Abstract
Studies on phytochemical properties and bioactivities of rice bran revealed the wealth of natural complex antioxidant compounds. The composition and the properties of the rice bran get altered after fermentation by several microbes. This study was designed to optimize the black rice bran fermentation conditions for the total anthocyanin (ACN) content, total antioxidant properties, and relative activity of β-glucosidase (BGS) by Saccharomyces cerevisiae. The Box–Behnken design and response surface methodology was employed to achieve the maximum response in fermentation. The kinetic analysis of HPLC based phytochemical determination and bioconversion of ACN, and in vitro antioxidant assays were performed during fermentation. The optimum pH, temperature and NaCl concentration to achieve maximum ACN content, antioxidant capacity, and BGS activity were pH 4.0, 40 °C, and 0.5%, respectively. Bioconversion of cyanidin-3-glucoside and peonidin-3-glucoside to cyanidin and peonidin was recorded at a significant level, respectively. The maximum activity of BGS on rice bran was noticed at 24 h of fermentation. The results suggested that phytochemical content was not changed significantly, whereas the antioxidant properties of rice bran were slightly enhanced after 24 h of fermentation. Additional detailed in vivo evaluation is required to explain the impact of submerged fermentation on the bioactivity of rice bran.
Electronic supplementary material
The online version of this article (doi:10.1186/s13568-017-0411-4) contains supplementary material, which is available to authorized users.
Keywords: Anthocyanin, Antioxidant capacity, Fermented rice bran, β-glucosidase, Saccharomyces cerevisiae
Introduction
Rice, especially colored or pigmented rice (purple, black, and red rice), is one of the major ration of South East Asian diet (Hu et al. 2003). The phytochemical constituents of pigmented rice are flavonoids, phenolics, tannin, sterols, tocols, γ-oryzanols, amino acids, and essential oils (Nakornriab et al. 2008; Min et al. 2009). Rice bran or rice coat is a waste product of agricultural milling process, which is highly enriched with phyto-antioxidant compounds, vitamin, and dietary fibers (Chen et al. 2008; Saenjum et al. 2012). The outcome of research on phytochemical properties and bioactivities of colored rice revealed the richness of natural complex antioxidant compounds, possibly due to the deposition of large amounts of anthocyanin (ACN) (Moldenhauer et al. 2003; Chaudhary 2003).
Anthocyanin are glycosides and belongs to the class of flavonoids (Kong et al. 2003). ACN are reported for its bioactive properties like antioxidant (Pengkumsri et al. 2015), cardiovascular disease prevention (Bell and Gochenaur 2006), anticancer, antitumor, antimutagenic (Marko et al. 2004), antidiabetes (Jayaprakasam et al. 2005), ocular impairment (Canter and Ernst 2004), aging treatments (Lau et al. 2007), and antibacterial activity (Puupponen-Pimia et al. 2005). ACN can be transformed to anthocyanidins (also known as aglycone forms of ACN) by the enzyme called β-glucosidases (BGS) (Fleschhut et al. 2006) and aglycone forms of ACN are more potent than the glycosylated forms (ACN) with respect to antioxidant property (Kahkonen and Heinonen 2003). Cyanidin, delphinidin, peonidin, petunidin, malvidin, and pelargonidin are commonly occurred as aglycone forms of ACN. While in plants, anthocyanidins occur as glycosylated forms, anthocyanins.
β-glucosidase specifically act on β-glucosidic linkages of di- and oligosaccharides, or other glucose conjugates. It comprises a heterogeneous group of enzymes that can cleave the glucosidic linkages and releases glycoside compounds (Hsieh and Graham 2001). BGS are ubiquitous in nature with a vast range of functionality. In bacteria and fungi, BGS are involved in the cellulolytic processes. Studies are representing the presence of BGS in several yeast species like Hanseniaspora, Pichia, Candida, Saccharomycodes, Metschnikowia and Brettanomyces, which are isolated from the grape (Giovanni et al. 2002). Saccharomyces cerevisiae is well known for the β-glucosidase activity (Delcroix et al. 1994). BGS can act on ginsenoside, natural steroid glycosides, complex and the hydrolysis of wheat bran ginsenosides by BGS has been reported (Jiang-Ning et al. 2008).
Production of most of the extracellular enzymes by microbes (fungi, yeast and bacteria) are performed using submerged fermentation technique, which provides various advantages including medium sterilization, cultivation of cultures due to easy monitoring and controlling of parameters like nutrients concentration, temperature, aeration, pH and moisture, and end product purification. Submerged fermentation technique had been reported for its use in the extracellular production of BGS by different Aspergillus strains (Gunata and Vallier 1999; Jager et al. 2001). Zahoor et al. (2011) reported the use of submerged fermentation technique for the maximum yield of BGS by Aspergillus niger in the Eggins and Pugh containing wheat bran (1%). A recent study by Mallek-Fakhfakh et al. (2017) have reported the BGS producing Talaromyces thermophilus with the agricultural waste as a substrate using submerged fermentation technique. The current investigation was conceived and the submerged fermentation of black rice bran with BGS producing S. cerevisiae was carried out. The impact of three variables such as pH, temperature, and concentration of co-factor (NaCl) on total ACN content and the antioxidant capacity of fermented rice bran have been evaluated. Moreover, the BGS activity during fermentation has also been reported in this study. Optimization and analysis of multiple variables in an experiment can be achieved by response surface methodology (RSM) and Box–Behnken design (BBD) (Woraharn et al. 2015). The basic theoretical and fundamental aspects of RSM have been reported previously (Chandrika and Fereidoon 2005; El-Naggar et al. 2015).
Materials and methods
Black rice bran and inoculum preparation
Fresh black rice bran (Chiang Mai province, Thailand) was obtained from rice milling process and sieved through a 60-mesh strainer, and sterilized by autoclave. Then, the sterile bran samples were used for the fermentation process. S. cerevisiae HII31 [strain deposited in Thailand Institute of Scientific and Technological Research (TISTR)] cells were grown in yeast extract-peptone-dextrose (YPD) broth [1% yeast extract (Difco Laboratories, Sparks, MD, USA), 2% peptone (Difco Laboratories), 2% glucose] at 30 °C for 48 h. Then the cell density was calculated by spectrophotometer at 600 nm. The cell suspension was adjusted with medium up to 1 × 108 cells/mL (Sirilun et al. 2016).
Experimental design
Stat-Ease software (Design-Expert 6.0.2, Delaware, USA Echip, 2000-trail version) was used for the experimental design and statistical analysis. A three-factor with three-level of Box–Behnken design was selected to evaluate the effect of the combination of three independent variables such as pH, temperature (°C), and NaCl concentration (%; w/v), coded as X1, X2, and X3, respectively. The values of pH, temperature, and NaCl concentration were set as 3.5–4.5, 35–45 °C, and 0.4–0.6% w/v, respectively (Additional file 1: Table S1). The complete design comprised of 17 experiments including five replicates of the center point. The Z (responses function) was divided into linear, quadratic and interactive values
β0, Bi, Bii, and Bij is constant, linear coefficient, quadratic coefficient, and cross-product coefficient, respectively. Xi and Xj are levels of the independent variables and k represents the number of the tested factors (k = 3) (Fan et al. 2008). RSM was employed to optimize the multiple variants that influence the β-glucosidase activity, anthocyanin content, and antioxidant activity.
Fermentation
About 25 mL of S. cerevisiae suspension (1 × 108 cells/mL) was used for every submerged fermentation process with 25 g of rice bran as a substrate. Fermentation conditions were denoted in Additional file 1: Table S2. The total reaction volume of all the fermentation was maintained as 60 mL, and pH of the mixture was adjusted respectively using 1.0 N HCl before the addition of inoculum. The bio-reaction was performed with constant aeration, and agitation at 150 rpm for 24 h. After 24 h of fermentation, content was separated into two parts like supernatant and bran residue. The supernatant was used for the assessment of β-glucosidase activity, and bran residues were subjected to total anthocyanin extraction subsequently anti-oxidant activity determination.
Beta-glucosidase activity determination and protein quantification
All the fermented rice bran (FRB) suspension was centrifuged at 5000×g for 10 min at 4 °C, and the clear supernatant was collected for the analysis of β-glucosidase activity. The β-glucosidase activity was evaluated by measuring the rate of hydrolysis of ρ-nitrophenyl-β-d-glucopyranoside (ρNPG) (Sigma) (Hernandez et al. 2003). In brief, 50 µL of the sample, 100 µL of 3.3 mM p-NPG, and 200 µL of 0.5 M Na2CO3 were mixed. The same mixture without a sample was served as blank. Then, the mixture was incubated at 40 °C for 25 min and measured at 405 nm using a spectrophotometer (DTX880 multimode detector, Beckman Coulter, USA with software version 2.0). The β-glucosidase activity was expressed as relative activity (%). The Lowry’s assay was performed to quantify the protein content in the sample (Lowry et al. 1951). BSA (20–200 µg/mL) has been used for the preparation of standard curve.
Fermented black rice bran extraction and determination of total anthocyanin
Fermented rice bran samples were extracted with 250 mL of 0.1 N HCl at 40 °C for 30 min. Then the solution was filtrated through 0.45 μm membrane and evaporated under reduced pressure at 40 °C. The recovered crude extracts were kept at −80 °C until use. Total anthocyanin content was determined as described previously (Pengkumsri et al. 2015). Total anthocyanin content was denoted as mg cyanidin chloride equivalent (mg CCE) per gram of FRB.
Anthocyanins and phenolic acids determination by HPLC
The FRB extracts were used for the determination of the content of anthocyanins and phenolic acids by reversed-phase HPLC as described previously (Pengkumsri et al. 2015). ACE® C18 column (250 mm × 4.6 mm; 5 μm) (Advanced Chromatography Technologies, Scotland) was used for the determination of anthocyanin (detected at a wavelength of 520 nm) and phenolic acids (detected at a wavelength of 280 nm). The mobile phase used for the determination of ACN includes acetonitrile, phosphoric acid (4%) and the rate of flow was set as 1.0 mL/min, and mobile phase used for the determination of phenolic acids includes acetonitrile, trifloroacetic acid (0.1%) and the rate of flow was set as 0.8 mL/min. The gradient elution was performed as described in the previous publication (Pengkumsri et al. 2015). The standards used for the determination of ACN are cyanidin-3-glucoside, peonidin-3-glucoside, cyanidin, and peonidin. The standards used for the determination of phenolic acids are protocatechuic acid, caffeic acid, syringic acid, and p-coumaric acid.
Antioxidant determination
Antioxidant capacity of FRB extracts were assessed by ABTS (2, 2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid), DPPH (1, 1-diphenyl-2-picryl-hydrazil), NO· (Nitric oxide), O·−2 (superoxide) radical scavenging assay, FRAP (ferric reducing antioxidant power) assay, and inhibition of lipid peroxidation (LPO) assay as described previously (Pengkumsri et al. 2015). The data obtained were expressed as antioxidant activity per gram of FRB.
Statistical analysis
The 3D response surface plots were showed in this study. Optimized parameters were defined by the Design-Expert software version 6.0.2-trail version and validated through wet lab experiments. The experimental data were established by second-order polynomial regressed equations. Analysis of variance (ANOVA) with a confident interval of 95% (p < 0.05) was reported, and all the experiments were performed in triplicates. The statistical program SPSS (v. 17.0) was used for the analysis of significant differences in response to different variables. ANOVA with least significant difference post hoc test was used for determining the significance difference between the respective anthocyanins or anthocyanidins or phenolic acids at different time points of fermentation. Significant difference between the IC50 values of the fermented black rice bran in the respective free radical scavenging assay (ABTS or DPPH or superoxide or nitric oxide inhibition assay or inhibition of lipid peroxidation) at 24 h of fermentation and IC50 values of fermented black rice bran in the respective free radical scavenging assay at 0 h of fermentation were analyzed by ANOVA with least significant difference post hoc test. The value of p < 0.05 (at confident interval of 95%) was considered significant.
Results
Experimental design and validation
The BBD of the current study was tabulated (Additional file 1: Table S2). The pH (X1), temperature (X2) and NaCl concentration (X3) were selected as three independent variables with the range of 3.5–4.5, 35–45 °C and 0.4–0.6%, respectively. A total of 17 different combinations of experiments were generated using design export software v.6. The total ACN content, antioxidant property and relative activity of BGS (%) were the desired outcome (dependent variables) of the current study. ACN content and the antioxidant property have been represented as the mg equivalent of cyanidin/g of FRB and mg TEAC/g of FRB, respectively. All the 17 combinations of experiments were carried out to evaluate the predicted values obtained from the design expert software with respect to all the dependent variables (Table 1). The optimum pH, temperature and NaCl concentration for the maximum recovery of total ACN, BGS activity, and TEAC were tabulated with actual and predicted values (Table 2). In the 17 combinations of experiments, five experiments (run order no. 3, 5, 11, 12, and 16) were with a same combinations of center points (4.0, 40 °C and 0.5%) of three independent variables (pH, temperature and NaCl concentration) that provided the same predicted values. Similarly, the actual values obtained from the five combinations of experiments (run order no. 3, 5, 11, 12, and 16) were found to be not statistically different (Table 1) and therefore, the five combinations of experiments of the center point of three independent variables acts as a biological replicates.
Table 1.
The predicted and actual values of ACN content, antioxidant capacity of FRB and BGS activity during fermentation
| Run order | IV | DV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | (°C) | NaCl (%) | Total ACN (mg CCE/g FRB) | Antioxidant capacity (mg TEAC/g FRB) | Relative activity (%) | ||||
| Actual | Predicted | Actual | Predicted | Actual | Predicted | ||||
| 1 | 3.5 | 45 | 0.5 | 78.50 | 78.15 | 60.00 | 59.99 | 84.08 | 84.08 |
| 2 | 3.5 | 35 | 0.5 | 82.10 | 79.70 | 61.00 | 60.99 | 84.34 | 84.34 |
| 3 | 4.0 | 40 | 0.5 | 100.30c | 100.88 | 70.50b | 70.10 | 99.90b | 98.54 |
| 4 | 4.5 | 40 | 0.6 | 95.50 | 92.78 | 67.10 | 67.33 | 90.70 | 90.70 |
| 5 | 4.0 | 40 | 0.5 | 100.00e | 100.88 | 71.00a | 70.10 | 97.20e | 98.54 |
| 6 | 4.5 | 45 | 0.5 | 82.50 | 83.05 | 62.50 | 62.26 | 86.13 | 86.13 |
| 7 | 4.5 | 40 | 0.4 | 91.70 | 92.78 | 64.50 | 64.25 | 89.56 | 89.56 |
| 8 | 4.5 | 35 | 0.5 | 83.50 | 84.60 | 63.00 | 63.26 | 86.39 | 86.39 |
| 9 | 4.0 | 45 | 0.4 | 90.00 | 91.15 | 64.00 | 63.78 | 88.83 | 88.83 |
| 10 | 4.0 | 35 | 0.4 | 91.00 | 92.70 | 65.00 | 65.24 | 89.09 | 89.09 |
| 11 | 4.0 | 40 | 0.5 | 100.50b | 100.88 | 69.50d | 70.10 | 98.10c | 98.54 |
| 12 | 4.0 | 40 | 0.5 | 104.50a | 100.88 | 69.50d | 70.10 | 100.00a | 98.54 |
| 13 | 3.5 | 40 | 0.4 | 86.00 | 87.88 | 64.00 | 64.24 | 87.51 | 87.51 |
| 14 | 3.5 | 40 | 0.6 | 87.00 | 87.88 | 63.00 | 63.25 | 88.65 | 88.65 |
| 15 | 4.0 | 35 | 0.6 | 93.10 | 92.70 | 67.00 | 66.51 | 90.23 | 90.23 |
| 16 | 4.0 | 40 | 0.5 | 100.20d | 100.88 | 70.00c | 70.10 | 97.50d | 98.54 |
| 17 | 4.0 | 45 | 0.6 | 92.50 | 91.15 | 65.50 | 65.51 | 89.97 | 89.97 |
IV independent variables, DV dependent variable, FRB fermented rice bran
a–eThe values of replicates of five combinations of the center points (run order no. 3, 5, 11, 12, and 16) showed no significant difference
Table 2.
The experimental values and predicted values of responses at the optimum condition
| Variables/responses | Optimum conditions | |
|---|---|---|
| Actual value | Predicted value | |
| pH | 4.00 | 4.06 |
| Temperature (°C) | 40.00 | 39.80 |
| NaCl (% w/v) | 0.50 | 0.52 |
| Total anthocyanin content (mg cyanidin equivalent/g of FRB) | 101.10 ± 1.91 | 101.04 |
| Antioxidant capacity TEAC (mg trolox equivalent/g of FRB) | 70.10 ± 0.65 | 70.30 |
| β-glucosidase activity (relative activity (%)) | 98.54 ± 1.33 | 98.57 |
The desirability value of optimum condition was 0.904
FRB fermented rice bran
The analysis of variance of the fitted quadratic polynomial model for total ACN content, TEAC, and BGS activity are shown in Additional file 1: Table S3 and the real factors for total ACN, TEAC, and BGS activity were respectively. The reliability of the model has been verified by the determination coefficient (R2). The R2 value closer to 1 represents the superior correlation between the investigational and predicted values (Jadhav et al. 2013). In this study, we obtained 0.965, 0.9873, and 0.9846 as R2 values for total ACN content, TEAC, and BGS activity, respectively. Moreover, the coefficient of variation (CV) was 2.02, 0.79, and 0.92, respectively (Additional file 1: Table S3). The lower value of CV indicates the relatively better reliability of the response model.
The ANOVA and F test were performed to evaluate the statistical significance of response surface quadratic model (Table 3). For total ACN content, the linear parameter (X1) and quadratic parameters (X21 and X22) were significant at the level of p < 0.01, but the linear parameter (X2) was not significant at the level of p > 0.05 and did not have interaction parameter power. For TEAC, the linear parameters (X1 and X3) and quadratic parameters (X21, X22, and X23) were significant at the level of p < 0.01, while the linear parameter (X2) was significant at the degree of p < 0.05. Also, the interaction parameter (X1X3) was significant at the level of p < 0.01.
Table 3.
Analysis of variance (ANOVA) for the quadratic model of ACN content, TEAC and BGS activity
| Source | Degree of freedom | Sum of squares | Mean square | f value | p value |
|---|---|---|---|---|---|
| Total anthocyanin | |||||
| Model | 4 | 908.37 | 227.09 | 66.01 | <0.0001 |
| X1 | 1 | 48.02 | 48.02 | 13.96 | 0.0028 |
| X2 | 1 | 4.80 | 4.80 | 1.40 | 0.2602 |
| X21 | 1 | 470.18 | 470.18 | 136.66 | <0.0001 |
| X22 | 1 | 338.41 | 338.41 | 98.36 | <0.0001 |
| Lack-of-fit | 8 | 26.71 | 3.44 | 0.92 | 0.5776 |
| Pure error | 4 | 14.58 | 3.64 | ||
| TEAC (antioxidant capacity) | |||||
| Model | 7 | 186.90 | 26.70 | 100.02 | <0.0001 |
| X1 | 1 | 10.35 | 10.35 | 38.78 | 0.0002 |
| X2 | 1 | 2.00 | 2.00 | 7.49 | 0.0230 |
| X3 | 1 | 3.25 | 3.25 | 12.18 | 0.0068 |
| X21 | 1 | 89.09 | 89.09 | 333.76 | <0.0001 |
| X22 | 1 | 63.22 | 63.22 | 236.84 | <0.0001 |
| X23 | 1 | 3.04 | 3.04 | 11.40 | 0.0082 |
| X1X3 | 1 | 3.24 | 3.24 | 12.14 | 0.0069 |
| Lack-of-fit | 5 | 0.70 | 0.14 | 0.33 | 0.8720 |
| Pure error | 4 | 1.70 | 0.42 | ||
| BGS activity (%) | |||||
| Model | 6 | 451.25 | 75.21 | 106.65 | <0.0001 |
| X1 | 1 | 8.40 | 8.40 | 11.92 | 0.0062 |
| X2 | 1 | 0.14 | 0.14 | 0.19 | 0.6708 |
| X3 | 1 | 2.60 | 2.60 | 3.69 | 0.0838 |
| X21 | 1 | 198.43 | 198.43 | 281.39 | <0.0001 |
| X22 | 1 | 174.63 | 174.63 | 247.63 | <0.0001 |
| X23 | 1 | 27.81 | 27.81 | 39.44 | <0.0001 |
| Lack-of-fit | 6 | 0.000 | 0.000 | 0.000 | 1.0000 |
| Pure error | 4 | 7.05 | 1.76 | ||
For BGS activity, the linear parameters (X1) and quadratic parameters (X21, X22, and X23) were significant at the level of p < 0.01, but the linear parameters (X2 and X3) were not significant at the degree of p > 0.05 and did not have interaction parameter power (Table 3).
Response surface plots
To optimize the desired outcome, the maximum level of responses were predicted by the interaction of two variables with a single constant variable using RSM and BBD. The response surface plots represent the optimum conditions (pH, temperature, and NaCl concentration) for total ACN content, TEAC, and BGS activity, and denoted as cyanidin equivalent, TEAC, and relative activity, respectively.
The optimum response of variables for the total ACN content was analyzed and found that the optimum condition was temperature 37.5–42.5 °C and pH 3.75–4.25 with constant cofactor concentration (0.5% NaCl) (Fig. 1a). At constant temperature (40 °C) with variable pH and NaCl concentration, the maximum ACN content was observed in the pH range of 3.75–4.35 (Fig. 1b). Likewise, the interaction of variable temperature and NaCl concentration with constant pH (4.0) condition showed the maximum ACN content at 36.5–43.0 °C (Fig. 1c), and at any case, the concentration of NaCl has no effect on ACN content (Fig. 1).
Fig. 1.
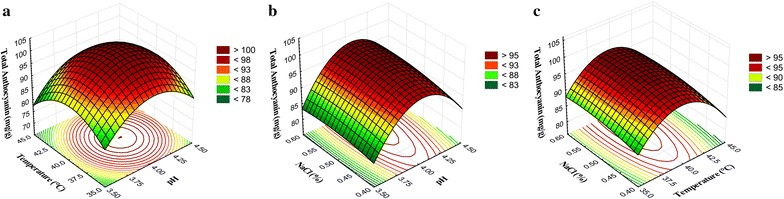
Response surface plot for the total ACN content of FRB describing the interaction of independent variables namely, pH, temperature, and NaCl concentration on ACN content. a Effect of temperature and pH on total ACN content of FRB, b effect of NaCl concentration (%) and pH on total ACN content of FRB, and c effect of NaCl concentration (%) and temperature on total ACN content of FRB
The optimum response of variables for TEAC was also found at 37.5–42.5 °C and pH 3.50–4.25 with constant cofactor concentration (0.5% NaCl) (Fig. 2a). At constant temperature (40 °C) with variable pH and NaCl concentration, the maximum TEAC was observed in the pH range of 3.50–4.25, and 0.43–0.60% of NaCl (Fig. 2b). Similarly, the interaction of variable temperature and NaCl concentration with constant pH (4.0) condition showed the maximum TEAC at 37.0–43.0 °C, and 0.43–0.60% of NaCl (Fig. 2c).
Fig. 2.
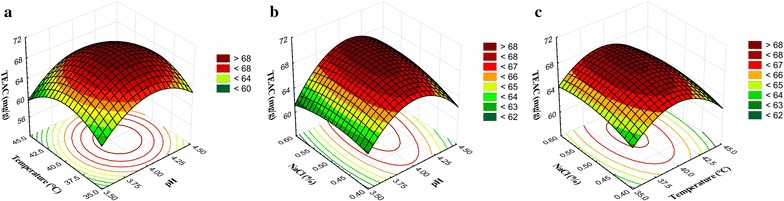
Response surface plot for the antioxidant capacity of FRB describing the interaction of independent variables namely, pH, temperature, and NaCl concentration on antioxidant capacity of FRB. The values were represented as trolox equivalent of antioxidant capacity (TEAC). a Effect of temperature and pH on antioxidant capacity of FRB, b effect of NaCl concentration (%) and pH on antioxidant capacity of FRB, and c effect of NaCl concentration (%) and temperature on antioxidant capacity of FRB
The BGS activity at a different temperature, pH, and NaCl concentration was also studied, and the optimum BGS activity was observed at 37.5–42.5 °C, and pH 3.75–4.35 with constant NaCl concentration (0.5%) (Fig. 3a). The optimum BGS activity was observed at the pH of 3.75–4.35 and 0.42–0.60% of NaCl at 40 °C (Fig. 3b). Similarly, the interaction of variable temperature and NaCl concentration with constant pH (4.0) condition represent the maximum BGS activity at 37.0–44.0 °C, and 0.42–0.60% of NaCl (Fig. 3c).
Fig. 3.
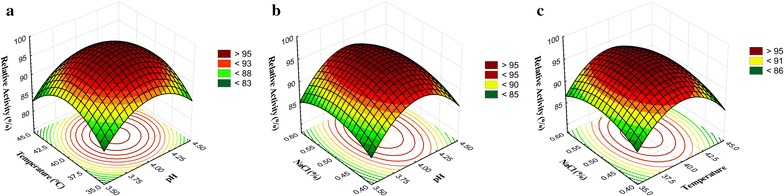
Response surface plot for the β-glucosidase activity during fermentation the interaction of independent variables namely, pH, temperature, and NaCl concentration on β-glucosidase activity during fermentation. The values were represented as relative activity (%). a Effect of temperature and pH on enzyme activity of S. cerevisiae, b effect of NaCl concentration (%) and pH on enzyme activity of S. cerevisiae, and c effect of NaCl concentration (%) and temperature on enzyme activity of S. cerevisiae
Anthocyanidins formation
Anthocyanin (cyanidin-3-glucoside and peonidin-3-glucoside) can be transformed to anthocyanidins (cyanidin and peonidin) by the enzyme BGS, which breaks the glycosidic bond of ACN and release the glucose group. The biotransformation of ACN to anthocyanidins during fermentation has been kinetically (0–24 h) evaluated (Additional file 1: Figure S1) and found that the concentration of cyanidin-3-glucoside and peonidin-3-glucoside are gradually decreased from 324.66 ± 16.23 to 190.03 ± 13.25 and 231.65 ± 11.58 to 163.66 ± 10.18 µg/g of FRB, respectively. In the meantime, the amount of cyanidin and peonidin has been increased from 226.73 ± 11.34 to 312.90 ± 11.65 and 86.73 ± 4.43 to 130.93 ± 4.55 µg/g of FRB, respectively. The activity of BGS during fermentation has also been recorded and about 85% of enzyme activity was noticed after 6 h of the process and 100% of relative activity was recorded at 24 h (Table 4). The transformation of ACN to anthocyanidins upon BGS activity was statistically significant (p < 0.05) between initial and end points.
Table 4.
Concentration of anthocyanins (glycoside forms), and anthocyanidins (aglycoside forms) during fermentation and β-glucosidase activity of S. cerevisiae
| Time (h) | Anthocyanins (µg/g of FRB) | Anthocyanidins (µg/g of FRB) | β-glucosidase [relative activity (%)] | ||
|---|---|---|---|---|---|
| Cyanidin-3-glucoside | Peonidin-3-glucoside | Cyanidin | Peonidin | ||
| 0 | 324.66 ± 16.23 | 231.65 ± 11.58 | 226.73 ± 11.34 | 86.73 ± 4.34 | 16.67 ± 2.08 |
| 1 | 319.96 ± 12.76 | 219.71 ± 10.99 | 229.74 ± 11.49 | 94.49 ± 4.72* | 41.67 ± 3.15 |
| 3 | 297.43 ± 14.87* | 215.74 ± 10.79 | 244.16 ± 11.21 | 97.07 ± 3.85* | 58.33 ± 3.92 |
| 6 | 275.23 ± 13.76* | 188.38 ± 10.42*** | 258.36 ± 11.92* | 114.86 ± 3.74*** | 85.00 ± 3.25 |
| 12 | 235.09 ± 15.22*** | 178.49 ± 8.92*** | 284.06 ± 13.20*** | 121.29 ± 4.06*** | 91.67 ± 4.83 |
| 18 | 194.93 ± 12.11*** | 171.31 ± 9.57*** | 309.76 ± 10.49*** | 125.95 ± 4.30*** | 95.00 ± 3.83 |
| 24 | 190.03 ± 13.25*** | 163.66 ± 10.18*** | 312.90 ± 11.65*** | 130.93 ± 4.55*** | 100.00 ± 4.00 |
The significant difference between the anthocyanins (cyanidin-3-glucoside or peonidin-3-glucoside) or anthocyanidins (cyanidin or peonidin) at different time points (1, 3, 6, 12, 18, and 24 h) during fermentation and the respective anthocyanins or respective anthocyanidins at 0 h of fermentation were represented as * (p < 0.05), ** (p < 0.01), and *** (p < 0.001)
Phenolic acid content
The changes in the representative phenolic acid (protocatechuic acid, caffeic acid, syringic acid, and p-coumaric acid) content were assessed during fermentation. The initial amount (at 0 h) of protocatechuic acid, caffeic acid, syringic acid, and p-coumaric acid were found as 23.03 ± 1.15, 26.74 ± 1.34, 5.23 ± 0.26, and 195.09 ± 9.75 µg/g of FRB, respectively and after 24 h, the concentration were noted as 22.99 ± 1.15, 27.07 ± 1.35, 5.49 ± 0.27, and 200.55 ± 10.03 µg/g of FRB, respectively (Table 5). Quantification of the phenolic acids was carried out by HPLC and the representative chromatograms was represented in Additional file 1: Figure S2.
Table 5.
The changes in the content of phenolic acids (µg/g of FRB) during biotransformation
| Time (h) | Protocatechuic acid | Caffeic acid | Syringic acid | p-coumaric acid |
|---|---|---|---|---|
| 0 | 23.03 ± 1.15 | 26.74 ± 1.34 | 5.23 ± 0.26 | 195.09 ± 9.75 |
| 1 | 22.56 ± 1.13 | 26.05 ± 1.35 | 5.40 ± 0.27 | 201.55 ± 10.08 |
| 3 | 21.88 ± 1.09 | 27.68 ± 1.32 | 5.00 ± 0.25 | 185.88 ± 9.95 |
| 6 | 24.00 ± 1.22 | 25.45 ± 1.26 | 5.44 ± 0.27 | 202.32 ± 10.12 |
| 12 | 22.75 ± 1.14 | 26.87 ± 1.29 | 5.11 ± 0.28 | 192.67 ± 9.63 |
| 18 | 23.18 ± 1.16 | 28.12 ± 1.34 | 4.97 ± 0.25 | 204.00 ± 10.10 |
| 24 | 22.99 ± 1.15 | 27.07 ± 1.35 | 5.49 ± 0.27 | 200.55 ± 10.03 |
Significant difference was not observed between the phenolic acids (protocatechuic acid or caffeic acid or syringic acid or p-coumaric acid) at different time points (1, 3, 6, 12, 18, and 24 h) during fermentation and the respective phenolic acids at 0 h of fermentation
Antioxidant properties
The antioxidant property of RB before and after the fermentation with BGS producing S. cerevisiae has been kinetically evaluated. The representative antioxidant assays such as ABTS, DPPH, inhibition of lipid peroxidation, superoxide, nitric oxide, and FRAP assays were performed. ABTS, DPPH, inhibition of lipid peroxidation, and superoxide assay results were represented as trolox equivalent of antioxidant capacity (mg/g of FRB), whereas the results of nitric oxide and FRAP assays were denoted as curcumin equivalent and FeSO4 equivalent (mg/g of FRB), respectively. The changes in the antioxidant properties were represented in Fig. 4.
Fig. 4.
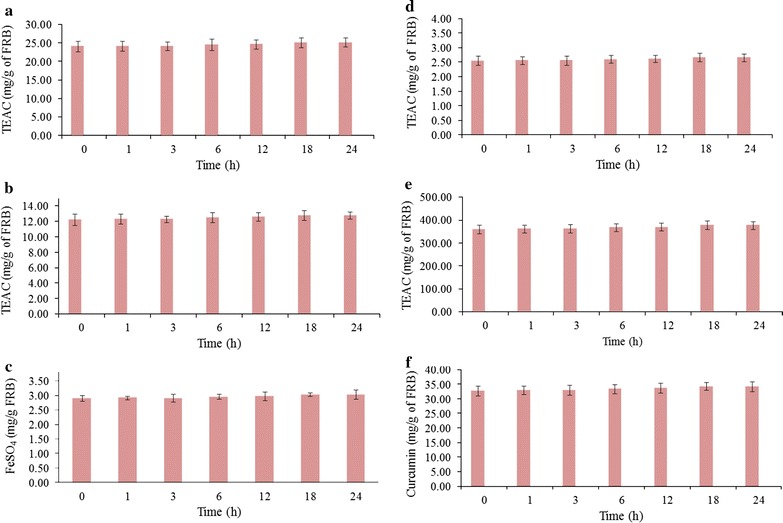
Assessment of free radical scavenging activity during S. cerevisiae mediated fermentation of rice bran. a ABTS assay, b DPPH assay, c FRAP assay, d inhibition of lipid peroxidation, e superoxide and f nitric oxide radical scavenging assays for fermented rice bran. a ABTS assay, b DPPH assay, d inhibition of lipid peroxidation, e superoxide radical scavenging assays results were represented as trolox equivalent of antioxidant capacity (TEAC). FRAP assay and nitric oxide scavenging assay results were denoted as FeSO4 equivalent/g of FRB and curcumin equivalent/g of FRB, respectively
The ABTS, DPPH, inhibition of lipid peroxidation, superoxide, nitric oxide, and FRAP assay results suggested that the free radical scavenging property of RB was not significantly altered after the fermentation (24 h), when compared with that at the 0 h of fermentation. The free radical inhibition ability of FRB has been represented as an IC50 (concentration of FRB that are capable of exhibiting 50% of inhibition of free radical) concentration for respective free radical assays. The IC50 values were not significantly altered after fermentation (Table 6).
Table 6.
The free radical inhibition activities of fermented black rice bran
| Time (h) | ABTS assay (µg of FRB) | DPPH assay (mg of FRB) | Inhibition of lipid peroxidation assay (mg of FRB) | Superoxide assay (µg of FRB) | Nitric oxide assay (mg of FRB) |
|---|---|---|---|---|---|
| 0 | 183.75 ± 7.19 | 1.06 ± 0.03 | 7.25 ± 0.32 | 88.72 ± 3.44 | 1.24 ± 0.07 |
| 1 | 182.81 ± 9.14 | 1.06 ± 0.05 | 7.22 ± 0.36 | 88.26 ± 4.41 | 1.24 ± 0.06 |
| 3 | 183.05 ± 7.15 | 1.06 ± 0.02 | 7.23 ± 0.30 | 88.38 ± 5.42 | 1.24 ± 0.08 |
| 6 | 180.07 ± 9.00 | 1.04 ± 0.05 | 7.11 ± 0.36 | 86.94 ± 4.35 | 1.22 ± 0.06 |
| 12 | 178.83 ± 8.94 | 1.04 ± 0.05 | 7.06 ± 0.35 | 86.34 ± 3.32 | 1.21 ± 0.05 |
| 18 | 175.70 ± 7.78 | 1.02 ± 0.04 | 6.94 ± 0.33 | 84.83 ± 4.24 | 1.19 ± 0.05 |
| 24 | 175.72 ± 8.79 | 1.02 ± 0.05 | 6.94 ± 0.33 | 84.84 ± 3.24 | 1.19 ± 0.06 |
The values are IC50 concentrations of FRB in the respective analysis
Significant difference was not observed between the IC50 values of fermented black rice bran in the respective free radical scavenging assay (ABTS or DPPH or lipid peroxidation or superoxide or nitric oxide inhibition assay) at 24 h of fermentation and IC50 values of fermented black rice bran in the respective free radical scavenging assay at 0 h of fermentation
Discussion
To study the influence of fermentation conditions on ACN content, antioxidant property (denoted as trolox equivalent of antioxidant capacity; TEAC) and BGS activity during black rice bran fermentation by S. cerevisiae, BBD and surface response methodology was employed. Extremely low probability values of the model for all decided responses (Total ACN, TEAC, and BGS activity) revealed that the current model is reliable and significant (p < 0.0001). The lack of fit values is typically used to measure the failure of the model generated by the software. This value should not be non-significant (Sharma et al. 2009). It is essential to validate the fitted model to confirm the created approximate values with wet lab results. The proposed model should be with acceptable fit value, if not the results are not reliable (Murugesan et al. 2007). The lack of fit values of total ACN, TEAC, and BGS activity (p value = 0.5776, 0.8720, and 1.000, respectively) suggested that the experimental model was statistically significant.
The response surface plots were represented to show the influence of independent variables on desired outcomes (Figs. 1, 2 and 3), which revealed the optimum conditions to achieve maximum anticipated products (total ACN content, TEAC, and BGS activity). Avila et al. (2009) reported about bioconversion of ACN by Lactobacillus (L. acidophilus, L. plantarum, and L. casei) and Bifidobacterium (B. lactis) with the involvement of BGS activity. These strains can release the metabolites like gallic, syringic and homogentisic acids by the enzymatic degradation of malvidin (an O-methylated anthocyanidin) and the authors claimed that B. lactis, L. casei, and L. plantarum strains are functional probiotics to acquire additional malvidin and phenolic acids (Avila et al. 2009). The results of the current study suggested that the BGS of S. cerevisiae also can stimulate the bioconversion of ACN, and the rate of conversion was directly proportional to the enzyme activity, and the maximum bioconversion rate was observed after 24 h of fermentation (Table 4).
Lactobacillus spp. mediated fermentation decreases the content of most of the polyphenols of red sorghum, whereas the antibiotic nature of the sorghum was not affected (Svensson et al. 2010). Ryan et al. (2011) demonstrated that Saccharomyces boulardii mediated fermentation altered the phytochemical nature of RB and also fermented RB showed the improved ability to suppress the growth of human B lymphomas. The phenolic content of RB was doubled after fermentation by Rhizopus oryzae and showed higher inhibition potential in DPPH and peroxidase assays (Schmidt et al. 2014). The results of the present study suggested that phenolic content of RB was not significantly altered after fermentation indicating that phytochemical nature of RB was not influenced by the BGS activity of S. cerevisiae.
The bioactivity of the anthocyanins also depends on the nature and location of the glycosidic groups, especially influences the antioxidant activity (Avila et al. 2009). Thus, the bioconversion slightly modifies the free radical scavenging activity of ACN. After fermentation, the IC50 values were not significantly changed (Table 6), which suggested that S. cerevisiae mediated fermentation process slightly increases the quality of the rice bran. Tsuda et al. (1994) reported the antioxidant activity of ACN pigments, and results suggested that cyanidin had stronger bioactivity than cyanidin 3-O-β-glucoside, and α-tocopherol especially in lipid peroxidation inhibition assay. The bioactivities of aglycons and glycosides are not fluctuated and influenced by emulsion, whereas the aglycons showed high low-density lipoprotein reduction activity than the glycosides (Kahkonen and Heinonen 2003). In the current study also, improved (statistically significant) bioactivity has been observed after fermentation, which suggested that the aglycone form of rice phytochemical are more potent than glycone form.
The optimum conditions for the ACN content, antioxidant activity and relative activity of BGS with RB as a substrate using RSM and BBD has been revealed. The optimum pH, temperature, and NaCl concentration for RB fermentation was 4, 40 °C, and 0.5%, respectively with respect to the ACN content, TEAC, and BGS activity. The bioconversion of ACN by BGS, phytochemicals, and free radical scavenging activity of fermented RB has been demonstrated. The results revealed that fermentation of RB with BGS producing S. cerevisiae HII31 enhanced the bioactivity of RB, significantly. Further, in vivo evaluation of bioactivity of FRB are required to explain the impact of fermentation on the ability of RB bioactivity.
Authors’ contributions
CC involved in the designing of the experiments, analysis of data, critical review of the manuscript, and support the manuscript writing. NP participated in the RSM study, phytochemical quantifications, and statistical analysis. SS involved in the microbiological experiments. SP was responsible for acquiring the raw experimental data and processing. SK participated in the analytical part of the study. BSS involved in the experimental design, data analysis, manuscript preparation, critical review of the results. All authors read and approved the final manuscript.
Acknowledgements
We gratefully acknowledge the Chiang Mai University grant (CMU-grant) for the support. We wish to acknowledge Faculty of Pharmacy and Chiang Mai University, Chiang Mai, Thailand for the necessary provision. BSS wish to thank CMU-Post Doctoral Fellowship for the support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data are fully available within the text or as a Additional files.
Ethics approval and consent to participate
No animal or human subjects were used in this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACN
anthocyanin
- BGS
β-glucosidase
- HPLC
high-performance liquid chromatography
- RSM
response surface methodology
- BBD
Box–Behnken design
- YPD
yeast extract-peptone-dextrose
- FRB
fermented rice bran
- CCE
cyanidin chloride equivalent
- ABTS
2, 2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid
- DPPH
1, 1-diphenyl-2-picryl-hydrazil
- FRAP
ferric reducing antioxidant power
- LPO
lipid peroxidation
- ANOVA
analysis of variance
- TEAC
trolox equivalent of antioxidant capacity
- R2
determination coefficient
- CV
coefficient of variation
- S. cerevisiae
Saccharomyces cerevisiae
- L. acidophilus
Lactobacillus acidophilus
- L. plantarum
Lactobacillus plantarum
- L. casei
Lactobacillus casei
- B. lactis
Bifidobacterium lacis
- S. boulardii
Saccharomyces boulardii
Additional file
Additional file 1: Figure S1. Representative Chromatograms showed the variation in ACN content of RB during fermentation. (a) ACN standards (1. Cyanidin-3-glucoside, 2. Peonidin-3-glucoside, 3. Cyanidin, 4. Peonidin), (b) 0 h sample (c) 12 h fermented samples (d) 24 h fermented samples. Figure S2. Representative Chromatograms showed the variation in phenolic acid content of RB during fermentation. (a) Phenolic acid standards (1. Protocatechuic acid, 2. Caffeic acid 3. Syringic acid, 4. p-coumaric acid), (b) 0 h sample (c) 12 h fermented samples (d) 24 h fermented samples. Table S1. The optimization code of independent variables and actual values. Table S2. The Box-Behnken design for anthocyanin content, antioxidant capacity of black RB, and β-glucosidase activity during fermentation. X1, X2, X3 is pH, temperature (°C), and NaCl concentration (%), respectively. Table S3. Codes and process variable levels used in experimental design.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13568-017-0411-4) contains supplementary material, which is available to authorized users.
Contributor Information
Chaiyavat Chaiyasut, Email: chaiyavat@gmail.com.
Noppawat Pengkumsri, Email: p_arkasus@hotmail.com.
Sasithorn Sirilun, Email: ssirilun@gmail.com.
Sartjin Peerajan, Email: sartjin_p@yahoo.com.
Suchanat Khongtan, Email: farfon1993@hotmail.com.
Bhagavathi Sundaram Sivamaruthi, Phone: +6653944341, Email: sivasgene@gmail.com, Email: sivamaruthi.b@cmu.ac.th.
References
- Avila M, Hidalgo M, Sanchez-Moreno C, Pelaez C, Requena T, Pascual-Teresa S. Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus. Food Res Int. 2009;42:1453–1461. doi: 10.1016/j.foodres.2009.07.026. [DOI] [Google Scholar]
- Bell DR, Gochenaur K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J Appl Physiol. 2006;100:1164–1170. doi: 10.1152/japplphysiol.00626.2005. [DOI] [PubMed] [Google Scholar]
- Canter PH, Ernst E. Anthocyanosides of Vaccinium myrtillus (bilberry) for night vision—A systematic review of placebo-controlled trials. Surv Ophthalmol. 2004;49:38–50. doi: 10.1016/j.survophthal.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Chandrika LP, Fereidoon S. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93:47–56. doi: 10.1016/j.foodchem.2004.08.050. [DOI] [Google Scholar]
- Chaudhary RC. Speciality rices of the world: effect of WTO and IPR on its production trend and marketing. J Food Agric Environ. 2003;1:34–41. [Google Scholar]
- Chen CR, Wang CH, Wang LY, Hong ZH, Chen SH, Ho WJ. Supercritical-carbon dioxide extraction and deacidification of rice bran oil. J Supercrit Fluids. 2008;45:322–331. doi: 10.1016/j.supflu.2008.01.006. [DOI] [Google Scholar]
- Delcroix A, Gunata Z, Sapis JC, Salmon JM, Bayonove C. Glycosidase activities of three enological yeast strains during winemaking: effect on the terpenol content of Muscat wine. Am J Enol Viticult. 1994;45:291–296. [Google Scholar]
- El-Naggar NEA, Haroun SA, Owis EA, Sherief AA. Optimization of β-glucosidase production by Aspergillus terreus strain EMOO 6-4 using response surface methodology under solid-state fermentation. Prep Biochem Biotechnol. 2015;45:568–587. doi: 10.1080/10826068.2014.940968. [DOI] [PubMed] [Google Scholar]
- Fan G, Han Y, Gu Z, Chen D. Optimizing conditions for anthocyanins extraction from purple sweet potato using response surface methodology (RSM) LWT-Food Sci Technol. 2008;41:155–160. doi: 10.1016/j.lwt.2007.01.019. [DOI] [Google Scholar]
- Fleschhut J, Kratzer F, Rechkemmer G, Kulling SE. Stability and biotransformation of various dietary anthocyanins in vitro. Eur J Nutr. 2006;45:7–18. doi: 10.1007/s00394-005-0557-8. [DOI] [PubMed] [Google Scholar]
- Giovanni S, Riccardo NB, Rosa P, Cristina R, Paolo G. Properties of endogenous β-glucosidase of a Saccharomyces cerevisiae strain isolated from Sicilian musts and wines. Enzym Microb Technol. 2002;31:1030–1035. doi: 10.1016/S0141-0229(02)00233-8. [DOI] [Google Scholar]
- Gunata Z, Vallier MJ. Production of a highly glucose-tolerant extracellular β-glucosidase by three Aspergillus strains. Biotechnol Lett. 1999;21(3):219–223. doi: 10.1023/A:1005407710806. [DOI] [Google Scholar]
- Hernandez LF, Espinosa JC, Fernandez-Gonzalez M, Briones A. β-Glucosidase activity in a Saccharomyces cerevisiae wine strain. Int J Food Microbiol. 2003;80:171–176. doi: 10.1016/S0168-1605(02)00149-6. [DOI] [PubMed] [Google Scholar]
- Hsieh MC, Graham TL. Partial purification and characterization of a soybean beta-glucosidase with high specific activity towards isoflavone conjugates. Phytochemistry. 2001;58:995–1005. doi: 10.1016/S0031-9422(01)00380-6. [DOI] [PubMed] [Google Scholar]
- Hu C, Zawistowski J, Ling WH, Kitts DD. Black rice (Oryza sativa L. indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model systems. J Agric Food Chem. 2003;51:5271–5277. doi: 10.1021/jf034466n. [DOI] [PubMed] [Google Scholar]
- Jadhav SB, Surwase SN, Phugare SS, Jadhav JP. Response surface methodology mediated optimization of Remazol Orange decolorization in plain distilled water by Pseudomonas aeruginosa BCH. Int J Environ Sci Technol. 2013;10:181–190. doi: 10.1007/s13762-012-0088-9. [DOI] [Google Scholar]
- Jager S, Brumbauer A, Feher E, Reczey K, Kiss L. Production and characterization of β-glucosidases from different Aspergillus strains. World J Microbiol Biotechnol. 2001;17(5):455–461. doi: 10.1023/A:1011948405581. [DOI] [Google Scholar]
- Jayaprakasam B, Vareed SK, Olson LK, Nair MG. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem. 2005;53:28–31. doi: 10.1021/jf049018+. [DOI] [PubMed] [Google Scholar]
- Jiang-Ning H, Xue-Mei Z, Ki-Teak L, Yi-Nan Z, Wei L, Li-Kun H, Zhe-Ming F, Li-Juan G, Bai-Sheng S, Chun-Yan W, Chang-Keun S. Optimization of ginsenosides hydrolyzing β-glucosidase production from Aspergillus niger using response surface methodology. Biol Pharm Bull. 2008;31:1870–1874. doi: 10.1248/bpb.31.1870. [DOI] [PubMed] [Google Scholar]
- Kahkonen M, Heinonen M. Antioxidant activity of anthocyanins and their aglycones. J Agric Food Chem. 2003;51:628–633. doi: 10.1021/jf025551i. [DOI] [PubMed] [Google Scholar]
- Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/S0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- Lau FC, Shukitt-Hale B, Joseph JA. Nutritional intervention in brain aging: reducing the effects of inflammation and oxidative stress. In: Harris RE, editor. Inflammation in the pathogenesis of chronic diseases. New York: Springer; 2007. pp. 299–318. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mallek-Fakhfakh H, Fakhfakh J, Masmoudi N, Rezgui F, Gargouri A, Belghith H. Agricultural wastes as substrates for β-glucosidase production by Talaromyces thermophilus: role of these enzymes in enhancing waste paper saccharification. Prep Biochem Biotechnol. 2017;47(4):414–423. doi: 10.1080/10826068.2016.1252928. [DOI] [PubMed] [Google Scholar]
- Marko D, Puppel N, Tjaden Z, Jakobs S, Pahlke G. The substitution pattern of anthocyanidins affects different cellular signaling cascades regulating cell proliferation. Mol Nutr Food Res. 2004;48:318–325. doi: 10.1002/mnfr.200400034. [DOI] [PubMed] [Google Scholar]
- Min B, Chen MH, Green BW. Antioxidant activities of purple rice bran extract and its effect on the quality of low-NaCl, phosphate-free patties made from channel catfish (Ictalurus punctatus) belly flap meat. J Food Sci. 2009;74:268–277. doi: 10.1111/j.1750-3841.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- Moldenhauer KA, Champagne ET, McCaskill DR, Guraya H (2003) Functional products from rice. In: Mazza G (ed) Functional foods. Technomic Publishing Co. Inc., Lancaster
- Murugesan K, Dhamija A, Nam I, Kim Y, Chang Y. Decolourization of reactive black 5 by laccase: optimization by response surface methodology. Dyes Pigments. 2007;75:176–184. doi: 10.1016/j.dyepig.2006.04.020. [DOI] [Google Scholar]
- Nakornriab M, Sriseadka T, Wongpornchai S. Quantification of carotenoid and flavonoid components in brans of some Thai black rice cultivars using supercritical fluid extraction and high-performance liquid chromatography-mass spectrometry. J Food Lipids. 2008;15:488–503. doi: 10.1111/j.1745-4522.2008.00135.x. [DOI] [Google Scholar]
- Pengkumsri N, Chaiyasut C, Saenjum C, Sirilun S, Peerajan S, Suwannalert P, Sirisattha S, Sivamaruthi BS. Physicochemical and antioxidative properties of black, brown and red rice varieties of northern Thailand. Food Sci Technol (Campinas) 2015;35:331–338. doi: 10.1590/1678-457X.6573. [DOI] [Google Scholar]
- Puupponen-Pimia R, Nohynek L, Alakomi HL, Oksman-Caldentey KM. Bioactive berry compounds—Novel tools against human pathogens. Appl Microbiol Biotechnol. 2005;67:8–18. doi: 10.1007/s00253-004-1817-x. [DOI] [PubMed] [Google Scholar]
- Ryan EP, Heuberger AL, Weir TL, Barnett B, Broeckling CD, Prenni JE. Rice bran fermented with Saccharomyces boulardii generates novel metabolite profiles with bioactivity. J Agric Food Chem. 2011;59:1862–1870. doi: 10.1021/jf1038103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenjum C, Chaiyasut C, Chansakaow S, Suttajit M, Sirithunyalug B. Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from Thai purple rice bran. J Med Plants Res. 2012;6:1070–1077. [Google Scholar]
- Schmidt CG, Gonçalves LM, Prietto L, Hackbart HS, Furlong EB. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014;146:371–377. doi: 10.1016/j.foodchem.2013.09.101. [DOI] [PubMed] [Google Scholar]
- Sharma P, Singh L, Dilbaghi N. Optimization of process variables for decolorization of disperse yellow 211 by Bacillus subtilis using Box-Behnken design. J Hazard Mater. 2009;164:1024–1029. doi: 10.1016/j.jhazmat.2008.08.104. [DOI] [PubMed] [Google Scholar]
- Sirilun S, Chaiyasut C, Pengkumsri N, Peerajan S, Chaiyasut K, Suwannalert P, Sivamaruthi BS. Screening and characterization of β-glucosidase production by Saccharomyces cerevisiae. J Appl Pharm Sci. 2016;6:029–035. doi: 10.7324/JAPS.2016.60505. [DOI] [Google Scholar]
- Svensson L, Sekwati-Monang B, Lutz DL, Schieber A, Gänzle MG. Phenolic acids and flavonoids in nonfermented and fermented red sorghum (Sorghum bicolor (L.) Moench) J Agric Food Chem. 2010;58:9214–9220. doi: 10.1021/jf101504v. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Watanabe M, Ohshima K, Norinobu S, Choi SW, Kawakishi S, Osawa T. Antioxidative activity of the anthocyanin pigments cyanidin 3-O-beta-D-glucoside and cyanidin. J Agric Food Chem. 1994;42:2407–2410. doi: 10.1021/jf00047a009. [DOI] [Google Scholar]
- Woraharn S, Lailerd N, Sivamaruthi BS, Wangcharoen W, Peerajan S, Sirisattha S, Chaiyasut C. Development of fermented Hericium erinaceus juice with high content of l-glutamine and l-glutamic acid. Int J Food Sci Technol. 2015;50:2104–2112. doi: 10.1111/ijfs.12873. [DOI] [Google Scholar]
- Zahoor S, Javed MM, Aftab S, Latif F, Ikram-ul-Haq Metabolic engineering and thermodynamic characterization of an extracellular β-glucosidase produced by Aspergillus niger. Afr J Biotechnol. 2011;10(41):8107–8116. doi: 10.5897/AJB11.186. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are fully available within the text or as a Additional files.


