumors composed of epithelioid or spindle cells possessing clear to focally granular eosinophilic cytoplasm, are centrally located, and have round to oval nuclei and inconspicuous nucleoli. The PEComa family includes lymphangioleiomyoma, angiomyolipoma, and clear cell “sugar” tumors.[1] The characteristic immunohistochemical profile includes positive melanocytic markers, such as human melanoma black 45 (HMB-45) and melanoma antigen (Melan-A), and occasionally myogenic markers, such as α smooth muscle actin (α-SMA) and desmin.
PEComas can occur in any part of the body and tend to arise especially in bone, soft tissue, abdominopelvic organs such as the uterus and gastrointestinal tract, or retroperitoneal organs such as the kidney. However, PEComas arising in the pancreas are extremely rare.[2] Until now, only 20 cases[3–22] have been reported, 18 of which were described in the form of case reports.
Here, we present a primary PEComa arising in the pancreatic head of a 43-year-old female without a history of tuberous sclerosis complexes (TSCs). The tumor in the present case is larger than any previously described. In addition, this is the first case described in which the tumor has invaded the liver, leading to a diagnosis of stage of M1. Due to the malignant tendency of the tumor and the late stage diagnosis, we treated the patient with chemotherapy after surgery. To our knowledge, this is the first case in which this treatment approach has been attempted.
2. Case report
A 43-year-old female was admitted to a local hospital presenting with epigastralgia for 1 year. An exploratory laparotomy had previously been performed at the local hospital, but failed to remove the tumor. A biopsy was taken, and the local hospital classified the tumor as a neuroendocrine neoplasm. Five months later, the patient came to the Chinese National Cancer Center for surgery. A pathology consultation indicated a diagnosis of pancreatic PEcoma.
Physical examination revealed mild epigastric pain and tenderness. No other signs or symptoms were present. The patient reported no tobacco or alcohol use. She did not have a history of malignancy, diabetes mellitus, or TSCs. Laboratory tests including hematology, serology, biochemistry, and tumor markers, shown in Table 1, revealed no significant abnormal findings.
Table 1.
Laboratory data.
Abdominal ultrasonographic imaging revealed an 11.8-cm-sized, oval-shaped, hypo- and iso-echoic mass located in the pancreatic head (Fig. 1). Color Doppler ultrasonography was used to visualize the blood flow signal in and around the mass. Neither large vessel involvement nor dilatation of common bile duct was observed.
Figure 1.
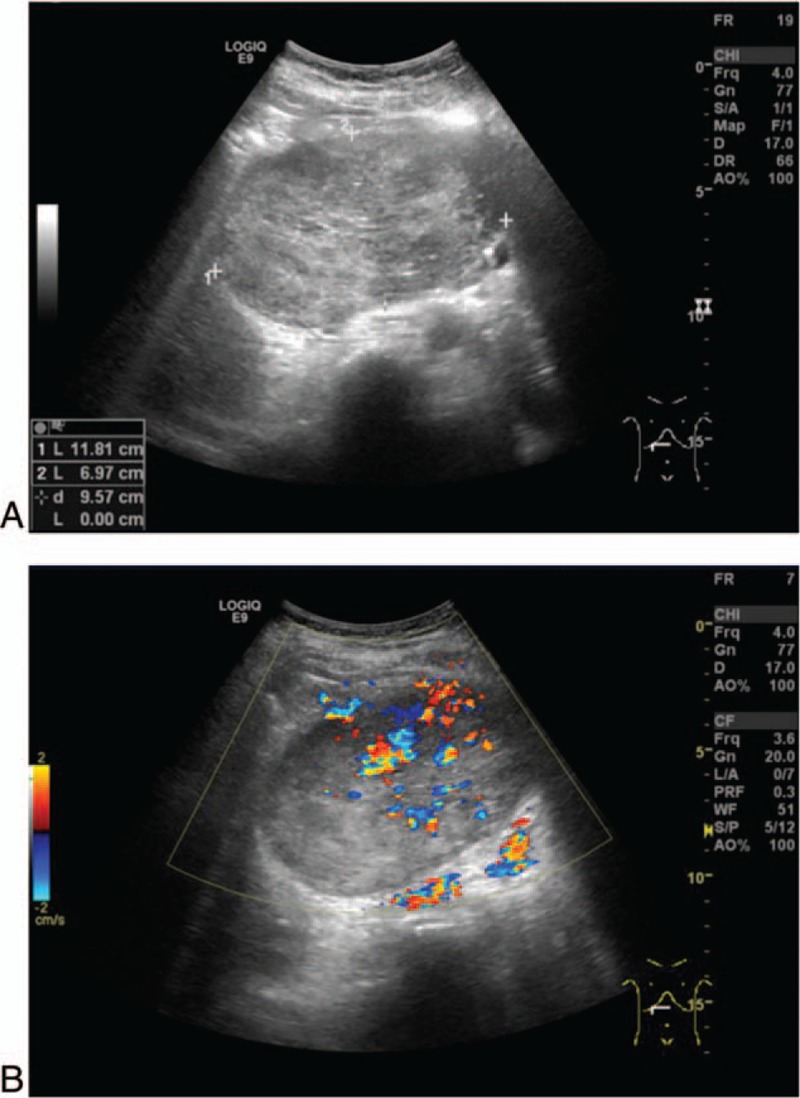
Imaging studies using abdominal ultrasonography. (A) Ultrasonography showing an oval-shaped hypo- and isoechoic mass, 11.8 cm in size, located in the pancreatic head. (B) Visualization of the blood flow signal in and around the mass using color Doppler ultrasonography.
Abdominal enhanced computed tomography (CT) imaging revealed a solid mixed-density mass, approximately 11.5 cm in diameter, located in the pancreatic head (Fig. 2). Dilatation of the upstream main pancreatic duct was also revealed. The tumor directly invaded adjacently into the liver and the superior mesenteric vein (SMV). In addition, no significant enlarged lymph nodes were detected in the peri-pancreatic region.
Figure 2.
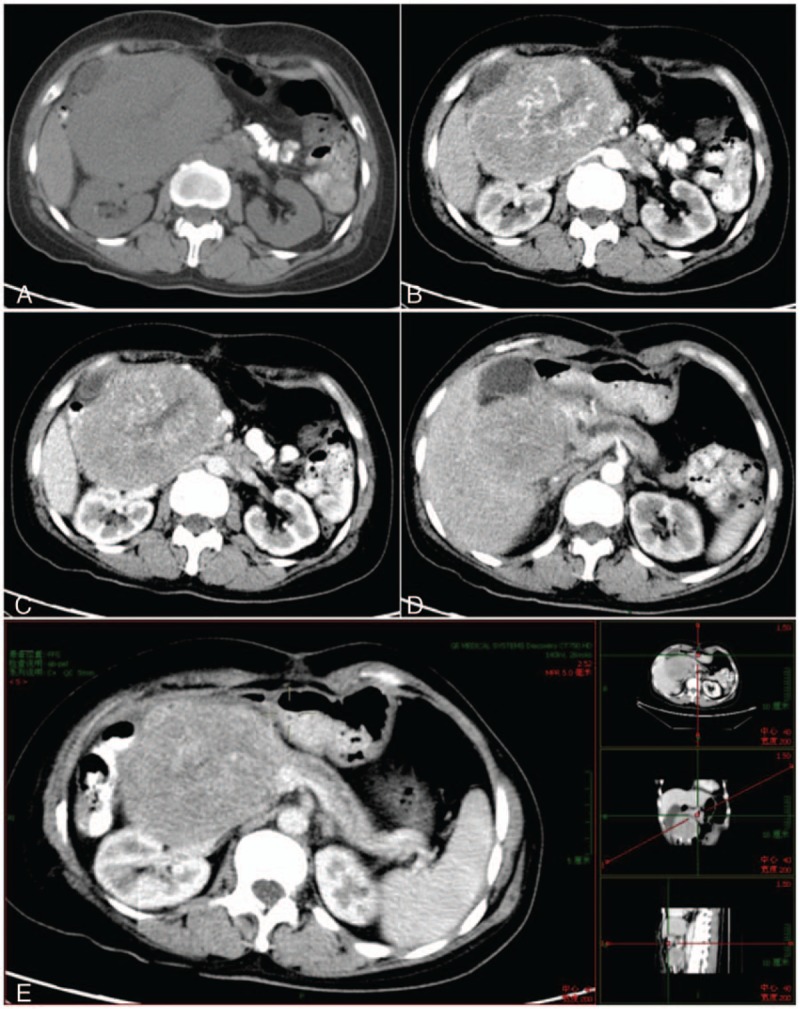
Imaging of abdominal enhanced CT scans. CT showed a solid oval-shaped mixed-density mass, 11.5 cm in size, located in the pancreatic head. (A) Plain scan. (B) Arterial phase. (C) Vein phase. (D) The tumor invaded into the liver. (E) MPR revealed that the tumor was located in the pancreatic head. Dilatation of the upstream main pancreatic duct was also revealed. CT = computed tomography.
Pancreaticoduodenectomy, partial hepatectomy, and vascular replacement of the involved SMV were performed. A macroscopically resected specimen showed a vaguely lobulated mass, which was 11.5 cm in diameter, distributed mainly in the pancreatic head. The cut surface of the tumor revealed the grayish red-colored solid mass with hemorrhage and necrosis at the center. The main pancreatic duct was dilated, while the common bile duct was not.
At the microscopic level, the tumors were composed of epithelioid or spindle cells possessing clear to focally granular eosinophilic cytoplasm, centrally located round to oval nuclei, and inconspicuous nucleoli which grew in a nested and alveolar pattern around blood vessels. Infiltrative growth patterns and mitotic figures were easily identified. Neither calcification nor lipomatous components were evident (Fig. 3). Results of immunohistochemical staining are shown in Fig. 4. The tumor cells had acid-Schiff positive granules, indicating the presence of glycogen, located in the cytoplasm. These tumor cells were positive for HMB-45 and progesterone receptors (PR) and cluster of differentiation 56 (CD56), but negative for Melan-A, a nervous system marker (S-100), epithelial markers (cytokeratin pan (AE1/AE3), cytokeratin 18 (CK18)), endocrine markers (synaptophysin (syn), chromogranin A (CgA), and somatostatin receptors (SSTR); Fig. 4). The Ki-67 labeling index was 5%. Based on the morphological features and the immunohistochemical profile, a diagnosis of PEComa was made. The final tumor stage was T3N1M1.
Figure 3.
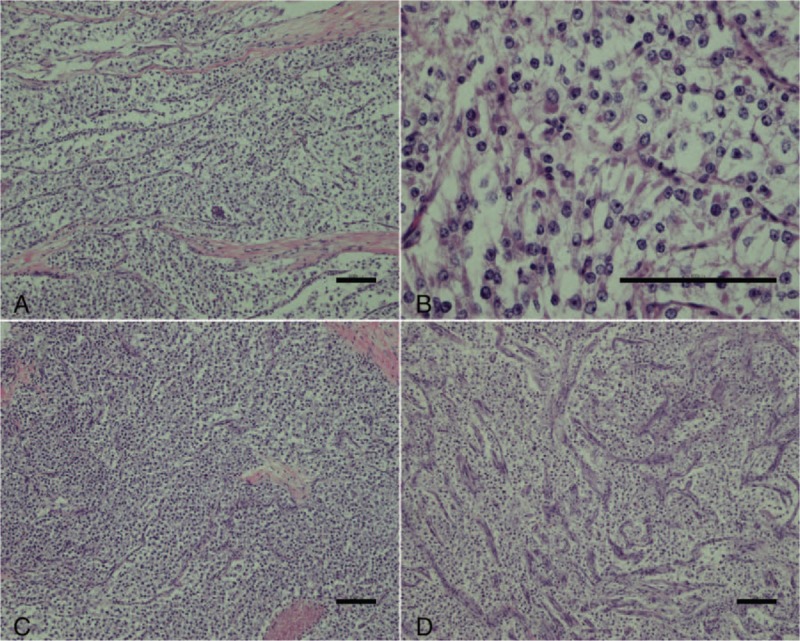
(A,B) At the microscopic level, tumors were composed of epithelioid or spindle cells possessing clear to focally granular eosinophilic cytoplasm, centrally located round to oval nuclei, and inconspicuous nucleoli. (C, D) The tumor cells were arranged in nests or bundles. Some slender branching capillaries were revealed. (A–D: hematoxylin and eosin, scale: 100 μm).
Figure 4.
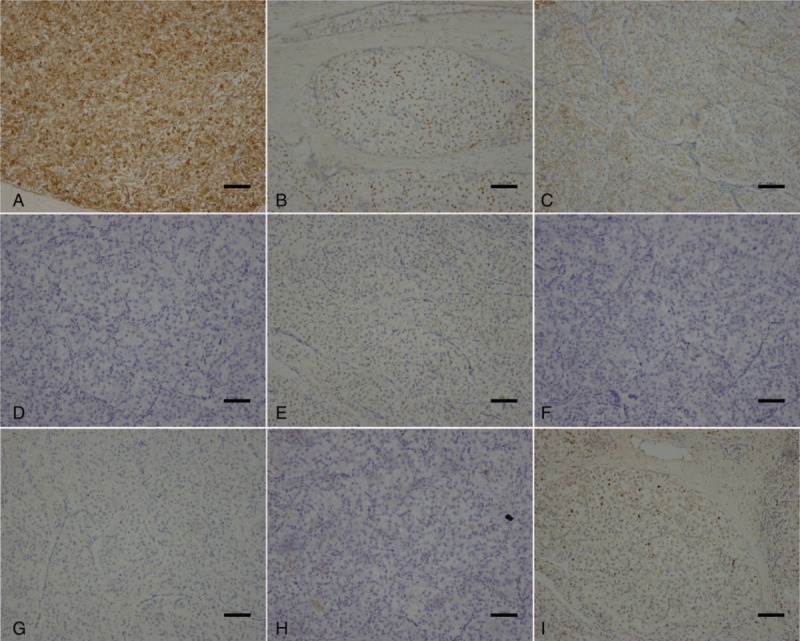
Following immunohistochemical staining, related tumor cells were positive for human melanoma black 45 (A), progesterone receptors (B) and cluster of differentiation 56 (CD56) (C). However, these tumor cells were negative for Melan-A (D), S-100 (E), cytokeratin pan (F), chromogranin A (G), and CD10 (H). The Ki-67 labeling index was 5% (I) (scale 100: μm).
The postoperative course was uneventful, and the patient was discharged 12 days after surgery. Chinese medicine (detailed ingredients being unclear) was also used due to the patient having poor gastrointestinal function postoperatively. Three months later, the patient received four 21-day cycles of chemotherapy. The treatment course included the intravenous administration of epirubicin (80 mg) on day 1, with ifosfamide (6.0 g) administered for 96 hours on days 1 to 4. In addition, endostar (15 mg) was administered every day from day 1 to 14. Drug-related toxic effects of the chemotherapy included gastrointestinal reactions and bone marrow depression. No recurrence of distant metastases was observed 1.5 years following surgery.
Because this report is a review of previously reported data and did not involve any human trials, an ethical review and ethical approval were not necessary. Informed consent was provided by the patient for the publication of the case report.
3. Discussion
PEComa is defined as “a mesenchymal tumor composed of histologically and immunohistochemically distinctive perivascular epithelioid cells,”[23] which include angiomyolipoma, lymphangioleiomyoma, and clear cell “sugar” tumors.[1] The tumor cells are composed of epithelioid or spindle-shaped cells with clear, eosinophilic, or granular cytoplasm, are centrally located, have round to oval nuclei, and inconspicuous nucleoli. Immunohistochemical staining shows that the tumor cells are positive for HMB-45, indicating that the tumor cells have melanocytic features. In addition they are occasionally positive for S-100, α-SMA, and desmin, but negative for epithelial and endocrine markers.[24]
PEComas arise in various organs, but PEComas arising in the pancreas are extremely rare.[2] Since Zamboni et al[3] first reported a clear cell “sugar” tumor in the pancreas in 1996, only 20 cases of PEComas arising in the pancreas have been reported.[3–22] Previously reported cases, as well as the present case, are summarized in Table 2. Pancreatic PEComas can arise in patients of any age (ages range from 17 to 74 years; mean age of 47.9 years). In addition, the patient group consists of 17 women, 3 men, and 1 unknown, indicating a strong female gender predilection. In 11 patients, the primary symptom was abdominal pain. One patient experienced lower back pain, tuberous sclerosis complex, diarrhea, melena, fever, and a bulge in the stomach. Three patients were asymptomatic.[11,16,19] Tumors were located in the pancreatic head and uncinated process in 10 patients, in the pancreatic body and tail in 8 patients, in the pancreatic head and body or neck in 2 patients, and was heterotopic in 1 patient, indicating that PEComas can arise anywhere in the pancreas. The mean tumor size was 4.0 cm (range from 0.38 to 11.5 cm), and the size of the present case was the largest reported. Pancreatic PEComas do not have special laboratory or imaging features, which leads to difficultly in making a conclusive preoperative diagnosis.
Table 2.
Summary of previous reports of pancreatic PEComas.
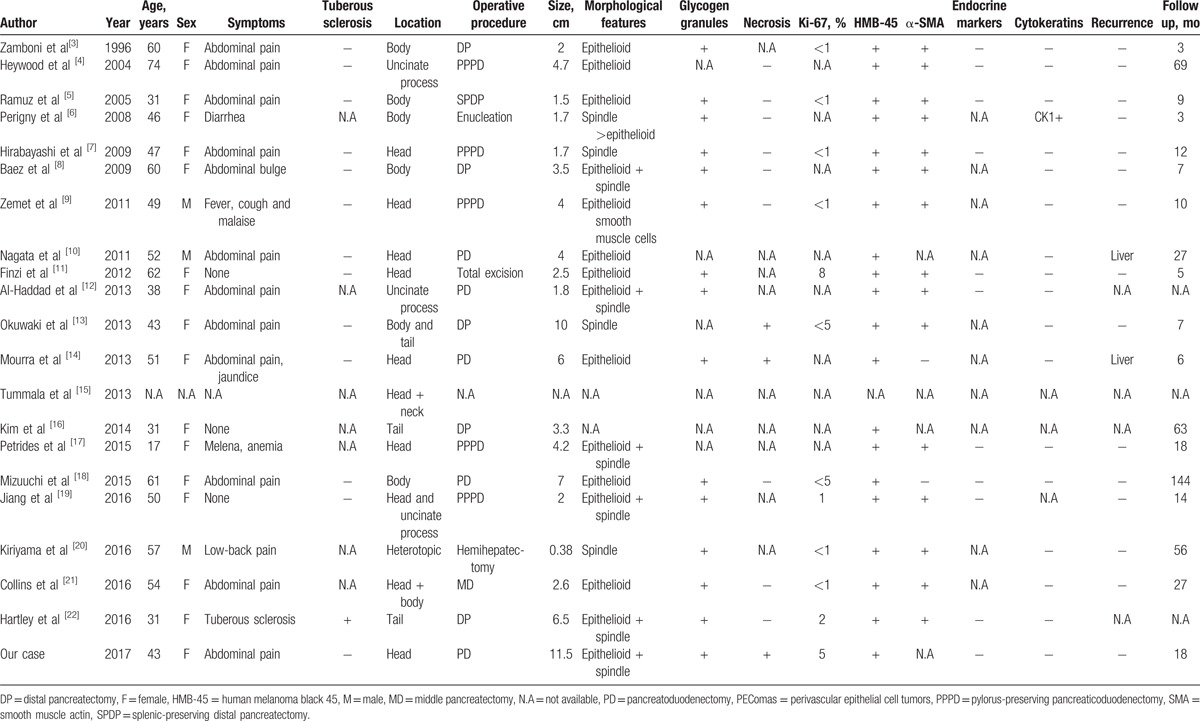
These pancreatic PEComa tumors contained different proportions of epithelioid cell and spindle-shaped cell components,[24] possessing glycogen granules in 15 patients. Necrosis was observed in only 3 cases. In our patient, the tumor was composed primarily of epithelioid cells and spindle-shaped tumor cells with clear cytoplasm. Hemosiderin deposition and glycogen granules were noted, and necrosis was also observed.
Immunohistochemically, all pancreatic PEComa tumor cells exhibited clear immunoreactivity for HMB-45, including our case. Neither epithelial nor endocrine markers exhibited immunoreactivity, with only 1 exception. Perigny et al[6] described a case report in which CK1 is positive. The myogenic marker, αSMA, exhibiting muscular features in spindle-shaped cells, was found in 88.2% (15/17) of PEComa patients.[24,25] In a large proportion of pancreatic PEComa patients, the Ki-67 labeling index was less than 1%, while in our patient it was around 5%.
TSC is a genetic disease which causes intellectual disability, seizures, and benign tumor development in multiple organs including the brain, kidney, and skin.[24,26,27] Although many PEComa lesions have been observed in TSC patients, there was only 1 pancreatic PEComa patient with a history of TSC, based on the available data, suggesting that pancreatic PEComa has only a weak association with TSC.
Due to its rarity and predominantly benign behavior, it is important to consider the PEComa in the differential diagnosis of solid pancreatic tumors. The main differential diagnosis of pancreatic PEComa includes endocrine tumors with clear cell change, epithelial tumors such as primary clear cell carcinoma, acinar cell carcinoma solid, pseudopapillary neoplasm, and metastatic renal cell carcinoma. Preoperative diagnosis is challenging, however it is relatively easy to distinguish among these after surgery by immunohistochemical staining for different markers. It is noteworthy that clear cell sarcoma arising in the pancreas, recently reported by Huang et al,[28] affects young adults, expresses melanocytic marker, HMB-45, and has a poor prognosis. In our case, we could rule out this disease based on low S-100 protein immunoreactivity in the tumor of our patient.
The great majority of PEComas are considered benign. Prognosis of pancreatic PEComa is relatively good, with most patients remaining free of relapse and metastases. As pancreatic PEComas are rare, it is necessary to define the worrisome features which predict recurrence and metastasis of pancreatic PEComa after surgery. Folpe et al[24] reported the features that could predict the presence of tumor recurrence or metastasis in PEComa. These features include large size (>5 cm), infiltrative growth, hypercellularity, high nuclear grade, high mitotic figures (>1/50 high-power field (HPF)), and necrosis. Malignant PEComas show the combination of 2 or more worrisome features. There were 2 cases which reported liver metastasis after the resection of pancreatic PEComas. We found necrosis and infiltrative growth in 1 patient, and mitotic figures were sparsely seen in both patients (2/50HPF and 1/50HPF, respectively), but the other features were lacking in these tumors. In our case, the tumor was 11.5 cm in size, demonstrated infiltrative growth, and necrosis and mitotic figures were seen, demonstrating 4 worrisome features of PEComa arising from the pancreas. Due to the presence of these features, additional attention was paid to the postoperative treatment.
In a case report by Mourra et al[14] describing a tumor which had invaded the duodenal wall with extensive necrosis and vascular invasion, the patient underwent a duodenopancreatectomy without adjuvant therapy. However, the patient developed liver metastases 6 months after surgery. In light of this outcome, we gave our patient additional chemotherapy after surgery. To our knowledge, this is the first case which describes invasion into liver at the time of diagnosis and the first case using postoperative chemotherapy for this tumor type. Because no previous cases could be used for reference, we opted to use the first line treatment regimen recommended for sarcoma. After surgery and chemotherapy, our patient has shown no sign of recurrence for 1.5 years. We will continue to follow up on this patient.
Here, we have presented a case of pancreatic PEComa with characteristic immunohistological features. We recommend that in the future, PEComa should be recognized as a preoperative differential diagnosis of pancreatic tumors. For treatment, removal of the tumor should be attempted, and in the case of tumors with malignant tendencies, the addition of chemotherapy should be considered.
Footnotes
Abbreviations: α-SMA = smooth muscle actin, AE1/AE3 = cytokeratin pan, CD = cluster of differentiation, CgA = chromogranin A, CK = cytokeratin, CT = computed tomography, HMB-45 = human melanoma black 45, HPF = high-power field, Melan-A = melanoma antigen, PEComas = perivascular epithelial cell tumors, PR = progesterone receptors, SMV = superior mesenteric vein, SSTR = somatostatin receptors, syn = synaptophysin, TSC = tuberous sclerosis complex.
FC and XH are co-first authors.
The authors have no conflicts of interest to disclose.
References
- [1].Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol 2010;41:1–5. [DOI] [PubMed] [Google Scholar]
- [2].Martignoni G, Pea M, Reghellin D, et al. PEComas: the past, the present and the future. Virchows Archiv 2008;452:119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zamboni G, Pea M, Martignoni G, et al. Clear cell “sugar” tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol 1996;20:722–30. [DOI] [PubMed] [Google Scholar]
- [4].Heywood G, Smyrk TC, Donohue JH. Primary angiomyolipoma of the pancreas. Pancreas 2004;28:443–5. [DOI] [PubMed] [Google Scholar]
- [5].Ramuz O, Lelong B, Giovannini M, et al. Sugar” tumor of the pancreas: a rare entity that is diagnosable on preoperative fine-needle biopsies. Virchows Archiv 2005;446:555–9. [DOI] [PubMed] [Google Scholar]
- [6].Perigny M, Larochelle O, Hammel P, et al. Pancreatic perivascular epithelioid cell tumor (PEComa). Ann Pathol 2008;28:138–42. [DOI] [PubMed] [Google Scholar]
- [7].Hirabayashi K, Nakamura N, Kajiwara H, et al. Perivascular epithelioid cell tumor (PEComa) of the pancreas: immunoelectron microscopy and review of the literature. Pathol Int 2009;59:650–5. [DOI] [PubMed] [Google Scholar]
- [8].Baez JC, Landry JM, Saltzman JR, et al. Pancreatic PEComa (sugar tumor): MDCT and EUS features. JOP 2009;10:679–82. [PubMed] [Google Scholar]
- [9].Zemet R, Mazeh H, Neuman T, et al. Asymptomatic pancreatic perivascular epithelial cell tumor (PEComa) in a male patient: report and literature review. JOP 2011;12:55–8. [PubMed] [Google Scholar]
- [10].Nagata S, Yuki M, Tomoeda M, et al. Perivascular epithelioid cell neoplasm (PEComa) originating from the pancreas and metastasizing to the liver. Pancreas 2011;40:1155–7. [DOI] [PubMed] [Google Scholar]
- [11].Finzi G, Micello D, Wizemann G, et al. Pancreatic PEComa: a case report with ultrastructural localization of HMB-45 within melanosomes. Ultrastruct Pathol 2012;36:124–9. [DOI] [PubMed] [Google Scholar]
- [12].Al-Haddad M, Cramer HM, Muram T, et al. Perivascular epithelioid cell tumor: an unusual pancreatic mass diagnosed by EUS-FNA. Gastrointest Endosc 2013;78:165.discussion 165–167. [DOI] [PubMed] [Google Scholar]
- [13].Okuwaki K, Kida M, Masutani H, et al. A resected perivascular epithelioid cell tumor (PEComa) of the pancreas diagnosed using endoscopic ultrasound-guided fine-needle aspiration. Int Med 2013;52:2061–6. [DOI] [PubMed] [Google Scholar]
- [14].Mourra N, Lazure T, Colas C, et al. Perivascular epithelioid cell tumor: the first malignant case report in the pancreas. Appl Immunohistochem Mol Morphol 2013;21:e1–4. [DOI] [PubMed] [Google Scholar]
- [15].Tummala Md P, Rao Md S, Agarwal Md B. Differential diagnosis of focal non-cystic pancreatic lesions with and without proximal dilation of pancreatic duct noted on CT scan. Clin Transl Gastroenterol 2013;4:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim JY, Song JS, Park H, et al. Primary mesenchymal tumors of the pancreas: single-center experience over 16 years. Pancreas 2014;43:959–68. [DOI] [PubMed] [Google Scholar]
- [17].Petrides C, Neofytou K, Khan AZ. Pancreatic perivascular epithelioid cell tumour presenting with upper gastrointestinal bleeding. Case Rep Oncol Med 2015;2015:431215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mizuuchi Y, Nishihara K, Hayashi A, et al. Perivascular epithelial cell tumor (PEComa) of the pancreas: a case report and review of previous literatures. Surg Case Rep 2016;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jiang H, Ta N, Huang XY, et al. Pancreatic perivascular epithelioid cell tumor: a case report with clinicopathological features and a literature review. World J Gastroenterol 2016;22:3693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kiriyama Y, Tsukamoto T, Mizoguchi Y, et al. Intrahepatic peribiliary perivascular epithelioid cell tumor (PEComa) associated with heterotopic pancreas: a case report. Diagn Pathol 2016;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Collins K, Buckley T, Anderson K, et al. Perivascular epithelioid cell tumor (PEComa) of pancreas diagnosed preoperatively by endoscopic ultrasound-guided fine-needle aspiration: a case report and review of literature. Diagn Cytopathol 2017;45:59–65. [DOI] [PubMed] [Google Scholar]
- [22].Hartley CP, Kwiatkowski DJ, Hamieh L, et al. Pancreatic PEComa is a novel member of the family of tuberous sclerosis complex-associated tumors: case report and review of the literature. Virchows Archiv 2016;469:707–10. [DOI] [PubMed] [Google Scholar]
- [23].Fletcher CD, Bridge JA, Hogendoorn P, et al. WHO Classification of Tumours of Soft Tissue and Bone. Lyon: International Agency for Research on Cancer; 2013. [Google Scholar]
- [24].Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol 2005;29:1558–75. [DOI] [PubMed] [Google Scholar]
- [25].Goldblum JR, Folpe AL, Weiss SW. Enzinger & Weiss's Soft Tissue Tumors. 6th ed.Philadelphia, PA: Elsevier Saunders; 2014. [Google Scholar]
- [26].Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 2006;355:1345–56. [DOI] [PubMed] [Google Scholar]
- [27].Dabora SL, Jozwiak S, Franz DN, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 2001;68:64–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Huang J, Luo RK, Du M, et al. Clear cell sarcoma of the pancreas: a case report and review of literature. Int J Clin Exp Pathol 2015;8:2171–5. [PMC free article] [PubMed] [Google Scholar]


