Abstract
Background
Few studies have examined maternal modifiers of temperature and adverse birth outcomes because of lack of data. We assessed the relationship between apparent temperature, preterm delivery (PTD) and maternal demographics, medical conditions, and behaviors.
Methods
A time-stratified case-crossover analysis was conducted using for 14,466 women who had a PTD (20 to less than 37 gestational weeks) from 1995 to 2009 using medical records from a large health maintenance organization in Northern California. Effect modifiers considered by stratification included several maternal factors: age, race/ethnicity, depression, hypertension, diabetes, smoking, alcohol use, pre-pregnancy body mass index, and Medicaid status. Difference by infant sex were also considered. Apparent temperature data for women who had a monitor located within 20 kilometers of their residential zip codes were included. All analyses were stratified by warm (May 1 through October 31) and cold (November 1 through April 30) seasons.
Results
Every 10°F (5.6°C) increase in average cumulatively weekly apparent temperature (lag06), a greater risk was observed for births occurring during the warm season (11.63%; 95% CI: 4.08, 19.72%) compared to the cold season (6.18%; −2.96, 16.18%), especially for mothers who were younger, Black, Hispanic, underweight, had Medicaid prior to giving birth, or had pre-existing or gestational hypertension or diabetes.
Conclusions
Our findings suggest that warmer apparent temperatures may exacerbate the risk of PTD, particularly for subgroups of women at higher risk.
Keywords: temperature, heat, preterm delivery, preterm birth, case-crossover, California, epidemiology
1. Introduction
Premature births, defined as births occurring before 37 gestational weeks, have been associated with increased risk of death, hospitalizations, and cognitive impairment throughout childhood as well as neurological effects that continue into adulthood.(Cuevas et al., 2005; Petrou et al., 2003) Approximately 12% of births in the US are currently preterm, and this estimate has remained relatively stable for the past five years (Martin et al., 2013). Many causes of preterm delivery (PTD) remain unknown, but factors such as maternal hypertension and chronic infections have been associated with increased risk (Mattison et al., 2003). In the past decade, researchers have made the connection between environmental exposures, such as air pollution and traffic exposure and PTD. More recently, positive associations between PTD and meteorology (Beltran et al., 2014), heat waves (Wang et al., 2013), and high ambient temperature (Carolan-Olah and Frankowska, 2014) have been found, identifying pregnant women as a vulnerable subgroup to heat exposure. With heat waves expected to increase in duration and frequency, it is essential to identify maternal risk factors to target mothers who are at higher risk and propose interventions to help prevent preterm births that are exacerbated by heat exposure. However, previous studies have relied primarily on birth certificate data that have limited information on maternal factors, such as health, demographic, and behavioral factors, that could modify risk.
In this study, we examined whether certain maternal demographics, behavioral factors, and medical conditions may increase pregnant women’s susceptibilities to heat-related PTDs using electronic health records from pregnant Kaiser Permanente Northern California (KPNC) members with delivery dates from 1995 through 2009. We also considered infant sex as a potential effect modifier, since previous studies have shown that male fetuses may be more vulnerable to ambient temperature exposure (Catalano et al., 2008; Mondal et al., 2014). All analyses were stratified by warm and cold season.
2. Methods
2.1 Exposure classification
Meteorologic data consisting of temperature and relative humidity were provided by the California Irrigation Management Information System (Office of Water Use Efficiency, 2014), the National Center for Environmental Information (NOAA, 2012) and the US Environmental Protection Agency Air Quality System (U.S. EPA, 2014) from 1995 through 2009. Apparent temperature was calculated using the following formula: −2.653 + (0.994 × temperature in °C) + 0.0153 *(dew-point temperature in °C)2. Each mother was assigned a value for mean daily apparent temperature from the weather monitor closest to the centroid of her reported residential zip code and in the same county where she gave birth. Only those cases residing in zip codes with centroids located within 20 km of a weather monitor, as identified by Hawth’s Tools for ArcGIS 9.3 (Beyer, 1995–2008), were eligible for this study. Warm season was defined as May 1 through October 31 and cold season from November 1 through April 30. We did not consider air pollutants in this analysis because no confounding or effect modification was found in a previous study using the same exposure data (Basu et al., 2010).
2.2 Outcome
KPNC is an integrated healthcare delivery system with more than three million members and 40 clinical facilities and 16 delivery hospitals covering membership population in urban, suburban, and rural areas. KPNC has more than 33,000 deliveries each year. Coverage is provided for approximately 30% of the northern California population and is similar demographically, racially and ethnically to the population living in the geographic area. Over 99% of participants had reliable information on gestational age at delivery ascertained through KPNC’s electronic health record (EHR) databases.
Gestational age at delivery was determined by obstetricians based on multiple sources including ultrasound dating and last menstrual period, and births were considered preterm if they occurred prior to 37 completed weeks of gestation. Other variables ascertained from the EHR included date of infant’s birth, infant’s sex, mother’s residential zip code when she gave birth, maternal demographics (age, race/ethnicity), behavioral factors during pregnancy collected by self-reported questionnaire routinely as part of prenatal care (smoking and alcohol use), Medicaid Status (prior to or during pregnancy) and pre-pregnancy Body Mass Index (BMI). Additionally, data were ascertained on medical and mental health conditions during pregnancy (depression, diabetes (pre-existing or gestational), hypertension (pre-existing or gestational), and pre-eclampsia). Depression was based on either ICD-9 code diagnoses (296.2–296.3 excluding 296.26 and 296.36, 300.4, 309.0–309.1, 648.4) or antidepressant medication dispensing during pregnancy. Hypertension and pre-eclampsia were based upon ICD-9 codes 401–405 and 642.4–642.7, respectively. Diabetes diagnoses were identified through KPNC’s Diabetes and Gestational Diabetes Registries. Only singleton births in counties with at least 15 preterm births for which data on the variables of interest were available were included in the primary analyses to ensure the stability of our estimates. In addition, there had to be at least 5 preterm births in each county of the specified variable to be included in analyses involving effect modifiers. Small neighboring counties such as El Dorado/Amador, Sonoma/Mendocino, and Tulare/King were combined so that they could be included in our study. Deliveries induced prematurely because of pregnancy complications were excluded (artificial rupture of membranes [PCT code: 73.0]; medical induction of labor [PCT code: 73.4]; and pre-labor C-section [PCT code 74.0 without code indicating labor or spontaneous delivery]), since these were likely due to medical issues rather than short-term apparent temperature exposure.
2.3 Study design
We used a time-stratified case-crossover method for data analysis.(Levy et al., 2001) Apparent temperature exposures for up to one week before a birth were compared with exposures for the same mother at other times in the infant’s birth month and year. Control periods were limited to the same day of the week as each case to inherently adjust for day of the week. Consequently, there could be a maximum of four control periods per case occurring a minimum of seven days and a maximum of 28 days before or after the case period.
A linear term for apparent temperature was included in a conditional logistic regression model by season of birth, and the log(odds) of PTD (yes/no) served as the outcome measure. We added a squared term for apparent temperature to see it provided a better model fit. All analyses were performed in two steps: first, we calculated the county-level estimate based on maternal residential zip codes; second, we combined the county-level estimates to produce an overall estimate using meta-analytical techniques for our all analyses and analyses stratified by season.(DerSimonian and Laird, 1986) In this study, all estimates are reported as percent change per 10°F (5.6°C) increase in apparent temperature with corresponding 95% confidence intervals (CI). SAS version 9.3 software PROC LOGISTIC matching by each ID for case/control pair was used to conduct the first stage of the analysis by county. A meta-analysis was conducted to create an overall estimate using Stata version 10.1 software.
In separate models, we stratified by the following demographic characteristics: maternal racial/ethnic group (non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian), maternal age (18–19, 20–24, 25–34, ≥35 years), and infant’s sex (male, female); dichotomized (yes/no) maternal pregnancy behavioral factors: smoking status and alcohol use; and maternal medical conditions: diabetes, hypertension, and depression during pregnancy. We also determined if Medicaid Status (prior to or during pregnancy vs. no) modified the association between temperature and PTD. Finally, we assessed the impact of pre-pregnancy body mass index (BMI) on heat-related preterm deliveries using the following categories: underweight (<18), normal weight (18–24), and overweight/obese (25+). This analysis was limited to pregnancies beginning in 2005 when pre-pregnancy BMI became available in the EHR databases. In sensitivity analyses, we added the induced pregnancies to our final data set to see how our results would be affected. We also considered the impact of a potential fixed cohort bias (Strand et al., 2011) by limiting women to those conception date, defined as 2 weeks post last menstrual period, was between 8/28/95 and 3/5/09.
Prior to beginning this study, the research protocol was approved by the KPNC Internal Review Board.
3. Results
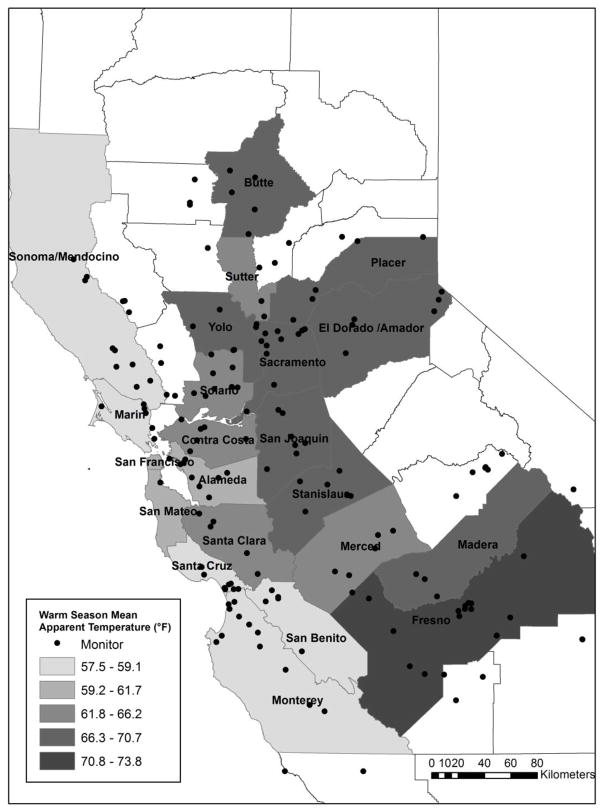
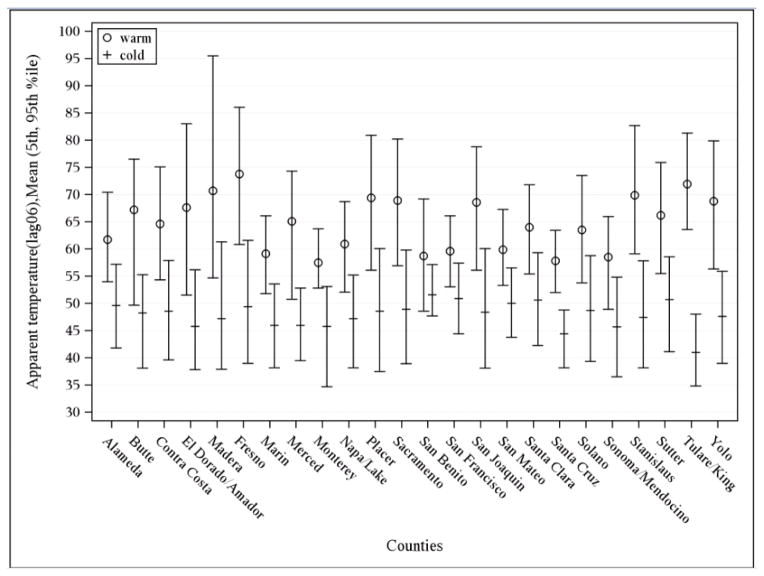
Our total study population consisted of 14,466 preterm births within KPNC from January 1, 1995 through December 31, 2009, after excluding 6,759 induced pregnancies and 1,647 women without monitors located within 20 km of their residential zip codes. Demographic characteristics of the women who were excluded because of monitors too far away were similar to those in our study population. Figure 1 depicts the map of the counties in Northern California that were included in our study based on the selection criteria described above. BMI data was available for 2,382 women. As shown in Table 1, most mothers were between 25–34 years of age (54%), White (40%), and gave birth to male infants (55%). The average warm season apparent temperature for all study areas was 64.5°F (5th, 95th percentile: 54.2°F, 77.1°F) and 49.2°F (39.7°F, 58.4°F) during the cold season (Figure 2).
Figure 1.
Map of northern California with counties included in the study population, 1995–2009.
Table 1.
Characteristics of the Study Population, by County in Northern California, 1995–2009.
| Maternal Age (years), % | Maternal Race/Ethnicity, % | Infant’s sex, % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N Preterm Births | 18–19 | 20–24 | 25–34 | ≥35 | White | Black | Hispanic | Asian | Male | |
| Alameda | 2,362 | 5 | 17 | 55 | 24 | 27 | 14 | 22 | 26 | 54 |
| Butte | 24 | 21 | 29 | 38 | 13 | 71 | 0 | 13 | 13 | 58 |
| Contra Costa | 1,467 | 5 | 16 | 54 | 24 | 45 | 11 | 18 | 16 | 55 |
| ElDorado/Amador | 95 | 5 | 6 | 66 | 22 | 82 | 0 | 7 | 6 | 60 |
| Fresno | 476 | 8 | 22 | 53 | 17 | 43 | 7 | 38 | 9 | 55 |
| Madera | 39 | 10 | 21 | 41 | 28 | 38 | 5 | 51 | 3 | 59 |
| Marin | 198 | 2 | 13 | 58 | 27 | 60 | 5 | 21 | 10 | 51 |
| Merced | 17 | 6 | 24 | 35 | 35 | 47 | 0 | 29 | 0 | 59 |
| Monterey | 17 | 0 | 35 | 41 | 24 | 35 | 0 | 47 | 12 | 59 |
| Napa | 166 | 6 | 19 | 54 | 20 | 62 | 0 | 33 | 2 | 58 |
| Placer | 311 | 5 | 18 | 59 | 18 | 70 | 2 | 13 | 11 | 50 |
| Sacramento | 2,489 | 8 | 21 | 51 | 20 | 49 | 14 | 17 | 17 | 56 |
| San Benito | 24 | 4 | 8 | 54 | 33 | 50 | 8 | 38 | 0 | 63 |
| San Francisco | 800 | 3 | 13 | 54 | 30 | 30 | 12 | 19 | 34 | 59 |
| San Joaquin | 661 | 7 | 17 | 57 | 19 | 31 | 10 | 33 | 14 | 55 |
| San Mateo | 878 | 4 | 15 | 55 | 26 | 30 | 2 | 28 | 34 | 56 |
| Santa Clara | 2,441 | 4 | 16 | 57 | 23 | 35 | 3 | 29 | 24 | 54 |
| Santa Cruz | 18 | 6 | 17 | 33 | 44 | 72 | 0 | 6 | 0 | 61 |
| Solano | 1,018 | 8 | 23 | 50 | 19 | 40 | 19 | 16 | 20 | 54 |
| Sonoma/Mendocino | 566 | 6 | 17 | 56 | 20 | 67 | 1 | 23 | 6 | 57 |
| Stanislaus | 211 | 3 | 19 | 62 | 16 | 33 | 3 | 30 | 6 | 48 |
| Sutter | 13 | 8 | 15 | 54 | 23 | 46 | 15 | 23 | 8 | 62 |
| Tulare/King | 17 | 6 | 41 | 35 | 18 | 24 | 0 | 65 | 0 | 59 |
| Yolo | 158 | 6 | 20 | 56 | 18 | 53 | 3 | 27 | 16 | 51 |
|
| ||||||||||
| Total | 14,466 | 6 | 18 | 54 | 22 | 40 | 9 | 23 | 20 | 55 |
Figure 2.
Apparent temperature distribution by county in Northern California for study period, 1995–2009.
All estimates presented correspond to weekly apparent temperature (lag06), since we determined that this weekly average cumulative lag had the best model fit in our previous study (Basu et al., 2010). A squared term was not necessary, as the p-value was not significant during the warm or cold season. Greater overall risk was observed during the warm season (11.63%; 4.08, 19.72%) compared to the cold season (6.18%; −2.96, 16.18%). Furthermore, no significant associations were found for the cold season, except for normal BMI and female infant sex. The effect estimate for female infant sex was identical to the warm season. During the warm season, the I2 for and p-value between the county-level estimates are 2.8% and 0.42, respectively.
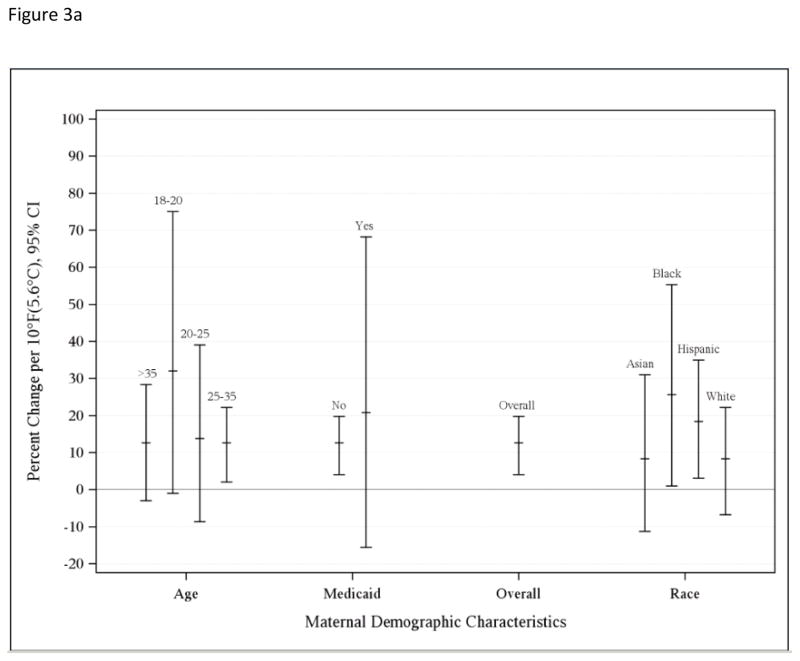
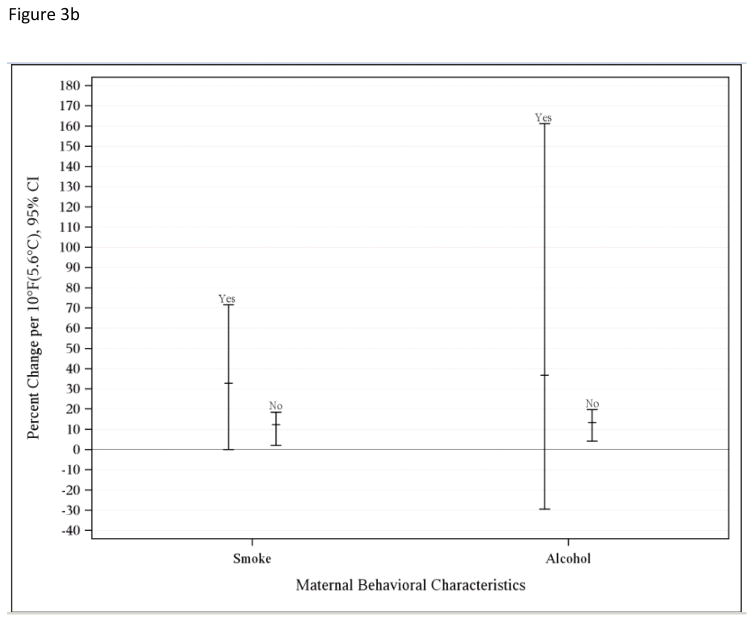
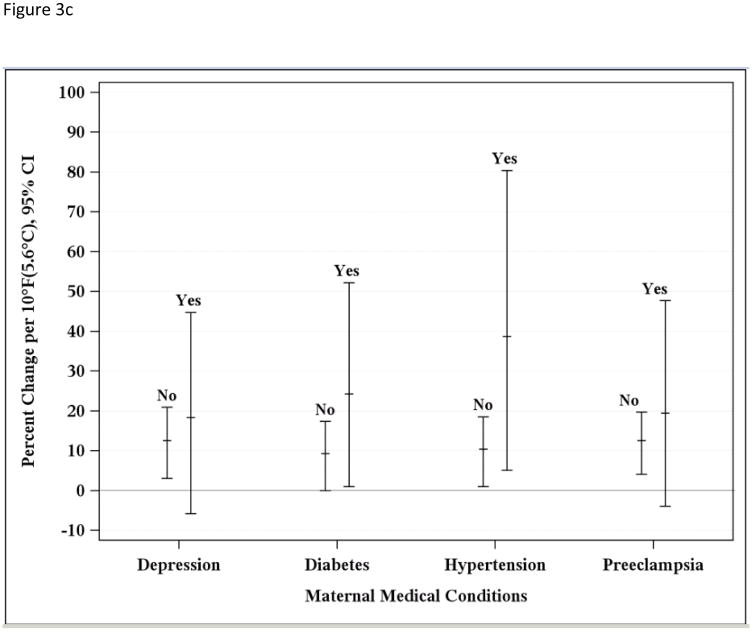
Figure 3a depicts effect modification by maternal demographics such as age and race/ethnic group, Figure 3b demonstrates maternal behaviors (smoking and alcohol use during pregnancy), and Figure 3c shows results for maternal medical conditions (hypertension, diabetes, depression, and pre-eclampsia) during the warm season. Greater associations were found for younger mothers, with those who were 18 and 19 years of age having the highest risk (30.99%; −1.00, 75.06%) compared to 20 to 24 years (12.74%; −8.61, 39.09%), 25 to 35 years (11.62%; 2.02, 22.14%), and more than 35 years (11.62%; −2.96, 28.40%) (p-values = 0.62, 0.97, 1.0, respectively, with 35+ years reference group). While the greatest risk of PTD associated with increasing temperature was found for Black (24.60%; 1.00, 55.27%) and Hispanic (17.35%, 3.04, 34.98%) mothers compared to Asian (7.25%; −11.31, 30.99) or White mothers (7.25%, −6.77, 22.14%) (p-values = 0.56, 0.64, and 1.0, respectively, with Whites as reference group). Although many of these associations were not statistically significant, a trend was noted suggesting increased risk of PTD associated with rising apparent temperature for mothers who smoked (30.99%; 0, 71.6%) or consumed alcohol (34.98%; −29.54, 161.16%) during pregnancy compared to those who did not (10.51%; 2.02, 18.53% and 11.62; 4.08, 19.72, respectively) (p-value for smoking = 0.55; p-value for alcohol use = 0.78). Hypertension (37.71%; 5.12, 80.39%), diabetes (23.36%, 1.00, 52.19%), or pre-eclampsia (18.53%, −3.92, 47.70%) during pregnancy resulted in greater risk (p-values = 0.42, 0.56, and 0.79, respectively). Mothers who were diagnosed with depression had a slightly greater risk (17.35%, −5.83, 44.77%) compared to those who did not (11.62%; 3.04, 20.92%) (p-value = 0.83). Having Medicaid prior to the birth date did not increase risk (not shown). Including the induced pregnancies still resulted in positive associations for both the warm (8.33%; 1.01, 15.03%) and cold (4.08%; −2.96, 11.63%) seasons, and addressing the fixed cohort bias yielded greater associations (11.63%; 3.05, 22.14% for warm and 6.18%; −2.96, 17.4% for cold season).
Figure 3.
a. Estimated percent change associated with a 10°F (5.6°C) increase in weekly average apparent temperature during the warm season and preterm birth for overall risk and by m aternal demographics, 1995–2009.
b. Estimated percent change associated with a 10°F (5.6°C) increase in weekly average apparent temperature during the warm season and preterm birth for overall risk and by maternal behavioral characteristics, 1995–2009.
c. Estimated percent change associated with a 10°F (5.6°C) increase in weekly average apparent temperature during the warm season and preterm birth for overall risk and by maternal medical conditions, 1995–2009.
4. Discussion
Our findings suggest several maternal behaviors, characteristics and medical conditions may increase women’s susceptibilities PTD associated with increasing temperatures, with some of these factors being more salient specifically during the warm season: race/ethnicity, smoking, alcohol use, hypertension, and diabetes. Although many results were statistically significant, they were not significantly different within each group because of small numbers, particularly in the stratified analyses. However, we should not rely solely on statistical significance because our findings show key differences that will help identify specific factors will help target pregnant women who are most vulnerable to heat-associated PTD as well as their medical practitioners and caretakers. Prevention strategies may include avoiding risk for heat exposure during pregnancy and being aware of symptoms of heat exhaustion and dehydration. We also found some associations during the cold season, but these effects were either not statistically significant or opposite of what we found during the warm season (i.e., older maternal age exhibited greater risk during the cold season, while younger maternal age was associated with greater risk during the warm season).
We found greater temperature associations for Black and Hispanic mothers during the warm season, as was found in a prior study conducted in California (Basu et al., 2010). Little research has focused on racial/ethnic disparities specifically for temperature and PTD, however, previous investigators have also indicated general disparities in birth outcomes by race/ethnicity (Shone et al., 2003). Some of this disparity may be due to lack of access to prenatal care specifically for Hispanic women or nutrient deficiencies in African-American women (Dunlop et al., 2011). The women in our study are all insured, so access to prenatal care would not be much of an issue. Younger maternal age was also found to have greater risk during the warm season. This finding is consistent with the literature showing that along with lower social class, less education, single marital status, low income, poor housing status, and younger maternal age are also generally associated with greater risk of preterm birth (Holditch-Davis et al., 2007). Younger maternal age has also been associated with more psychological and social stigma (Whitley and Kirmayer, 2008). Older maternal age (≥45 years of age), however, has also been associated with adverse birth outcomes, including preterm birth (Carolan, 2013), and greater racial disparities (Buescher and Mittal, 2006), which is consistent with our findings during the cold season.
Women who smoked or consumed alcohol during pregnancy had greater risk for heat-associated PTD in our study. These maternal groups may already be at higher risk for PTD. While low to moderate drinking (up to an average of 1.5 drinks per day) did not show any effects in a published meta-analysis, heavier drinking had an increased risk for preterm birth and small-for-gestational age infants (Patra et al., 2011) as well as for miscarriages (Avalos et al., 2014). Smoking has similarly shown a greater risk for adverse birth outcomes (National Center for Chronic Disease et al., 2014). Mothers who smoke are more likely to consume higher levels of alcohol during pregnancy and also have lower socioeconomic status (SES) compared to the general population (Cannon et al., 2012). Thus, younger maternal age, race/ethnicity, maternal smoking and alcohol use all serve as markers of lower SES, but other psychosocial factors and higher exposure may also be responsible for vulnerability (Schwartz et al., 2011). Nonetheless, we found that mothers who were on Medicaid before birth of their babies had greater risk for PTD associated with apparent temperature, particularly during the warm season.
We observed greater risk from increased apparent temperature during the warm season for mothers with diabetes and hypertension, including gestational-onset and pre-existing types. Since adult-onset (Type 2) diabetes and its co-morbidities may cause dehydration, decreased skin blood flow, and reduced sweating, the thermoregulatory response may be impaired (Yardley et al., 2013). Thus, pregnant women with prior or gestational diabetes should be targeted for interventions, particularly those who enter the second half of their pregnancy during the warm season. Women with chronic hypertension have been shown to have increased risk for PTD as well as other adverse birth outcomes (Bramham et al., 2014), although heat exposure may increase hypotension, ischemic stroke, and ischemic heart disease; the latter two outcomes are driven by increased cholesterol. We observed mothers with low pre-pregnancy BMI having a greater risk of PTD during the warm season. Since BMI data were only available beginning in 2005, only 41 women were categorized as having low BMI, and thus, this finding had a wide confidence interval. In previous studies, mothers who were overweight and/or had other co-morbidities such as gestational diabetes and hypertension, were more likely to have preterm PTDs (McDonald et al., 2010).
Since PTD generally has multiple etiologies, a clear biologic mechanism or cause is unknown. One possible explanation may be increased dehydration with heat exposure, which could decrease uterine blood flow and increase pituitary secretion of antidiuretic hormone and oxytocin to induce labor (Stan et al., 2002). There is also evidence that pregnant women may not be able to thermoregulate efficiently. When body temperatures rise, the body generally shifts blood flow from the vital organs to the skin’s surface in an effort to cool down (Bouchama and Knochel, 2002). Thermoregulation may be inadequate when too much blood is diverted from the vital organs of the mother and the developing fetus (Bouchama and Knochel, 2002), or when a sudden rise in temperature or extreme heat causes heat stress (Carolan-Olah and Frankowska, 2014). Finally, increased prenatal inflammation may be another factor to promote PTD or adverse birth outcomes (Bastek et al., 2014). Increased blood viscosity, elevated cholesterol levels associated with higher temperatures, and a higher sweating threshold have also been reported in susceptible subgroups in general (Astrand et al., 2003). Meanwhile, short-term colder temperatures have been associated with greater diastolic, systolic and pulse pressures in adults (Halonen et al., 2011), particularly among those with Type-2 diabetes mellitus (Lanzinger et al., 2014).
There are several limitations to this study. We relied on ambient monitoring data rather than on individual monitoring to assign exposure. By limiting the study to mothers whose residential zip codes were within 20 km of a meteorologic monitor, we attempted to reduce exposure misclassification, but it still could have been an issue. For most cases, utilizing the zip code for the residential address at the time of delivery should be sufficient to characterize exposure. Although we had data on several maternal factors, we did not have information on occupational address, air conditioning status, or time-activity patterns, so we could not account for these factors to capture a better understanding of maternal effect modifiers. While we did have information on maternal smoking status and alcohol use, these variables were based on self-report on a prenatal care questionnaire. Since these behaviors are not socially desirable, particularly during pregnancy, there may have been some exposure misclassification from mothers who smoked or used alcohol but reported less or no exposure. Thus, the estimates for maternal smoking and alcohol use may be biased toward the null, but we observed very high associations for both of these maternal behaviors in this study.
This study adds to the growing body of literature to find an association between ambient temperature and PTD. We determined that several maternal factors modified the association to inform pregnant women and their healthcare professionals, particularly those who are most vulnerable to heat and PTD. In addition, other caretakers, family members, and employers should be aware of the potential impacts of heat exposure among pregnant women. Because we used the case-crossover method, time-stable confounders were controlled for by study design. During heat advisory warnings, pregnant women should be included as a vulnerable subgroup to take extra precautions. The Intergovernmental Panel on Climate Change states that hot days and nights and heat waves have become more frequent in recent years, and their duration and intensity are likely to increase in the future (Ipcc, 2014). This study focused on high apparent temperature, and the association during a heat wave is likely to be substantially greater. Given the significant associations for apparent temperature and PTD found in this study, more large-scale studies of temperature and PTD, including modifiers of the association, in other locations are warranted.
Highlights.
Greater risk of preterm delivery from apparent temperature during the warm season.
Most vulnerable were younger, Black, and Hispanic mothers.
Mothers who were underweight or had Medicaid also an increased risk.
Maternal alcohol use or smoking also increased risk.
Pre-existing or gestational maternal hypertension, diabetes, or preeclampsia increased risk.
Acknowledgments
The authors would like to thank Mr. Brian Malig for creating the map of California.
Funding Source: This work was supported by the The National Institute of Environmental Health Sciences Grant R21 ES021368 (MPI, Avalos and Basu).
Footnotes
Disclaimers: The opinions expressed in this article are those of the authors, and do not represent those of the California Environmental Protection Agency or the Office of Environmental Health Hazard Assessment. The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astrand P-O, et al. Textbook of Work Physiology: Physiological Bases of Exercise. McGraw-Hill; Canada: 2003. [Google Scholar]
- Avalos LA, et al. Volume and type of alcohol during early pregnancy and the risk of miscarriage. Subst Use Misuse. 2014;49:1437–45. doi: 10.3109/10826084.2014.912228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastek JA, et al. Prenatal inflammation is associated with adverse neonatal outcomes. Am J Obstet Gynecol. 2014;210:450e1–10. doi: 10.1016/j.ajog.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Basu R, et al. High ambient temperature and the risk of preterm delivery. Am J Epidemiol. 2010;172:1108–1117. doi: 10.1093/aje/kwq170. [DOI] [PubMed] [Google Scholar]
- Beltran AJ, et al. Associations of meteorology with adverse pregnancy outcomes: a systematic review of preeclampsia, preterm birth and birth weight. Int J Environ Res Public Health. 2014;11:91–172. doi: 10.3390/ijerph110100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer HL. Environmental Systems Research Institute. Hawth's Analysis Tools for ArcGIS. :1995–2008. [Google Scholar]
- Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–88. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Bramham K, et al. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301. doi: 10.1136/bmj.g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher PA, Mittal M. Racial disparities in birth outcomes increase with maternal age: recent data from North Carolina. N C Med J. 2006;67:16–20. [PubMed] [Google Scholar]
- Cannon MJ, et al. Characteristics and behaviors of mothers who have a child with fetal alcohol syndrome. Neurotoxicol Teratol. 2012;34:90–5. doi: 10.1016/j.ntt.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Carolan-Olah M, Frankowska D. High environmental temperature and preterm birth: a review of the evidence. Midwifery. 2014;30:50–9. doi: 10.1016/j.midw.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Carolan M. Maternal age >/=45 years and maternal and perinatal outcomes: a review of the evidence. Midwifery. 2013;29:479–89. doi: 10.1016/j.midw.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Catalano R, et al. Ambient temperature predicts sex ratios and male longevity. Proc Natl Acad Sci U S A. 2008;105:2244–2247. doi: 10.1073/pnas.0710711104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas KD, et al. The cost of prematurity: hospital charges at birth and frequency of rehospitalizations and acute care visits over the first year of life: a comparison by gestational age and birth weight. Am J Nurs. 2005;105:56–64. doi: 10.1097/00000446-200507000-00031. quiz 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dunlop AL, et al. Racial disparities in preterm birth: an overview of the potential role of nutrient deficiencies. Acta Obstet Gynecol Scand. 2011;90:1332–41. doi: 10.1111/j.1600-0412.2011.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, et al. Relationship between outdoor temperature and blood pressure. Occup Environ Med. 2011;68:296–301. doi: 10.1136/oem.2010.056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holditch-Davis D, et al. Correlates of mother-premature infant interactions. Res Nurs Health. 2007;30:333–46. doi: 10.1002/nur.20190. [DOI] [PubMed] [Google Scholar]
- Barros VR, Field CB, Dokken DJ, Mastrandrea MD, Mach KJ, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL, editors. Ipcc. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, United Kingdom and New York, NY, USA: 2014. [Google Scholar]
- Lanzinger S, et al. Short-term effects of air temperature on blood pressure and pulse pressure in potentially susceptible individuals. Int J Hyg Environ Health. 2014;217:775–84. doi: 10.1016/j.ijheh.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Levy D, et al. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology. 2001;12:186–192. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Martin JA, et al. Births: final data for 2011. Natl Vital Stat Rep. 2013;62:1–69. 72. [PubMed] [Google Scholar]
- Mattison DR, et al. The Role of Environmental Hazards in Premature Birth. National Academies Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- McDonald SD, et al. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ. 2010;341:c3428. doi: 10.1136/bmj.c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D, et al. Elevated risk of stillbirth in males: systematic review and meta-analysis of more than 30 million births. BMC Med. 2014;12:220. doi: 10.1186/s12916-014-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Chronic Disease, P., et al. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention (US); Atlanta (GA): 2014. Reports of the Surgeon General. [Google Scholar]
- National Oceanic and Atmospheric Administration (NOAA), National Centers for Environmental Information (NCEI) 2012 [Google Scholar]
- Office of Water Use Efficiency, California Department of Water Resources, California Irrigation Management System (CIMIS) 2014 [Google Scholar]
- Patra J, et al. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG. 2011;118:1411–21. doi: 10.1111/j.1471-0528.2011.03050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou S, et al. The impact of preterm birth on hospital inpatient admissions and costs during the first 5 years of life. Pediatrics. 2003;112:1290–7. doi: 10.1542/peds.112.6.1290. [DOI] [PubMed] [Google Scholar]
- Schwartz J, et al. Exploring potential sources of differential vulnerability and susceptibility in risk from environmental hazards to expand the scope of risk assessment. Am J Public Health. 2011;101(Suppl 1):S94–101. doi: 10.2105/AJPH.2011.300272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shone LP, et al. The role of race and ethnicity in the State Children's Health Insurance Program (SCHIP) in four states: are there baseline disparities, and what do they mean for SCHIP? Pediatrics. 2003;112:e521. [PubMed] [Google Scholar]
- Stan C, et al. Hydration for treatment of preterm labour. Cochrane Database Syst Rev. 2002:CD003096. doi: 10.1002/14651858.CD003096. [DOI] [PubMed] [Google Scholar]
- Strand LB, et al. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol. 2011;11:49. doi: 10.1186/1471-2288-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA. Air Quality System Data Mart. 2014. [Google Scholar]
- Wang J, et al. Maternal exposure to heatwave and preterm birth in Brisbane, Australia. BJOG. 2013;120:1631–41. doi: 10.1111/1471-0528.12397. [DOI] [PubMed] [Google Scholar]
- Whitley R, Kirmayer LJ. Perceived stigmatisation of young mothers: an exploratory study of psychological and social experience. Soc Sci Med. 2008;66:339–48. doi: 10.1016/j.socscimed.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley JE, et al. Do heat events pose a greater health risk for individuals with type 2 diabetes? Diabetes Technol Ther. 2013;15:520–9. doi: 10.1089/dia.2012.0324. [DOI] [PubMed] [Google Scholar]