Abstract
Diagnosis of feline immunodeficiency virus (FIV) infection by polymerase chain reaction (PCR) has recently become available, but little is known about the performance of this assay. The purpose of this study was to determine the sensitivity and specificity of PCR diagnosis of FIV infection. Replicate aliquots of blood samples from cats identified as FIV positive or negative by 2 previous enzyme-linked immunosorbent assay (ELISA) results, and from clinically healthy dogs, were submitted to different laboratories for FIV serologic diagnosis and PCR. The PCR products obtained in 1 laboratory were sequenced to determine the FIV subtype. The PCR assays correctly identified 100%, 80%, and 50% of the FIV-positive samples, and 100%, 90%, and 70% of FIV-negative samples. Each dog sample was reported as FIV PCR positive at least once, and FIV subtypes A, B, and C were identified. It was concluded that PCR tests currently available for FIV infection are unreliable, with highly variable sensitivity and specificity.
Abstract
Résumé — Variabilité des diagnostics sérologique et moléculaire de l’infection par le virus de l’immunodéficience féline. Le diagnostic de l’infection par le virus d’immunodéficience féline (VIF) par la réaction de polymérisation en chaîne (PCR) est récemment devenu disponible, mais on connaît peur de chose du rendement de ce test. Le but de cette étude était de déterminer la sensibilité et la spécificité du diagnostic par analyse PCR de l’infection virale d’immunodéficience féline. Des aliquots répliqués d’échantillons sanguins provenant de chats identifiés comme étant positifs ou négatifs pour le VIF tel que décelé par deux dosages antérieurs ELISA, et aussi provenant de chien cliniquement en santé, ont été soumis à différents laboratoires pour des diagnostics sérologiques et par analyse PCR du VIF. Les produits de la PCR obtenus dans un laboratoire ont été séquencés pour déterminer le sous-type du VIF. Les analyses par le PCR ont identifié correctement 100 %, 80 % et 50 % des échantillons positifs du VIF et 100 %, 90 % et 70 % des échantillons négatifs du VIF. Chaque échantillon canin était positif par analyse PCR pour le VIF au moins une fois, et les sous-types A, B et C du VIF ont été identifiés. On a conclu que les tests par la réaction de polymerization en chaîne actuellement disponibles pour l’infection par le VIF sont peu fiables et que leur sensibilité et leur spécificité sont fortement variables.
(Traduit par Docteur André Bisaillon)
Introduction
The feline immunodeficiency virus (FIV) is a retrovirus in the subgroup of lentiviruses distantly related to the feline leukemia virus (FeLV), an oncogenic retrovirus of cats. Both viruses occur in cats of North America, but FIV is more common (1). The FIV has a genetic structure very similar to that of the human immunodeficiency virus (HIV), and infection of cats serves as a small animal model of HIV infection (2). Cats are typically infected by FIV through being bitten, but vertical in utero transmission (3), mucosal infection through sexual contact (4), and infections of neonates through suckling have all been reported (5). Soon after infection, there is marked viremia, during which the virus disperses throughout the body from the site of entry (6). The FIV targets lymphocytes and monocytes/macrophages with resultant lymph node enlargement, thymic atrophy, and central nervous system abnormalities. Two to 8 wk after infection, specific antiviral antibodies and cytotoxic lymphocytes appear, and virus becomes difficult to detect in blood. Infected cats next enter a period spanning several years with few clinical abnormalities; however, progressive immunodeficiency, as measured by decreased numbers of CD4+ T-lymphocytes, develops. Eventually, most FIV-infected cats develop overt disease from secondary infections, neoplasia, or persistent neurological abnormalities (7). With few exceptions, cats remain FIV-infected with readily detectable serum antibodies against the virus for life.
Most available FIV diagnostic tests detect serum, plasma, or whole blood antibodies to FIV. An enzyme-linked immunosorbent assay (ELISA) consisting of labeled antigens that are immobilized on either a membrane or a plastic well, and bind to antibodies, is the only available FIV test for commercial or in-practice laboratories in North America. The test comes in kit format for in-practice use (SNAP FIV Antibody/FeLV Antigen Combo Test; IDEXX, Westbrook, Maine, USA), or in a microwell format for laboratory use (PetChek FIV Antibody Test Kit; IDEXX). This ELISA is based on detection of antibodies against a core viral protein, p24, and a positive or negative result depends on color development relative to a positive control (8). Other available serological tests include a Western blot and an immunofluorescent antibody (IFA) test (9). Advantages of the Western blot are that antibodies reactive with a range of viral proteins, derived from nondomestic cat FIV, are detectable; however, it is technically more demanding and more costly. Immunofluorescent antibody tests are not commonly used to diagnose FIV infection, and little is known regarding their performance. The microwell ELISA used in veterinary laboratories has been described as having high sensitivity and specificity (9). False negative results are rare and due mostly to acute infection prior to the generation of specific antibodies. False positive results have been attributed to poor technique and nonspecific reactivity against tissue culture components following vaccination (10). Similar information is not available for the in-practice ELISA kit; however, up to 20% of in-practice positive samples submitted for Western blot confirmation were identified as false positives (10). This discrepancy has been attributed to more frequent operator error in veterinary practices as opposed to veterinary laboratories (10). Based on these test characteristics, it was recommended that a positive in-practice ELISA in a healthy cat be confirmed with a Western blot (10).
Recently, veterinary diagnostic laboratories in North America have offered both individual and panels of tests for infectious diseases of animals (“DNA test,” “Genetic test”) based on the detection of nucleic acids by polymerase chain reaction (PCR). The PCR technology is a key research tool in modern life sciences and is frequently used to detect, quantify, and assess genetic variation of infectious agents (11). The PCR diagnosis of infection depends on an organism having sufficiently conserved gene regions to allow amplification of a wide range of field strains, presence of the organism in samples available for routine testing, stringent contamination precautions in the laboratory, controls to verify the quality of the sample, and the artifactual absence or presence of the nucleic acid of interest (12). The FIV is a difficult organism to detect reliably by PCR, since gene sequences differ by as much as 20% among field isolates (13), and the amount of virus in blood is low during the clinically silent phase of infection when most testing is performed (6). These features often necessitate nested PCR approaches, which increase possibilities for false positive results. Recently, an inactivated whole FIV vaccine that induces strong antibody responses has been licensed, rendering current serologic methods for distinction between vaccinated and infected animals ineffective (14). The PCR or virus isolation may thus become necessary despite the above limitations. Virus isolation is technically demanding, expensive, and takes several weeks. Several PCR assays for FIV are commercially available, are priced comparably with ELISAs, but little is known about their performance. Thus, the objectives of this study were to assess the performance of PCR and antibody tests for FIV diagnosis, to determine the specificity of the PCR diagnosis for feline samples, and to identify whether test variability can be attributed to different FIV subtypes.
Materials and methods
Samples
Cats were categorized as “FIV-positive,” if they had 2 or more positive ELISA results, or a positive ELISA and a positive Western blot result, in the 2 y preceding this study. Cats were categorized as “FIV-negative,” if they had 2 negative ELISA results within the 2 y preceding this study and had not been in contact with other cats since the last test. Blood samples (6.0 mL) were collected by jugular venipuncture, and 1.0 mL was dispensed into each of 3 ethylenediaminetetraacetic acid (EDTA)- containing tubes (Vacutainer 3.0 mL; Becton Dickinson, Mississauga, Ontario), and 1.5 mL into each of 2 serum tubes (Vacutainer 3.0 mL; Becton Dickinson). The blood samples with anticoagulant were coded, and then immediately submitted by the veterinary practitioners to 1 research laboratory and 2 veterinary diagnostic laboratories for PCR testing for FIV infection. The serum samples were allowed to clot for approximately 60 min at room temperature and then centrifuged for 5 min at 800 ×g. Then, the supernatants were transferred to fresh serum tubes, coded, and stored for 7 to 10 d at −20°C, after which they were submitted to a veterinary laboratory for FIV ELISA testing, and to a United States (US) state veterinary laboratory for a kinetic ELISA (KELA), a Western blot, or both. The latter laboratory performs the multiwell FIV ELISA and takes measurements of optical density throughout the antibody-antigen reaction period. Protocols validated in the US state laboratory have determined an optical density cutoff for the KELA that yields a result of equivalent specificity and sensitivity to the Western blot, and an optical density range that is considered equivocal and requires further testing by Western blot (10). Testing of samples was performed in accordance with these protocols.
Three blood samples were obtained from healthy dogs living in cat-free households. These samples were processed and submitted in the same manner as the cat samples. The identity of all samples was revealed only after all results had been obtained.
Sequencing and subtyping
The PCR assay in the research laboratory targeted a region encompassing 1127 base pairs (bp) of the viral LTR-gag region. Products of the correct size or differing by up to 100 bp were either sequenced directly by using the amplification primers or cloned into a plasmid vector (TOPO cloning kit; Invitrogen, Burlington, Ontario) and sequenced with universal plasmid primers. The sequences obtained were assembled, aligned, and analyzed with sequence analysis software (Vector NTI; Invitrogen). The FIV subtype was assigned according to homology with prototypes FIV-A (Petaluma, GenBank M25381; National Center for Biotechnology Information, Bethesda, Maryland, USA), FIV-B (USIL2489, GenBank U11820), and FIV-C (GenBank AF474246). The PCR products lacking homology to any FIV sequences were assessed for homology with other sequences available in the GenBank database by using a file automation transfer tool (15).
Results
The veterinary diagnostic laboratories reported results as “FIV-positive” or “FIV-negative,” the research laboratory reported results as “positive,” “negative,” or “equivocal.” An “equivocal” result referred to a distinct PCR product that differed by 100 bp or less from the predicted size.
Ten cats categorized as “FIV-positive” were all confirmed as positive by KELA. The ELISA results from the veterinary diagnostic laboratory agreed with the KELA result in all 10 FIV positive samples (Table 1). The PCR results from laboratories X, Y, and Z were in concordance with the infection category and KELA result in 100%, 80%, and 50% of cases, respectively. There was no consistency in the identification of false negative samples by PCR between the 2 different laboratories.
Table 1.
Results of the polymerase chain reaction (PCR) and serological assays for feline immunodeficiency virus (FIV) infection
| PCR testing | Antibody testing | ||||||
|---|---|---|---|---|---|---|---|
| Category | Laboratory Xa | Laboratory Yb | Laboratory Zc | ELISAb | KELAd | Western blote | Subtype |
| FIV-positive samples | |||||||
| FIV+ | positive | positive | negativef | positive | positive | ND | A |
| FIV+ | positive | positive | positive | positive | positive | ND | A |
| FIV+ | positive | positive | positive | positive | positive | ND | C |
| FIV+ | positive | positive | negativef | positive | positive | ND | A |
| FIV+ | positive | positive | negativef | positive | positive | ND | B |
| FIV+ | positive | positive | positive | positive | positive | ND | B |
| FIV+ | positive | positive | negativef | positive | positive | ND | A |
| FIV+ | positive | positive | negativef | positive | positive | ND | A |
| FIV+ | positive | negativef | positive | positive | positive | ND | C |
| FIV+ | positive | negativef | positive | positive | positive | ND | B |
| FIV-negative samples | |||||||
| FIV− | negative | negative | positivef | negative | negative | ND | ND |
| FIV− | negative | negative | positivef | negative | negative | ND | ND |
| FIV− | negative | negative | negative | negative | negative | ND | ND |
| FIV− | negative | negative | negative | negative | negative | ND | ND |
| FIV− | negative | negative | negative | negative | negative | ND | ND |
| FIV− | negative | negative | negative | negative | negative | ND | ND |
| FIV− | negative | negative | negative | negative | negative | ND | ND |
| FIV− | negative | negative | positivef | negative | negative | ND | ND |
| FIV− | negative | positivef | negative | positivef | negative | ND | ND |
| FIV− | negative | negative | negative | negative | negative | ND | ND |
| FIV-discordant samples | |||||||
| FIV− negative | negative | positive | negative | negative | positive | equivocal | ND |
| FIV− equivocal | equivocal | positive | negative | negative | equivocal | equivocal | non-FIV |
| FIV− negative | negative | negative | negative | positive | equivocal | equivocal | ND |
| FIV− negative | negative | negative | negative | positive | equivocal | positive | ND |
| Dog samples | |||||||
| Dog 1 equivocal | equivocal | negative | positivef | negative | negative | ND | non-FIV |
| Dog 2 negative | negative | positivef | positivef | negative | negative | ND | ND |
| Dog 3 negative | negative | positivef | positivef | positivef | negative | ND | ND |
ELISA — Enzyme-linked immunosorbent assay; KELA — kinetic ELISA; ND — not done
aResearch laboratory
bCommercial laboratory Y
cCommercial laboratory Z
dKinetic ELISA
eWestern blot
fDiscordant with laboratory X and KELA
Sequence analysis of the PCR products from the research laboratory identified FIV-subtypes A, B, and C (Table 1). The sequences clearly clustered with established FIV subtypes (Figure 1).
Figure 1.
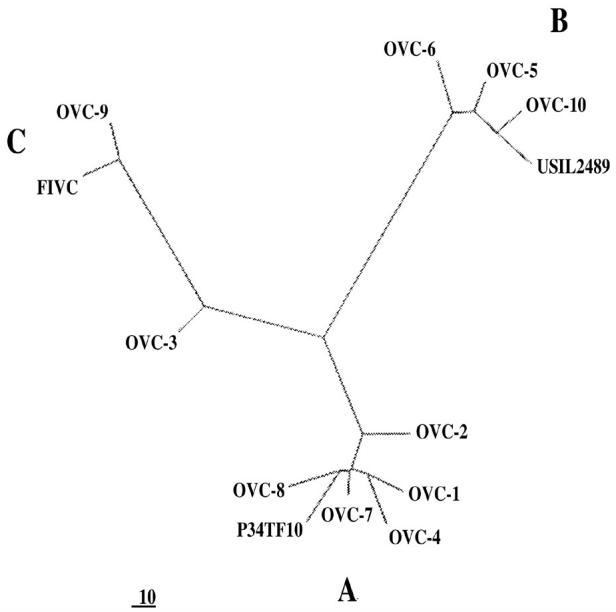
Unrooted parsimony tree of 1042 base pairs from the LTR-gag region of 10 feline immunodeficiency virus (FIV) sequences from Ontario cats. The sequences cluster with prototypes FIV A (P34TF10), FIV B (USIL2489), and FIV C. Relative distances are indicated.
Among 14 cats categorized as “FIV-negative,” 10 confirmatory negative KELA results and 4 discordant or equivocal results were obtained. The ELISA result agreed with the negative KELA result for 9 of these 10 samples. Among the cats with concordant FIV category and KELA result, the PCR results from labs X, Y, and Z agreed in 100%, 90%, and 70% of cases, respectively (Table 1). There was no apparent correlation between the false positive results obtained by either of the 2 diagnostic laboratories.
Four samples were categorized as “FIV-negative,” according to criteria described previously, but yielded positive or equivocal KELA results (Table 1). Among these discordant samples, 1 was categorized as “FIVnegative,” yielded a positive KELA result, and tested negative in 2 PCR assays and the ELISA. The Western blot was equivocal in this case. Three other samples categorized as “FIV-negative” yielded equivocal KELA results, and 2 could not be resolved by Western blot testing either. One sample with an equivocal KELA result yielded a positive Western blot interpretation. The PCR and ELISA results were variable for these 4 cases (Table 1). The FIV infection status could not be resolved for these 4 cats through these tests, and they were therefore considered “discordant.”
Blood samples from the dogs yielded negative KELA results in all 3 cases, 1 positive ELISA result, and a range of positive, negative, or equivocal PCR results (Table 1). The sequence of the 2 “equivocal” PCR products from 1 cat and 1 dog obtained in the research laboratory had a similar sequence but no homology with retroviral sequences in GenBank. Each had short stretches (25 to 40 bp) of homology with feline major histocompatibility (MHC) class II genes and canine sequences of unidentified origin.
Discussion
Data concerning the performance of PCR assays are conspicuously lacking in veterinary medicine, although a range of veterinary laboratories offer such tests. The PCR identification of FIV may be more challenging than that of other infectious agents, due to the marked genetic variability characteristic of lentiviruses and the relatively low proviral load during a protracted period of infection. However, obtaining a “reliable” diagnosis is important, since the infection is, for the lifetime of the cat, essentially untreatable and poses opportunity for transmission to other cats. Recognition of the limitations of serological testing, in particular with in-practice kits, was the basis for recommending Western blot confirmation of positive ELISA results (16). The findings in this study were somewhat surprising with regard to confirmation of previous ELISA results. All cats categorized as “FIV-positive” uniformly tested positive on KELA and, therefore, were not further investigated by Western blot. However, 4 out of 14 cats categorized as “FIV-negative” yielded equivocal or positive KELA results, and Western blotting did not resolve the infection status for 3 of these 4 cats. In the KELA, a specific amount of p24 antigen is immobilized on the plastic well and incubated with a predetermined amount of test serum (9). Antigen-antibody interaction results in the development of a colored product, which is measured over several time points in the KELA but at only one time point in the ordinary ELISA. However, in this format, only a single ratio of antigen to antibody is assessed, when the ideal concentration of either component may vary widely, and high concentrations of antibody may produce erroneous prozone-like effects (17). Equivocal KELA results from some cats with high levels of antibody to FIV, and from cats lacking specific antibodies, could be resolved by diluting the serum sample (10).
Western blotting yielded equivocal results in 3 out of 4 cats in this study. Samples showing reactivity with 2 or more virus-specific proteins in the Western blot were interpreted as positive, while reactivity with a single viral protein, or concurrent reactivity with nonviral proteins, was considered equivocal (10). The Western blot depends on a concentrated source of antigen encompassing the full range of viral proteins. The FIV antigen used in the Western blot at the diagnostic laboratory is of nondomestic cat origin, which likely has subtle antigenic differences to domestic cat FIV (18). Furthermore, the antigens have to be separated clearly by electrophoresis, blotted to membranes, and incubated with cat serum; then, color development at bands of specific size has to be interpreted individually. Thus, the blot technique is a relatively manual procedure with opportunity for operator variability (19). Possible explanations for the equivocal Western blot results obtained in this study were non-specific reactivity of the test sera with tissue culture components, such as bovine serum proteins or with non-viral cellular components. Antibodies against bovine albumin were common in farm workers, and could be removed by pre-adsorption of the serum (20). Other concurrent infectious diseases resulted in false positive or equivocal HIV serological results (21–23), and concurrent FeLV, FIP, or Toxoplasma gondii infections have yielded equivocal FIV serological results (10). One “FIV-negative” cat had a positive Western blot assay, which could only be explained by possible sample mishandling, operator variability in the assay, or interpretation. Thus, serological testing employing 3 different assays did not resolve the FIV infection status of 4 cats in this study; therefore, retesting, serum dilutions, or an alternate Western blot format might have been necessary to resolve their FIV status.
The PCR diagnosis of FIV infection is available through several Canadian veterinary laboratories. Little is known regarding this diagnostic approach, and in 2002 the American Association of Feline Practitioners cautioned that a validated PCR test for FIV infection is not yet available (24). In this study, multiple submissions of identical samples for FIV PCR testing resulted in widely variable results. One laboratory reported half of the samples as FIV negative, although these samples were positive by all serological tests. This is likely attributable to poor sensitivity of the assay, in particular regarding FIV subtype A. Possible reasons for false negative PCR results are that the sample quality was inadequate, the primers employed did not match the target sequence adequately, there was insufficient template, or basic components of the reaction were missing (11). Careful validation of a PCR assay and routine inclusion of control samples may identify these problems.
The 10 FIV-positive samples from Ontario included all 3 FIV subtypes present in North America, suggesting that there may be a relatively great diversity of FIV sequences in Ontario. Nevertheless, there was no apparent correlation with lack of detection of specific FIV subtypes by laboratories, suggesting that primer match was not the reason for false negative results. The same laboratory reporting 50% false negative results yielded 30% false positive results. Again, this would have to be considered inadequate specificity of a test. False positive PCR results are most commonly due to contamination of the laboratory environment with amplified DNA sequences (25). This problem is widely recognized in diagnostic, as well as research, laboratories, and stringent containment and quality control measures are necessary to prevent generation of false positive test results (25,26). The range of positive FIV PCR results obtained from dog samples further indicated a marked lack of specificity of the PCR reaction for the target sequence, since a canine lentivirus has not yet been conclusively identified and genetic differences between lentiviruses of different animal species are marked (27).
The number of samples investigated in this study precluded meaningful statistical analysis of test performance. Nevertheless, the prevalence of FIV infection among owned cats is low (1) and would reduce predictive values for both negative and positive test results from commercial veterinary laboratories. Therefore, the results of this study indicate the need for caution when choosing a PCR approach for FIV testing.
Footnotes
Reprints will not be available from the authors.
Funds for this study were provided by the Pet Trust Foundation at the University of Guelph and the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Lee IT, Levy JK, Gorman SP, Crawford PC, Slater MR. Prevalence of feline leukemia virus infection and serum antibodies against feline immunodeficiency virus in unowned free-roaming cats. J Am Vet Med Assoc. 2002;220:620–622. doi: 10.2460/javma.2002.220.620. [DOI] [PubMed] [Google Scholar]
- 2.Overbaugh J, Luciw PA, Hoover EA. Models for AIDS pathogenesis: simian immunodeficiency virus, simian-human immunodeficiency virus and feline immunodeficiency virus infections. AIDS. 1997;11:47–54. [PubMed] [Google Scholar]
- 3.Jordan HL, Liang Y, Hudson LC, Tompkins WA. Feline immunodeficiency virus is shed in semen from experimentally and naturally infected cats. AIDS Res Hum Retroviruses. 1998;10:1087–1092. doi: 10.1089/aid.1998.14.1087. [DOI] [PubMed] [Google Scholar]
- 4.Stokes CR, Finerty S, Gruffydd-Jones TJ, Sturgess CP, Harbour DA. Mucosal infection and vaccination against feline immunodeficiency virus. J Biotechnol. 1999;20:213–221. doi: 10.1016/s0168-1656(99)00139-x. [DOI] [PubMed] [Google Scholar]
- 5.Allison RW, Hoover EA. Covert vertical transmission of feline immunodeficiency virus. AIDS Res Hum Retroviruses. 2003;15:421–434. doi: 10.1089/088922203765551764. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen NC, Yamamoto JK, Ishida T, Hansen H. Feline immunodeficiency virus infection. Vet Immunol Immunopathol. 1989;21:111–129. doi: 10.1016/0165-2427(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann K. Feline immunodeficiency virus infection — causative agent of an acquired immunodeficiency syndrome in cats. Eur J Med Res. 1996;25:547–550. [PubMed] [Google Scholar]
- 8.O’Connor TP, Jr., Tanguay S, Steinman R, et al. Development and evaluation of immunoassay for detection of antibodies to the feline T-lymphotropic lentivirus (feline immunodeficiency virus) J Clin Microbiol. 1989;27:474–479. doi: 10.1128/jcm.27.3.474-479.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barr MC, Pough MB, Jacobson RH, Scott FW. Comparison and interpretation of diagnostic tests for feline immunodeficiency virus infection. J Am Vet Med Assoc. 1991;199:1377–1381. [PubMed] [Google Scholar]
- 10.Barr MC. FIV, FeLV, and FIPV: interpretation and misinterpretation of serological test results. Semin Vet Med Surg (Small Anim) 1996;11:144–153. doi: 10.1016/s1096-2867(96)80026-0. [DOI] [PubMed] [Google Scholar]
- 11.Belak S, Thoren P. Molecular diagnosis of animal diseases: some experiences over the past decade. Expert Rev Mol Diagn. 2001;1:434–443. doi: 10.1586/14737159.1.4.434. [DOI] [PubMed] [Google Scholar]
- 12.Elnifro EM, Ashshi AM, Cooper RJ, Klapper PE. Multiplex PCR: optimization and application in diagnostic virology. Clin Microbiol Rev. 1999;13:559–570. doi: 10.1128/cmr.13.4.559-570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachmann MH, Mathiason-Dubar C, Learn GH, et al. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J Virol. 1997;71:4241–4253. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhl EW, Heaton-Jones TG, Pu R, Yamamoto JK. FIV vaccine development and its importance to veterinary and human medicine: a review. FIV vaccine 2002 update and review. Vet Immunol Immunopathol. 2002;90:113–32. doi: 10.1016/S0165-2427(02)00227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BLAST 2.2.6 [Genbank Nucleotide]. Bethesda, Maryland, USA: National Library of Medicine (US). 2001 [cited 2003 Sept 18]. Available from: http://www.ncbi.nlm.nih.gov/
- 16.Levy J, Richards J, Edwards D, et al. 2001 Report of the American Association of Feline Practitioners and Academy of Feline Medicine Advisory Panel on feline retrovirus testing and management. J Feline Med Surg. 2003;5:3–10. doi: 10.1053/jfms.2002.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nygren H. Kinetics of antibody binding to surface-immobilized antigen. Analysis of data and an empiric model. Biophys Chem. 1994;52:45–50. doi: 10.1016/0301-4622(94)00064-6. [DOI] [PubMed] [Google Scholar]
- 18.Barr MC, Zou L, Long F, Hoose WA, Avery RJ. Proviral organization and sequence analysis of feline immunodeficiency virus isolated from a Pallas’ cat. Virology. 1997;228:84–91. doi: 10.1006/viro.1996.8358. [DOI] [PubMed] [Google Scholar]
- 19.George R, Pope V, Fears M, Morrill B, Larsen S. An analysis of the value of some antigen-antibody interactions used as diagnostic indicators in a treponemal Western blot (TWB) test for syphilis. J Clin Lab Immunol. 1998;50:27–44. [PubMed] [Google Scholar]
- 20.Revelen R, Bordron A, Dueymes M, Youinou P, Arvieux J. False positivity in a cyto-ELISA for anti-endothelial cell antibodies caused by heterophile antibodies to bovine serum proteins. Clin Chem. 2000;46:273–278. [PubMed] [Google Scholar]
- 21.Hsia J. False-positive ELISA for human immunodeficiency virus after influenza vaccination. J Infect Dis. 1993;167:989–990. doi: 10.1093/infdis/167.4.989. [DOI] [PubMed] [Google Scholar]
- 22.Challakere K, Rapaport MH. False-positive human immunodeficiency virus type I ELISA results in low-risk subjects. West J Med. 1993;159:214–215. [PMC free article] [PubMed] [Google Scholar]
- 23.Wai CT, Tambyah PA. False-positive HIV-1 ELISA in patients with hepatitis B. Am J Med. 2002;112:737. doi: 10.1016/s0002-9343(02)01113-0. [DOI] [PubMed] [Google Scholar]
- 24.American Association of Feline Practitioners Information Brief: In response to inquiries regarding Fel-O-Vax® FIV September 2002 [homepage on the Internet]. Nashville, Tennessee. [cited 2003 Sept 23] Available from: http://www.aafponline.org/fiv_info_brief.htm
- 25.Hartley JL, Rashtchian A. Dealing with contamination: enzymatic control of carryover contamination in PCR. PCR Methods Appl. 1993;3:10–14. doi: 10.1101/gr.3.2.s10. [DOI] [PubMed] [Google Scholar]
- 26.Bastien P, Chabbert E, Lachaud L. Contamination management of broad-range or specific PCR: is there any difference? J Clin Microbiol. 2003;41:2272. doi: 10.1128/JCM.41.5.2272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell RS, Robinson WF. The comparative pathology of the lentiviruses. J Comp Pathol. 1998;119:333–395. doi: 10.1016/s0021-9975(98)80033-9. [DOI] [PubMed] [Google Scholar]


