Supplemental Digital Content is available in the text
Keywords: colorectal cancer, lymphocyte-to-monocyte ratio, prognosis
Abstract
Background:
Although previous meta-analyses have proved that lymphocyte-to-monocyte ratio (LMR) is a prognostic factor in solid cancers, its prognostic role in colorectal cancer (CRC) remains controversial. We, therefore, conducted this up-to-date meta-analysis to evaluate the prognostic role of the LMR in CRC.
Methods:
A systematic search was performed in PubMed and Embase for relevant studies in November 2016. Article assessing the prognostic role of LMR in CRC was enrolled in this meta-analysis. Data and characteristics of each study were extracted. A meta-analysis was performed to generate pooled hazard ratio (HR) and 95% confidence intervals (95% CIs) for overall survival (OS) and disease-free survival. Begg funnel plot was used to evaluate publication bias.
Results:
Eleven studies published between 2014 and 2016 with a total of 9045 patients were enrolled in this meta-analysis. Our findings indicated that a low LMR predicted a worse OS (HR 1.57, 95% CI 1.30–1.90, P < .001) and disease-free survival. (HR 1.25, 95% CI 1.13–1.39, P < .001) for patients with CRC. Subgroup analyses according to stage (I–III and IV) and LMR cut-off value (<3.00 and ≥3.00) showed a significant prognostic value of LMR on OS. Begg funnel plot showed that publication bias existed in this meta-analysis.
Conclusions:
This up-to-date meta-analysis shows that a low LMR is associated with poor survival in patients with CRC, although the publication bias is existed. Large-sample multicenter prospective cohort is needed to assess the role of the LMR in CRC patients.
1. Introduction
Colorectal cancer (CRC) is 1 of the most common cancers and 1 of the leading causes of cancer death worldwide.[1] About 1.36 million were diagnosed with CRC and 0.7 million died of it in 2012.[1] Although the therapeutic strategies have been developed in recent decades, the 5-year overall survival (OS) of CRC is unsatisfactory because of local recurrence or metastasis. Many factors can predict the prognosis of CRC, for instance tumor stage, cell differentiation grade, vascular invasion, and neural invasion. However, some patients with good prognostic factors still have poor prognosis. Thus, there is an urgent need to find other new biomarker to predict the prognosis of CRC and help choose the optimal therapeutic strategies.
Since the first report by Virchow[2] in 1881 described the association between inflammation and tumorigenesis, strong evidence has suggested that inflammation plays a critical role in cancer onset, development, and therapeutic response.[3–6] Published studies have demonstrated that several systemic inflammatory factors can be used to predict the prognosis for CRC patients, such as platelet-to-lymphocyte ratio[7] and neutrophil-to-lymphocyte ratio.[8,9] As a new factor of systemic inflammatory, lymphocyte-to-monocyte ratio (LMR) has been drawing increasing attention lately.
The LMR is the ratio calculated by dividing the absolute lymphocyte counts by the absolute monocyte counts from the blood test. Lymphocytes participate in cytotoxic cell death and inhibition of tumor cell proliferation and migration.[10,11] Lymphopenia usually indicates disease severity and can make cancer cells escape from the immune of tumor-infiltrating lymphocytes (TILs).[12] TILs are formed by lymphocytes migrating into the tumor microenvironment.[13] It has been proved that decreased levels of TILs predict a worse survival in patients with CRC.[14–16] Conversely, monocytes can promote tumor progression and metastasis.[6,17] Several proinflammatory cytokines, secreted from monocytes, are associated with poor prognosis in cancer patients, such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1.[18] Besides, tumor-associated macrophages, derived from circulating monocytes, have a role in suppressing adaptive immunity and promoting angiogenesis, invasion, and migration.[19] From the above, a decreased LMR could generate a favorable immune microenvironment that promotes cancer development. In other words, a decreased LMR could be associated with poor prognosis in cancer patients.
Previous literatures have proved that an elevated pretreatment LMR is associated with survival benefit in hematologic malignancies.[20–22] In addition, 2 meta-analyses also have revealed that elevated pretreatment LMR can predict a good prognosis in patients with solid cancers.[23,24] One meta-analysis[23] included 3 studies focusing on CRC and did not analyze the association between LMR and CRC; the other[24] did analyze the association between LMR and CRC, but it only enrolled four studies. Since there have been published several other studies assessing the prognostic role of LMR in CRC in the past 2 years,[25–28] and the results of those studies remains controversial, we conducted an up-to-date meta-analysis to investigate the association between the LMR and the survival in CRC.
2. Materials and methods
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Supplemental 1 PRISMA Checklist). The ethical approval was not necessary because this study was a meta-analysis.
2.1. Search strategy
A systematic literature search with no limits was performed in PubMed (Medline) and Embase. Our search strategy included terms “LMR, lymphocyte-to-monocyte ratio, lymphocyte to monocyte ratio, or lymphocyte monocyte ratio,” and “rectal cancer, rectal carcinoma, colon cancer, colon carcinoma, CRC, or colorectal carcinoma” (Supplemental 2 Search Strategy). The last search was performed on November 10, 2016. Besides, a manual search of references of articles and reviews was also performed for additional potentially eligible studies.
2.2. Inclusion and exclusion criteria
The inclusion criteria for selecting studies for this meta-analysis were as follows: all patients were pathologically diagnosed and did not have any tumors besides CRC; the lymphocyte and monocyte were measured before treatment; cohort studies reporting the association between LMR and OS or disease-free survival (DFS) was reported; hazard ratio (HR) and 95% confidence intervals (CIs) of OS or DFS was reported.
The exclusion criteria studies were as follows: studies that did not report HR or 95% CI; abstracts, letters, editorials, reviews, expert opinions, or case reports; studies with a sample size less than 20.
2.3. Data extraction
Two independent reviewers (Q.W. and T.H.) reviewed all candidate articles. Discrepancies were resolved by discussion. If agreement could not be reached, a third reviewer (Z.W.) would be required. The following items were collected from each study: first author's name, year of publication, country of the study population, cancer location, stage, main treatment, sampling time, cut-off value for LMR, number of patients, and the HRs with 95% CI, and median survival time of OS and DFS.
2.4. Quality assessment
We used the Newcastle–Ottawa Scale (NOS) to assess the quality of enrolled studies.[29] The total scores were 9, and study with scores ≥7 was considered as high quality study.
2.5. Statistical analysis
The primary objective of this meta-analysis was to evaluate the association of pretreatment LMR and OS in patients with CRC. The DFS was the secondary outcome. A pooled HR with 95% CI was calculated according to HR and 95% CI from each study. Multivariate analysis was selected when both univariate and multivariate analyses were existed. Higgins I2 statistic and Cochran Q test were used for heterogeneity test. A fixed-effects model was applied if I2 ≤ 50% and P ≥ .10. Correspondingly, the random-effects model was applied if I2 ≥ 50% and P ≤ .10. If high heterogeneity existed, the sensitivity analysis was conducted by removing 1 study each time to decrease heterogeneity. Begg funnel plot was used to evaluate publication bias. All statistical analyses were carried out using the comprehensive meta-analysis program (Version 2, Biostat, Englewood, NJ).
3. Results
3.1. Description of included studies
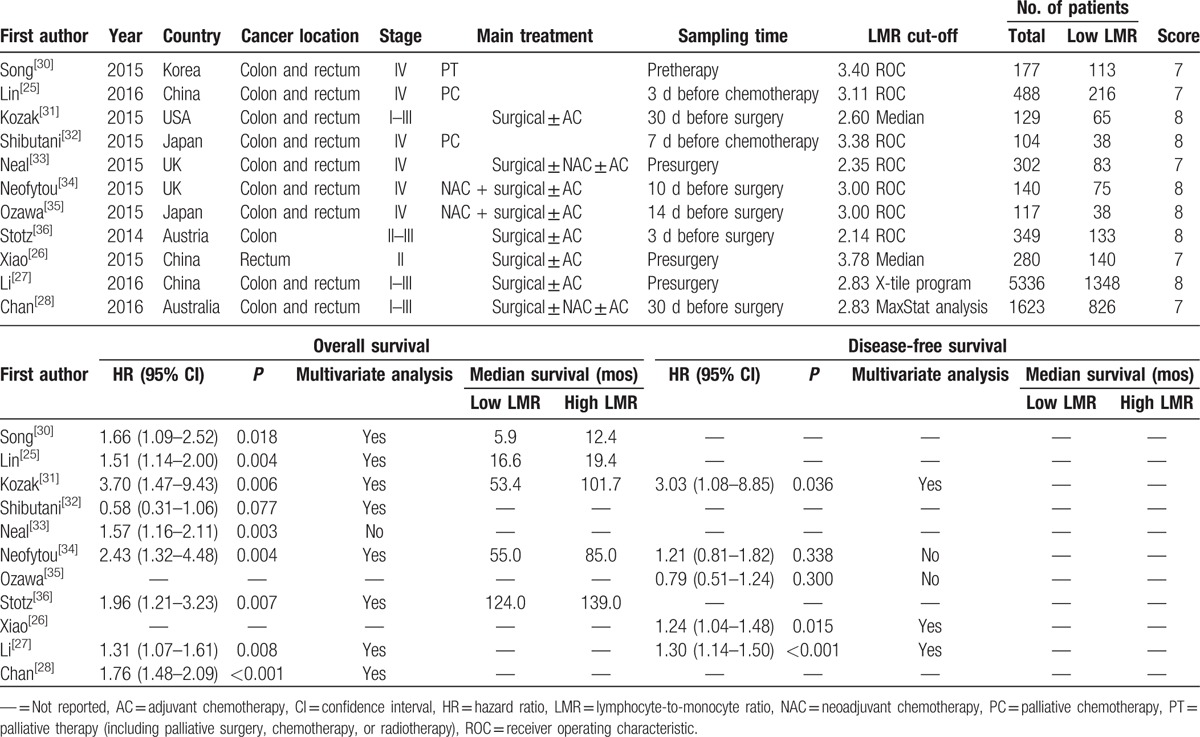
A flow chart of the literature search was shown in Fig. 1. The initial search algorithm retrieved a total of 82 studies. Besides, 1 additional record was identified through other sources. There existed 62 studies after duplicated removed. After the initial review, only 23 relevant studies were further evaluated. Of these studies, 12 reports were excluded due to following reasons: 1 was irrelevant article; 1 was letter; 4 included overlap patients; 4 did not provide sufficient data for estimating the HR and 95% CI; and 2 were meta-analysis. Thus, 11 studies[25–28,30–36] published between 2014 and 2016 were included in our meta-analysis. The characteristics of the included studies were summarized in Table 1. A total of 9045 patients were enrolled. The studies came from the USA (n = 1), UK (n = 2), Austria (n = 1), Japan (n = 2), South Korea (n = 1), Australia (n = 1), and China (n = 3). Seven studies reported that blood test was done within 30 days before treatment, whereas other 4 studies only reported blood test was done before treatment without time. LMR was calculated using the white blood cell counts. All enrolled studies had high quality (NOS scores ≥7).
Figure 1.
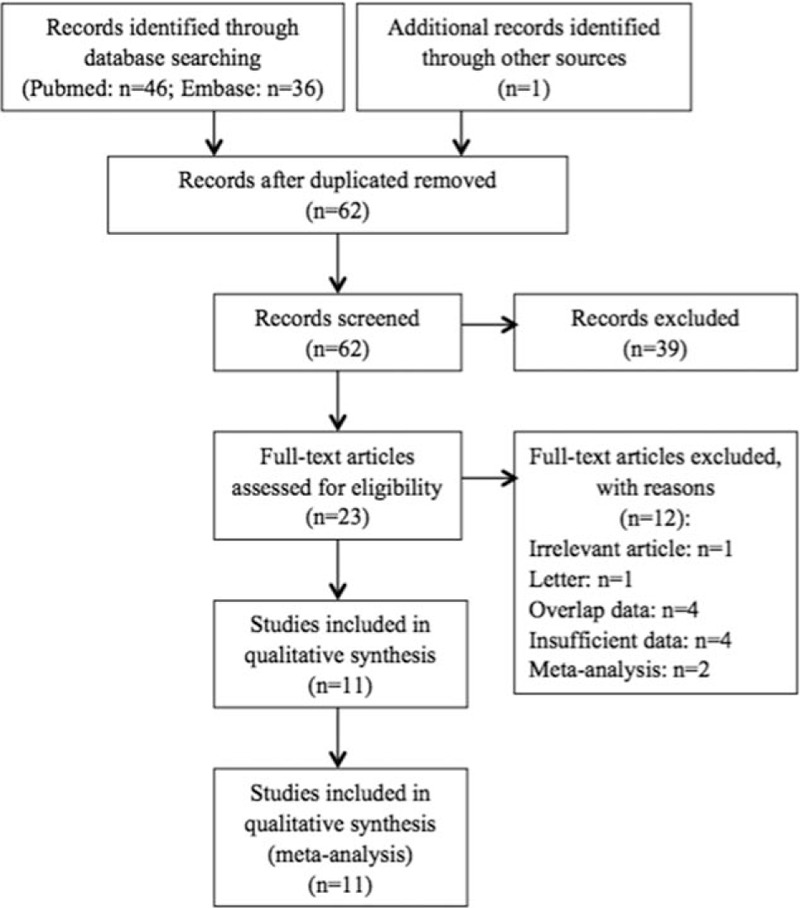
PRISMA diagram. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Characteristics of all identified studies.
3.2. Primary outcome: OS
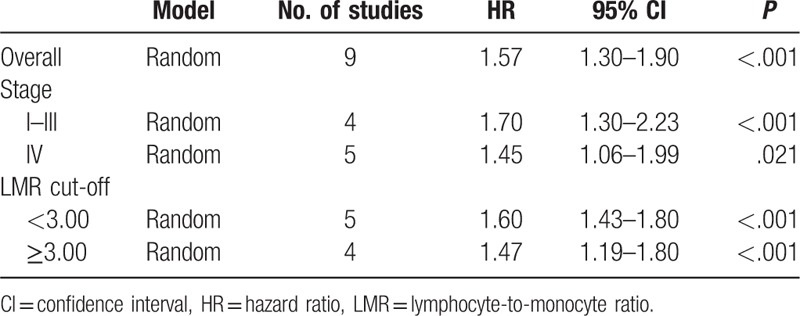
Nine studies enrolling 8648 patients presented the data on LMR and OS. The random-effects model was used for the analysis due to the significant heterogeneity (Q = 20.94, P = .007, I2 = 61.79). A pooled HR of 1.57 (95% CI 1.30–1.90, P < .001) showed that patients with low LMR have worse OS after treatment (Fig. 2). We conducted sensitivity analysis by removing 1 study each time, and the outcomes remained unchanged. Exploratory subgroup analyses according to stage and LMR cut-off values were performed (Table 2). In the subgroup analysis by stage, a prognostic role of LMR was observed for stage I to III and IV CRC (HR 1.70, 95% CI 1.30–2.23, P < .001; and HR 1.45, 95% CI 1.06–1.99, P = .021, respectively). The cut-off values used in included studies ranged from 2.14 to 3.78. Thus, we divided enrolled studies into 2 groups according to cut-off values: <3.00 and ≥3.00. Subgroup analysis showed a low LMR was associated with worse OS in <3.00 group (HR 1.60, 95% CI 1.43–1.80), and also in ≥3.00 group (HR 1.47, 95% CI 1.19–1.80, P < .001).
Figure 2.
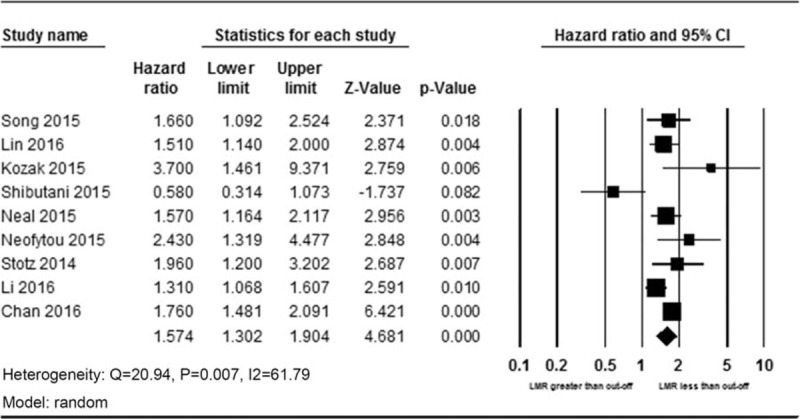
Forest plot of HR and 95% CI for overall survival. CI = confidence interval, HR = hazard ratio.
Table 2.
Subgroup analyses according to stage and LMR cut-off value.
A total of 5 studies enrolling 6002 patients presented the data on LMR and DFS. Because a minor heterogeneity (Q = 7.17, P = .127, I2 = 44.19) was observed, a fixed-effects model was used. A pooled HR of 1.25 (95% CI 1.13–1.39, P < .001) showed that patients with a low LMR have shorter DFS after treatment (Fig. 3).
Figure 3.
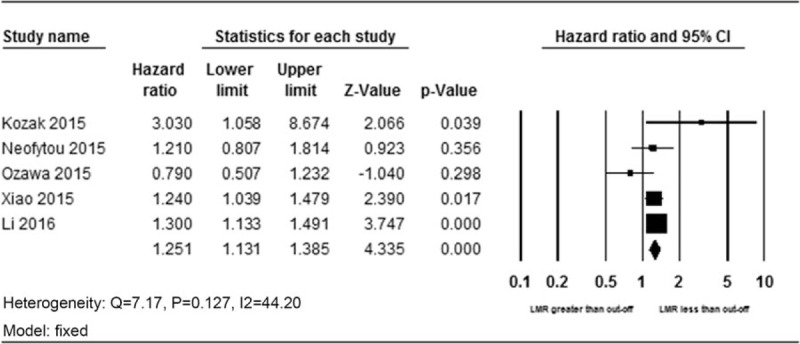
Forest plot of HR and 95% CI for disease-free survival. CI = confidence interval, HR = hazard ratio.
3.3. Publication bias
A Begg funnel plot was used for the assessment of potential publication bias according to primary outcome (Fig. 4). According to the result, we observed evidence of publication bias (P < .05).
Figure 4.
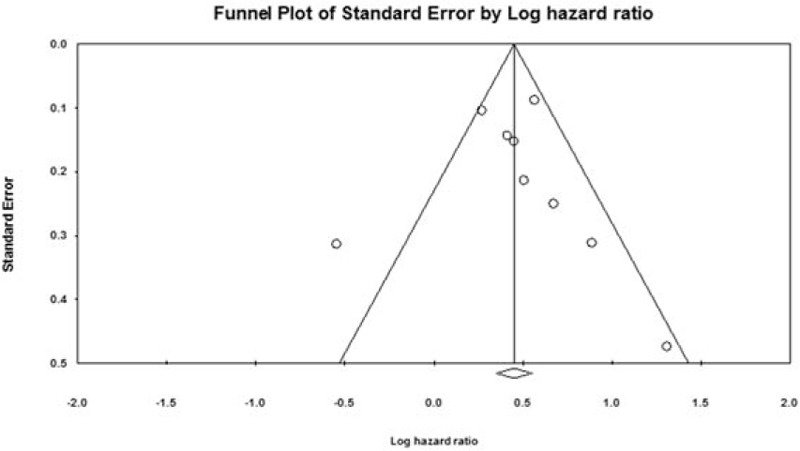
Begg funnel plot for the assessment of potential publication bias according to the primary outcome.
4. Discussion
The systemic inflammation is a key component of cancer progression since it can not only destroy cancer cells but also establish the tumor microenvironment to aids cancer cells proliferation and metastasis.[6,37,38] Literatures have demonstrated that several systemic inflammatory factors can be used to predict the prognosis of CRC patients, such as platelet-to-lymphocyte ratio[7] and neutrophil-to-lymphocyte ratio.[8,9] As a new factor of systemic inflammatory, LMR has been proved to be a predictor for haematologic malignancies,[39] and also for solid cancers.[23,24] Nishijima et al[24] showed that LMR was a prognostic factor for CRC patients in subgroup analysis, which included 4 studies. However, several other studies published later, and the results of those studies remain controversial.[25–28] Therefore, we conducted this up-to-date meta-analysis to investigate the prognostic role of LMR in CRC patients.
In our meta-analysis, we enrolled 11 articles comprising 9045 patients. According to the results, CRC patients with a low LMR had significantly worse OS, and also DFS. Additionally, to investigate the impact of different stage and cut-off values on the prognostic effect of LMR, we conducted subgroup analyses by stage and cut-off values. In the subgroup analysis, we found that the results remained unchanged that low LMR was an unfavorable predictor regardless of the different cut-off values and metastasis or not.
Although there have been 2 meta-analyses focusing on the prognostic role of LMR in solid cancer patients, both of them have limitations when it comes to the association of LMR and CRC patients. Teng et al[23] only included 3 reports focusing on CRC and did not analyze the association between LMR and CRC. The other study[24] did analyze the association between LMR and CRC, the results of which are in line with our results, but it only enrolled 4 studies. Besides, it did not do subgroup analysis for CRC patients. Our meta-analysis has following merits to cover these shortages. First, we included 11 studies with 9045 CRC patients, which is far more than previous meta-analysis. Second, we did subgroup analyses by stage and cut-off value, and the results remained unchanged.
Though this meta-analysis proved that LMR could be a prognostic factor for patients with CRC, it had some limitations that called for cautious interpretation of the results. First, there existed significant heterogeneity when analyzing the relationship between LMR and OS. Thus, the sensitivity analysis was conducted by removing 1 study each time. The outcomes remained unchanged compared with primary outcome. Therefore, we speculated that the heterogeneity might be caused by factors such as age, sex, stage, and cut-off value. Second, besides articles with OS or DFS as an endpoint, we also searched for articles with cancer-specific survival, postrecurrence survival, time to recurrence, or progression-free survival as an endpoint. However, we found only 3 articles for cancer-specific survival[33–35]—1 for postrecurrence survival,[34] 1 for time to recurrence,[36] and 1 for progression-free survival.[25] Given the small number, we did not analyze these endpoints in the meta-analysis. Third, all enrolled studies were retrospective study, which might induce patient selection bias. Fourth, there existed publication bias. The possible reason might be that the studies with negative results were difficult to publish. Despite these limitations, we believe that our results provide valuable support for the prognostic role of LMR in CRC patients.
In conclusion, this meta-analysis shows that a low LMR is associated with poor survival in patients with CRC, although the publication bias is existed. Large-sample multicenter prospective cohort is needed to assess the role of the LMR in CRC patients.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CRC = colorectal cancer, DFS = disease-free survival, HR = hazard ratio, LMR = lymphocyte-to-monocyte ratio, OS = overall survival, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, TILs = tumor-infiltrating lymphocytes.
Q.W. and T.H. contributed equally to this work.
Funding: This work was supported by the Science and Technology Support Program of the Science & Technology Department of Sichuan Province (Grant numbers: 2016SZ0043) and the National Natural Science Foundation of China (Grant numbers: 81172373).
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Virchow R. An address on the value of pathological experiments. Br Med J 1881;2:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature 2001;411:380–4. [DOI] [PubMed] [Google Scholar]
- [4].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [5].Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–48. [DOI] [PubMed] [Google Scholar]
- [6].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [7].Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One 2014;9:e101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li MX, Liu XM, Zhang XF, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer 2014;134:2403–13. [DOI] [PubMed] [Google Scholar]
- [9].Malietzis G, Giacometti M, Kennedy RH, et al. The emerging role of neutrophil to lymphocyte ratio in determining colorectal cancer treatment outcomes: a systematic review and meta-analysis. Ann Surg Oncol 2014;21:3938–46. [DOI] [PubMed] [Google Scholar]
- [10].Terzic J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology 2010;138:2101–14.e2105. [DOI] [PubMed] [Google Scholar]
- [11].Lin EY, Pollard JW. Role of infiltrated leucocytes in tumour growth and spread. Br J Cancer 2004;90:2053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Waldner M, Schimanski CC, Neurath MF. Colon cancer and the immune system: the role of tumor invading T cells. World J Gastroenterol 2006;12:7233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073–81. [DOI] [PubMed] [Google Scholar]
- [14].Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998;58:3491–4. [PubMed] [Google Scholar]
- [15].Huh JW, Lee JH, Kim HR. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg 2012;147:366–72. [DOI] [PubMed] [Google Scholar]
- [16].Prall F, Duhrkop T, Weirich V, et al. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol 2004;35:808–16. [DOI] [PubMed] [Google Scholar]
- [17].Evani SJ, Prabhu RG, Gnanaruban V, et al. Monocytes mediate metastatic breast tumor cell adhesion to endothelium under flow. FASEB J 2013;27:3017–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anand M, Chodda SK, Parikh PM, et al. Abnormal levels of proinflammatory cytokines in serum and monocyte cultures from patients with chronic myeloid leukemia in different stages, and their role in prognosis. Hematol Oncol 1998;16:143–54. [DOI] [PubMed] [Google Scholar]
- [19].Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263–6. [DOI] [PubMed] [Google Scholar]
- [20].Porrata LF, Ristow K, Colgan JP, et al. Peripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin's lymphoma. Haematologica 2012;97:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li ZM, Huang JJ, Xia Y, et al. Blood lymphocyte-to-monocyte ratio identifies high-risk patients in diffuse large B-cell lymphoma treated with R-CHOP. PLoS One 2012;7:e41658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rambaldi A, Boschini C, Gritti G, et al. The lymphocyte to monocyte ratio improves the IPI-risk definition of diffuse large B-cell lymphoma when rituximab is added to chemotherapy. Am J Hematol 2013;88:1062–7. [DOI] [PubMed] [Google Scholar]
- [23].Teng JJ, Zhang J, Zhang TY, et al. Prognostic value of peripheral blood lymphocyte-to-monocyte ratio in patients with solid tumors: a meta-analysis. Oncotargets Ther 2015;9:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nishijima TF, Muss HB, Shachar SS, et al. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev 2015;41:971–8. [DOI] [PubMed] [Google Scholar]
- [25].Lin GN, Liu PP, Liu DY, et al. Prognostic significance of the pre-chemotherapy lymphocyte-to-monocyte ratio in patients with previously untreated metastatic colorectal cancer receiving FOLFOX chemotherapy. Chin J Cancer 2016;35:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xiao WW, Zhang LN, You KY, et al. A low lymphocyte-to-monocyte ratio predicts unfavorable prognosis in pathological T3N0 rectal cancer patients following total mesorectal excision. J Cancer 2015;6:616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer 2016;139:220–31. [DOI] [PubMed] [Google Scholar]
- [28].Chan JC, Chan DL, Diakos CI, et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [30].Song A, Eo W, Lee S. Comparison of selected inflammation-based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World J Gastroenterol 2015;21:12410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kozak MM, von Eyben R, Pai JS, et al. The prognostic significance of pretreatment hematologic parameters in patients undergoing resection for colorectal cancer. Am J Clin Oncol 2015. [DOI] [PubMed] [Google Scholar]
- [32].Shibutani M, Maeda K, Nagahara H, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with metastatic colorectal cancer. World J Gastroenterol 2015;21:9966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Neal CP, Cairns V, Jones MJ, et al. Prognostic performance of inflammation-based prognostic indices in patients with resectable colorectal liver metastases. Med Oncol 2015;32:144. [DOI] [PubMed] [Google Scholar]
- [34].Neofytou K, Smyth EC, Giakoustidis A, et al. The preoperative lymphocyte-to-monocyte ratio is prognostic of clinical outcomes for patients with liver-only colorectal metastases in the neoadjuvant setting. Ann Surg Oncol 2015;22:4353–62. [DOI] [PubMed] [Google Scholar]
- [35].Ozawa T, Ishihara S, Kawai K, et al. Impact of a lymphocyte to monocyte ratio in stage IV colorectal cancer. J Surg Res 2015;199:386–92. [DOI] [PubMed] [Google Scholar]
- [36].Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer 2014;110:435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Karin M. Nuclear factor-kappaB in cancer development and progression. Nature 2006;441:431–6. [DOI] [PubMed] [Google Scholar]
- [38].Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007;7:41–51. [DOI] [PubMed] [Google Scholar]
- [39].Sun HL, Pan YQ, He BS, et al. Prognostic performance of lymphocyte-to-monocyte ratio in diffuse large B-cell lymphoma: an updated meta-analysis of eleven reports. Onco Targets Ther 2016;9:3017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


