Abstract
Objective:
To understand the efficacy of cladribine (CLAD) treatment in MS through analysis of lymphocyte subsets collected, but not reported, in the pivotal phase III trials of cladribine and alemtuzumab induction therapies.
Methods:
The regulatory submissions of the CLAD Tablets Treating Multiple Sclerosis Orally (CLARITY) (NCT00213135) cladribine and Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis, study one (CARE-MS I) (NCT00530348) alemtuzumab trials were obtained from the European Medicine Agency through Freedom of Information requests. Data were extracted and statistically analyzed.
Results:
Either dose of cladribine (3.5 mg/kg; 5.25 mg/kg) tested in CLARITY reduced the annualized relapse rate to 0.16–0.18 over 96 weeks, and both doses were similarly effective in reducing the risk of MRI lesions and disability. Surprisingly, however, T-cell depletion was rather modest. Cladribine 3.5 mg/kg depleted CD4+ cells by 40%–45% and CD8+ cells by 15%–30%, whereas alemtuzumab suppressed CD4+ cells by 70%–95% and CD8+ cells by 47%–55%. However, either dose of cladribine induced 70%–90% CD19+ B-cell depletion, similar to alemtuzumab (90%). CD19+ cells slowly repopulated to 15%–25% of baseline before cladribine redosing. However, alemtuzumab induced hyperrepopulation of CD19+ B cells 6–12 months after infusion, which probably forms the substrate for B-cell autoimmunities associated with alemtuzumab.
Conclusions:
Cladribine induced only modest depletion of T cells, which may not be consistent with a marked influence on MS, based on previous CD4+ T-cell depletion studies. The therapeutic drug-response relationship with cladribine is more consistent with lasting B-cell depletion and, coupled with the success seen with monoclonal CD20+ depletion, suggests that B-cell suppression could be the major direct mechanism of action.
MS is a CNS demyelinating disease responding to immunosuppression.1 Pulsed induction therapies with cladribine (CLAD)2,3 and alemtuzumab (ALEM)4–6 can induce long-term remission,3,6 while reducing risks of a permanent immunosuppressive state through continuous drug use.7 Pivotal trials of an oral CLAD, 2-chlorodeoxyadenosine triphosphate, prodrug8 and ALEM, CD52-depleting antibody,9 suggest that both drugs have comparable clinical efficacy in controlling relapses,2–5 but markedly different side-effect profiles.2,4,5 Although both drugs induce lymphocyte depletion,8–11 only ALEM causes significant secondary B-cell autoimmunity (SAI) in people with MS.6,9 It was suggested that CLAD may create a cancer risk,2 which in the absence of additional trial data caused regulators to refuse licensing and halted CLAD development in 2011.12 However, a subsequent CLAD trial13 and meta-analysis14 indicated that the CLAD-associated cancer frequency was no different to natural aging or other pivotal MS-drug trials.14 This suggested that even in the absence of oral CLAD, injectable generic CLAD may still have value in treating active MS.15,16
Although the mechanism of action of CLAD in MS is unclear,17 efficacy of ALEM has been attributed to CD4+ T-cell deletion and relative sparing of T-regulatory cells9,18 and SAI to homeostatic T-cell proliferation and lack of thymic repopulation.19 Although immune-reconstitution kinetics after ALEM have been reported,10,18,20 the lymphocyte subset of pivotal CLAD/ALEM trials was only partially disclosed,2,4,5 yet meeting abstracts indicated that lymphocyte subset data were collected and analyzed years ago.21,22 We hypothesized that differences in the CLAD/ALEM lymphocyte repopulation kinetics may offer insights into the efficacy of CLAD and adverse-effect profile of ALEM.
METHODS
Standard protocol approvals, registrations, and patient consents.
Freedom of Information (FOI) requests to the European Medicines Agency (EMA) for the full regulatory submissions of phase III “CLAD Tablets Treating Multiple Sclerosis Orally” (CLARITY; NCT00213135)2 and “Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis, study one” (CARE-MS I; NCT00530348)4 trials were made. Although these trials were recruited following ethical approval of the trials and informed consent, as previously reported,2,4 no specific ethical approval was obtained or required to view and use these “public domain” documents. The details of participants were anonymous. Information relevant to study design, setting, participants, eligibility, variables, randomization, blinding, study size, bias reduction, flow diagrams of participants, and the elements relating to the CONSORT and STROBE reporting guidelines can be obtained from the original CLARITY2 and CARE-MS I4 publications.
Trial designs.
The full details of the trials have been reported previously.2,4 Briefly, in the 96-week CLARITY trial, people with relapsing MS (pwRMS) were randomized 1:1:1 to receive either placebo or one of 2 doses of oral CLAD. Patients were given tablets containing either 10 mg/d (60–69.9 kg body weight) or 20 mg/d (70–79 kg body weight) CLAD prodrug administered for 4–5 days in weeks 0 and 5 (year 1) and weeks 48 and 52 (year 2) to result in a total cumulative dose of 3.5 mg/kg. Those randomized to the 5.25 mg/kg arm were given additional doses in weeks 9 and 13.2 In the CARE-MS I, pwRMS were randomly allocated 1:1 to receive either interferon β-1a (Rebif 44 mg tiw) or ALEM 12 mg/d on days 1–5 in year 1, followed by 12 mg/d on days 1–3 one year later.4
FOI requests.
After termination of the commercial development of oral CLAD in 2011, and subsequent conversations with the UK Medicines and Healthcare products Regulatory Agency about approaches to develop generic CLAD, the full regulatory submission of the CLARITY trial2 was obtained through a FOI request (Submitted May 2013, obtained November 2013). The data set was provided in portable document format (PDF). Files containing relevant data were identified and converted into Microsoft Excel spreadsheets using a PDF parser developed on a Python 2.7 platform at the MidPlus computational facilities at Queen Mary University of London (code available on request). The converted data were validated by comparing sample records between PDF and spreadsheet versions of the files. In addition, we obtained redacted copies of the regulatory submission of the CARE-MS I trial.4 The data were provided in PDF batches during the third and fourth quarter of 2016. However, primary (raw) white cell counts were not included in this package. The data presented here were therefore extracted from the tabulated documents.
Lymphocyte phenotyping data.
In both CLARITY and CARE-MS I, lymphocyte subsets were analyzed using flow cytometry. Both data sets included the following: CD3+, CD4+ and CD8+ T cells; CD19+ B cells; CD16+/CD56+ (natural killer [NK]) cells; CD4+/CD45RA+ (naive T-helper) cells; and CD4+/CD45RO+ (memory T) cells. The data were presented as absolute numbers/unit volume in both studies.
Statistical analysis.
Statistical analysis comparing 2 or more unpaired independent nominal variables was performed using χ2 test for heterogeneity. If statistical significance was detected for comparison of more than 2 variables, post hoc χ2 test for heterogeneity with Bonferroni correction for multiple comparisons was applied. Data are represented as mean ± SD unless described otherwise. For comparison of unpaired independent continuous variables, a 2-tailed Student t test for unpaired samples assuming unequal variances was used. For comparison of more than 2 unpaired independent continuous variables, 1-way analysis of variance (ANOVA) with post hoc Tukey was used. It was evident that the whole population was not analyzed at every time point as indicated in the text; no adjustments for such missing data were made.
RESULTS
In total, 309/1,326 pwRMS from the CLARITY trial2 had lymphocyte subsets analyzed (figure 1), with n = 101 in the placebo arm, n = 103 in the CLAD 3.5 mg/kg arm, and n = 105 in the CLAD 5.25 mg/kg arm (tables e-1 and e-2 at Neurology.org/nn). There was no significant difference for any demographics or clinical characteristics among the study arms (table e-2). It is important that, compared with placebo, both doses of CLAD caused significant and comparable (p = 0.953, ANOVA post hoc Tukey) reduction in the annualized relapse rate (relative reduction compared with placebo 55% and 61% for CLAD 3.5 mg/kg and 5.25 mg/kg, respectively, over 96 weeks) and MS-related MRI parameters (table e-2). The sample was representative of the overall population in the CLARITY study.2
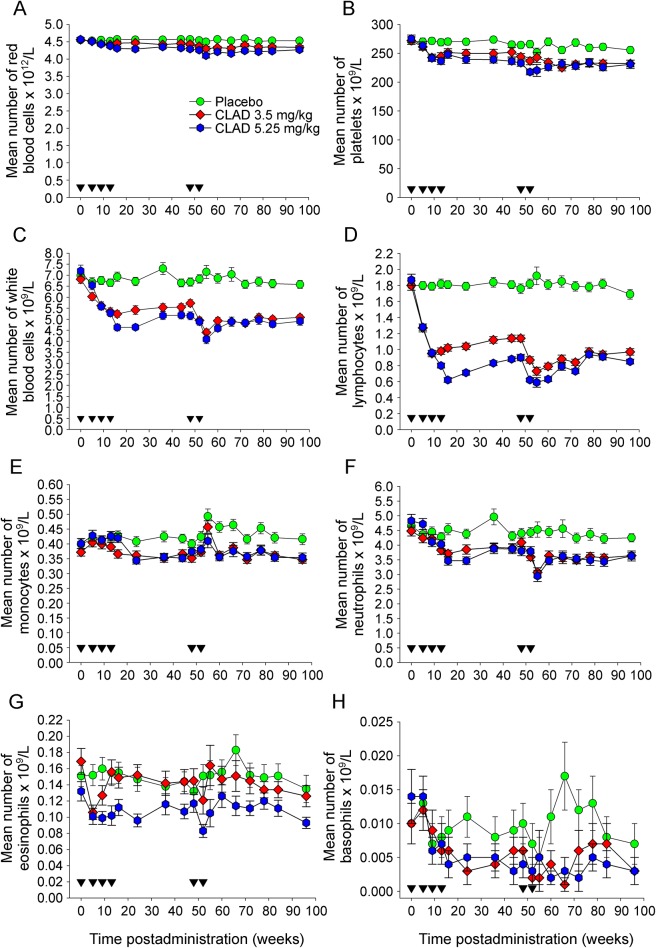
Figure 1. Cladribine targets mainly lymphocytes.
Mean number of red blood cells and leucocytes following treatment with either placebo (n = 42–101. Typically, the lower limit of sample size was n = 63, except week on 55) or a total doses of either 3.5 mg/kg (n = 47–103. Typically, the lower limit of sample size was n = 67, except on week 55) or 5.25 mg/kg (n = 38–104. Typically, the lower limit of sample size was n = 62, except on week 55). Placebo (circle) or cladribine (CLAD) that was administered in monthly courses (inverse triangle) at 0, 5 and 48 and 52 weeks (diamond; 3.5 mg per dose) and additionally at 9 and 13 weeks (hexagon; 5.25 mg per dose). The results show the mean ± SEM of (A) red blood cells (B) platelets (C) white blood cells, (D) lymphocytes, (E) monocytes, (F) polymorphonuclear neutrophils, (G) eosinophils, or (H) basophils.
Full blood count.
Following administration of CLAD, only minor and nonsignificant depletion of platelets and red blood cells occurred. However, there was significant (p < 0.01) depletion of the total leukocyte population within a month of the second cycle of both doses of CLAD (figure 1A). As expected, lymphocytes (figure 1B)2 were markedly depleted over the 96-week observation period (p < 0.01) compared with more subtle influences on monocytes (figure 1C), polymorphonuclear neutrophils (figure 1D),2 eosinophils (figure 1E), and basophils (figure 1F). Following lymphocyte depletion with 2 doses of CLAD, there was further depletion following the use of 2 additional doses (figure 1B).
Lymphocyte phenotyping.
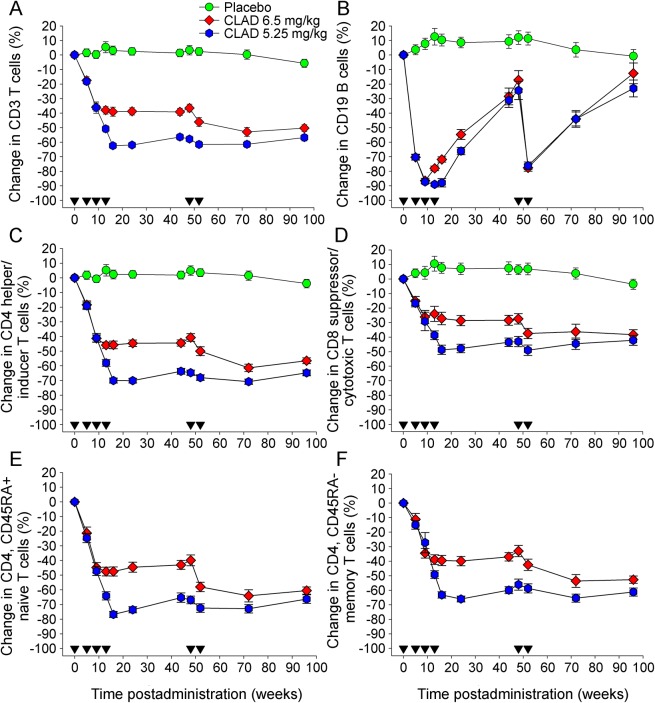
Although reduction of CD3+ T cells showed some dose dependency (figure 2A), depletion of CD19+ B cells was very similar with both dosing schedules (figure 2B). B-cell numbers dropped markedly following the first course of CLAD with a nadir (85%–90% depletion) at the time of the second dose of cycle 1. B cells did not drop further following administration of additional CLAD doses as part of the 5.25 mg/kg schedule and recovered to 30% of baseline prior to the second treatment cycle after 48 weeks, which again led to significant depletion (80% of baseline; figure 2B). In contrast to the B-cell population, a dose-response effect with CD4+ and CD8+ T cells was detected (figure 2, C and D). In the 3.5 mg/kg group, CD4+ T-cell depletion by 20% occurred following the first dose and by 45% following the second dose of cycle 1. This level of depletion was maintained until the second cycle of treatment was given, which led to a maximum depletion by 60% of baseline, over the duration of the study (96 weeks). In the 5.25 mg/kg group, depletion was more pronounced, by 70% of baseline after the first cycle, remaining at this level until the end of the observation period (figure 2C). CD8+ T cells followed a rather similar kinetic as CD4+ T cells, although depletion was overall less pronounced. In the 3.5 mg/kg group, CD8+ T cells were reduced by 30% and 40% of baseline following treatment cycles 1 and 2, respectively. In the 5.25 mg/kg group, CD8+ T cells were reduced by 50% of baseline after the first treatment cycle with no further depletion following the second cycle. Again, similar to CD4+ T cells, CD8+ T cells did not show any significant recovery during the observation period (figure 2D). The two CD4+ T-cell subsets analyzed (naive, i.e., CD45RA+ and memory, i.e., CD45RA−) T cells were both affected and revealed rather similar kinetics of depletion and recovery (figure 2, E and F). In the 3.5 mg/kg group, naive T cells were depleted by 45% and 60% of baseline following treatment cycles 1 and 2, respectively (figure 2E) with reductions in memory T cells being slightly less pronounced (figure 2F). Maximum reduction of naive T cells in the 5.25 mg/kg group was nearly 80% depletion from the baseline within 12 weeks after treatment cycle 1, subsequently remaining at 70% throughout the remainder of the study, whereas memory T cells described a similar curve of depletion and (minor) recovery at 60%–65%. Three months after treatment initiation, naive CD8+ T cells in the 3.5 mg/kg group were reduced by 26.5% and memory CD8+ T cells by less than 10% of baseline levels. No difference in CD3, CD4, CD8, or CD19 lymphocyte counts was detected between the patient cohorts remaining free of relapses and patients who developed at least 1 relapse (figure 3).
Figure 2. Cladribine preferentially depletes B lymphocytes compared with a modest depletion of T cells.
The results represent the mean percentage ± SEM of blood lymphocytes compared with baseline following treatment with either placebo (circle; n = 56–79) or total doses of either 3.5 mg/kg (diamond; n = 62–82) or 5.25/kg (hexagon; n = 66–81) cladribine (CLAD) administered in monthly courses (inverse triangle) at 0, 5 and 48 and 52 weeks (3.5 mg per dose) and additionally at 9 and 13 weeks (5.25 mg per dose). Results show the numbers of (A) CD3+ T cells, (B) CD19+ B cells, (C) CD4+ T cells (D) CD8+ T cells, and (E) CD4+-naive and (F) CD4+ memory T cells.
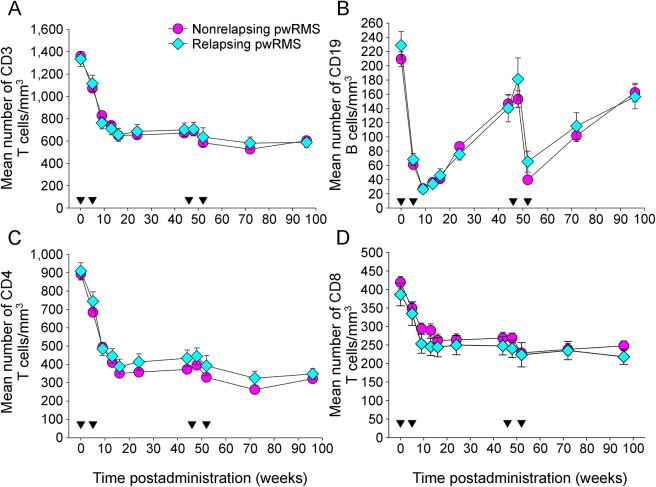
Figure 3. The incidence of relapse does not relate to peripheral blood CD3, CD4, CD8, CD19, T- and B-cell levels.
Cladribine (CLAD) was administered as weekly courses at 0, 5 and 48 and 52 weeks. The results represent the mean ± SEM absolute number (per cubic millimeter) of peripheral blood: (A) CD3, (B) CD19, (C) CD4, and (D) CD8 lymphocytes following treatment (inverse triangles) with oral CLAD in the CLAD Tablets Treating Multiple Sclerosis Orally (CLARITY) trial administered with 3.5 mg/kg CLAD and those divided into groups who remained healthy (circle; n = 121–136 per group) or those who had at least 1 relapse (diamond; n = 29–34). pwRMS = people with relapsing MS.
Comparison of lymphocyte kinetics after CLAD and ALEM administration.
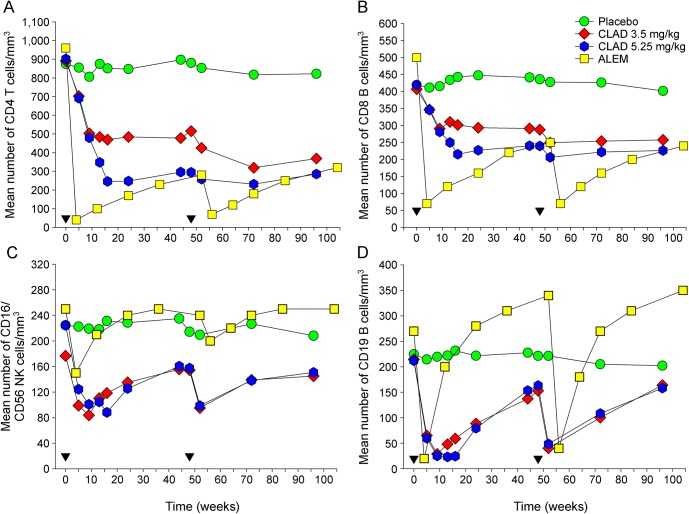
Although the demographics of pwRMS were different between CLARITY2 and CARE-MS I4 studies (table e-1), both CLAD and ALEM exhibited high efficacy.2–5 However, analysis of lymphocyte subsets following CLAD and ALEM treatment revealed several differences (figure 4). (1) One month after the first treatment cycle with ALEM, CD4+ T cells were almost completely (by over 95% of baseline) depleted (figure 4A). Even 1 year after treatment, just before the second treatment cycle, CD4+ T cells remained depleted by over 70% of baseline. The 5.25 mg/kg dose of CLAD led to a reduction of CD4+ T cells by 70% of baseline within 3 months, with very little recovery until the second treatment cycle nearly 9 months later. (2) With the 3.5 mg/kg dose of CLAD, which was clinically as effective as the 5.25 mg/kg dose3,4 (table e-2), depletion of CD4+ T cells during year 1 was much less pronounced than that with ALEM or CLAD 5.25 mg/kg. The gap between the CD4+ T-cell depletion curves induced by the 2 different CLAD dosing schedules only narrowed during year 2, although neither dose ever caused a nadir similar to ALEM (reduction by >90% of baseline). (3) The kinetics of CD8+ T-cell depletion were comparable for both ALEM and CLAD (high and low doses) with their respective CD4+ T-cell behavior (figure 4B). (4) Although ALEM, as well as both doses of CLAD, induced depletion of NK cells, this cell type rapidly recovered following ALEM treatment and was even slightly above baseline at 6 and 12 months. By contrast, both doses of CLAD caused pronounced and virtually identical NK cell depletion, by 47% of baseline, within 9 weeks of treatment cycle 1 (n = 71–72) (figure 4C). (5) The most striking difference became evident, comparing the effect of ALEM and CLAD on the CD19+ B-cell population (figure 4D). Although the level of depletion was initially similar between ALEM and both doses of CLAD, the repopulation characteristics were very different. After CLAD, B cells slowly repopulated, remaining significantly below their baseline level until the second treatment cycle reduced this subset yet again. By contrast, B-cell numbers after ALEM repopulated back to baseline within less than 6 months and then hyperrepopulated well above baseline by 9 and 12 months (figure 4D). Surprisingly, neither of the peer-reviewed phase III trial reports of ALEM in MS provided any indication of the latter but only reported that B cells reach normal levels 6 months after drug administration.4,5
Figure 4. Depletion of lymphocyte subsets following alemtuzumab and cladribine treatment.
Cladribine (CLAD) was administered as weekly courses of CLAD at 0, 5 and 48 and 52 weeks (time of initiation of cycle; inverse triangle) or weekly courses of alemtuzumab (ALEM) at 0 and 52 weeks. The results represent the mean absolute number of peripheral blood lymphocytes (per cubic millimeter) during the CLAD Tablets Treating Multiple Sclerosis Orally (CLARITY) trial in people with relapsing MS (pwRMS) treated with either placebo (circle; n = 68–80), CLAD 3.5 mg/kg (diamond; n = 77–86), CLAD 5.25 mg/kg (hexagon; n = 79–84), and pwRMS in the Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis, study one (CARE-MS I) trial treated with 12 mg/d ALEM (square; n = 171–184).
DISCUSSION
We analyzed the lymphocyte repopulation kinetics following depletion with oral CLAD prodrug and IV ALEM using data sets from their regulatory submissions. Using data mining of these publicly available documents, it was possible to obtain additional information not previously published in peer-reviewed papers. This may provide value for understanding the mechanisms of action and side-effect profiles of these disease-modifying therapies (DMTs), which have focused on the pathogenic role of T cells.23 However, the therapeutic drug–response relationship with CLAD is perhaps more consistent with lasting B-cell depletion of both doses and helps create a focus on the role of the B cells in control of MS, which may or may not act via T cells.24 The data obtained with ALEM are consistent with previous smaller scale studies.10,25
There is unequivocal evidence from the marked reduction of disease following hematopoietic stem-cell treatment that relapsing MS is due to aberrant function of the immune system.26 CLAD induced a modest depletion in T and NK cells, but a more marked depletion of B cells. This was consistent with the known selective lymphocyte-depleting effect because they express high levels of deoxycytidine kinase that phosphorylates CLAD, a deoxyadenosine analog, to cytotoxic 2-chlorodeoxyadenosine triphosphate.8 Although causality cannot be ascertained, the phase III trial data suggest that lymphocyte depletion may contribute to the reduction in disease activity in pwRMS.2,3 CLAD also effectively inhibited the evolution from a clinically isolated syndrome of demyelination to definite MS.13 Most of the ongoing disease activity in that trial occurred within the first 3 months of the study.13 Based on the data presented here, maximum depletion using the dosing schemes used takes between 1 and 3 months. Rebaselining of efficacy data at 3 months after first treatment course would have made the suppression of disease activity even more impressive.13 Furthermore, the slower depletion kinetics of CLAD probably contribute to the lack of administration-related reactions with CLAD that are reported in approximately 90% of pwRMS treated with ALEM.4,5
Although MS has commonly been considered to be a CD4+ T cell–mediated (Th1/Th17) disease,23 a concept supported by the marked (95%) CD4+ T-cell depletion induced by ALEM, CD4+ T-cell depletion in isolation has arguably failed to control MS.24,27 Depletion of CD4+ T cells using a CD4-specific antibody, dosed to maintain CD4+ T-cell numbers above 250 cells/mm3 (equivalent to a depletion of approximately 70%) to limit immunodeficiency thresholds,27 did not effectively suppress the development of new lesions on MRI and was considered to have failed in MS.27 Based on our analysis, the 45%–50% depletion of CD4+ T cells achieved by CLAD 3.5 mg/kg leaves these cells above the threshold required for optimal disease inhibition in both CD4 T cell–mediated experimental autoimmune encephalomyelitis in animals28 and MS.27 Therefore, CD4+ T-cell depletion may not account for the efficacy of this drug, in terms of relapse reduction and its effect on new MRI lesions, given a strong treatment effect of CLAD was already detectable within the first year of treatment.2,3,13
The perceived failure of CD4-depleting monoclonal antibody led some groups to speculate that CD8+ T cells may be the pathogenic drivers of MS as suggested by the predominance of this cell type in MS lesions.29 Again, given the depletion threshold established for CD4 depletion,27,28 we hypothesize that this is unlikely to be a key mechanism of action of CLAD, given CLAD 3.5 mg/kg had only a minor (15%–28%) CD8+ T cell–depleting effect in year 1, when a strong disease-modifying effect was already evident.3,13 However, again, this comes with the proviso that an effective threshold of CD8+ T-cell depletion required for effective immunotherapy in MS has yet to be defined. By contrast, ALEM markedly depletes CD8+ T cells. This, coupled with depletion of other immune subsets, may potentially contribute to the high number of reported infections that occurred in 77% of pwRMS participating in CARE-MS I, compared with only 48% in CLARITY.2,4 In addition, ALEM is more likely to effect CD8+ regulatory/suppressor-cell responses that can control tolerance and potential SAI induction.28
Although it has been shown that the cancer risk in pwRMS on CLAD was no different compared with all contemporaneously licensed DMT,14 NK cells represent an important part of cancer immunosurveillance. As CLAD induced a modest depletion of NK cells, vigilance is, therefore, advised over the intermediate and longer term to truly establish safety, whether or not oral CLAD becomes a licensed DMT for pwRMS, or a generic preparation of CLAD is being used off-label.16,30
CLAD induced a marked and long-lasting CD19+ B-cell depletion that did not reach baseline levels within the 12-month treatment cycle, a repopulation kinetic evidently contrasting with ALEM. Although the capacity of ALEM for hyperrepopulation of B cells has been reported previously,10,20 it was subsequently largely ignored, notably in the phase III trial reports.4,5 We have recently reported that CD8+ T-cell depletion by CD52-specific antibodies can block immunologic tolerance induction,28 suggesting that it could contribute to the rapid hyperrepopulation of B cells that may be the prelude of SAI. Such hyperrepopulation of CD19+ B cells is not a feature of treatment with CLAD or CD20-specific B cell–depleting therapies31 neither of which are associated with B cell–driven autoimmunities2,32 or significant T-cell depletion.31 Following ALEM, the CD19+ B-cell hyperrepopulation into the blood is due to the accumulating immature B cells, probably from the bone marrow, where they differentiate into mature/naive B cells.20 When this occurs in the absence of T-cell regulation, previous studies have shown that B-cell autoreactivity can develop, and this may be a cause of SAI following the use of ALEM in pwRMS.33,34 Previously, it has been shown that the apparent increase in the number of CD19+ B cells, generated by the overproduction of immature/mature B cells following ALEM infusion, masks a long-lasting depletion of CD19+/CD27+ memory B cells,20 which also occurs at varying levels with other agents that control MS,24,31 suggesting that these cells may be important in disease control by DMT.24
However, it must be recognized that there are limitations of solely examining peripheral blood immune subset levels, when it is likely that the pathogenic cells form only a minor population within any single subtype. It is therefore perhaps not be surprising that peripheral blood CD4+, CD8+, and CD19+ cell levels have not demonstrated biomarker activity to predict disease activation following immune reconstitution after CLAD and ALEM treatment,25 suggesting that further functional analysis may be required to define the mechanisms of action. Furthermore, without access to all of the primary data, we were unable to directly compare all the subset analysis performed to clinical efficacy and safety, so their true relationship remains to be established. The lack of peripheral blood biomarker activity may also be due to compartmentalization of autoimmunity outside the peripheral blood. This could be due to the actions of immune cells sequestered within the CNS during MS, which trigger disease activity.24 Alternatively, compartmentalization of the immune response within lymphoid tissues may also be a reason for the inability to detect a biomarker of autoimmune activity within the blood. As such, it is of interest that sequestration of immune cells in lymphoid tissue, such as in the spleen and notably bone marrow, following sphingosine-1-phosphate receptor modulation35 may limit the capacity of ALEM to control MS,36 as ALEM may deplete less effectively in lymphoid tissues compared with the blood.36,37 Alternatively, it is possible that the important pathogenic cells may not yet have been adequately analyzed, as it is likely that they form only a small component within the pool of immune cells. Furthermore, it appears that in addition to any T-cell inhibitory activity,23 most agents that inhibit MS deplete memory B cells.24 Relating levels of memory B cells to therapeutic activity have yet to be performed in MS, but this has been used to personalize retreatment to limit relapse in other autoimmune diseases that are sensitive to CD20-depleting antibodies.38,39 Although it is clear that ALEM markedly depletes memory B cells,20 the memory B cell–depleting capacity of CLAD is unknown. It is tempting to speculate that suppression of MS disease activity by CLAD may relate to long-term depletion of memory B cells. Further work, including functional studies and cytokine analysis, additional to that already known9,17,18 will be required to fully understand the definitive mechanism of action of these treatments, as both qualitative and quantitative changes in lymphocyte subsets will be important in defining their therapeutic activity.9,18
ACKNOWLEDGMENT
This research used MidPlus computational facilities at Queen Mary, supported by QMUL Research-IT and funded by EPSRC grant EP/K000128/1. The authors thank the European Medicines Agency for supplying the trial documents.
GLOSSARY
- ALEM
alemtuzumab
- ANOVA
analysis of variance
- CARE-MS I
Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis, study one
- CLAD
cladribine
- CLARITY
CLAD Tablets Treating Multiple Sclerosis Orally
- DMT
disease-modifying therapy
- FOI
Freedom of Information
- NK
natural killer
portable document format
- pwRMS
people with relapsing MS
- SAI
secondary B-cell autoimmunity
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
Freedom of Information requests: Klaus Schmierer. Concepts: David Baker and Klaus Schmierer. Data extraction: Lukasz Zalewski. Data Analysis: David Baker, Samuel S. Herrod, and Cesar Alvarez Gonzalez. Drafting: David Baker, Samuel S. Herrod, Cesar Alvarez Gonzalez, and Klaus Schmierer.
STUDY FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
DISCLOSURE
None considered relevant; however, D.B. received a grant from Sanofi Genzyme with the past 3 years. K.S. has been involved in trials sponsored by, and received meeting support from, Sanofi Genzyme and received honoraria from Merck KGaA. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Scolding N, Barnes D, Cader S, et al. Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol 2015;1:273–279. [DOI] [PubMed] [Google Scholar]
- 2.Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010;362:416–426. [DOI] [PubMed] [Google Scholar]
- 3.Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol 2011;10:329–337. [DOI] [PubMed] [Google Scholar]
- 4.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380:1819–1828. [DOI] [PubMed] [Google Scholar]
- 5.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829–1839. [DOI] [PubMed] [Google Scholar]
- 6.Tuohy O, Costelloe L, Hill-Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry 2015;86:208–215. [DOI] [PubMed] [Google Scholar]
- 7.Levin SN, Kaplan TB. Infectious complications of novel multiple sclerosis therapies. Curr Infect Dis Rep 2017;19:7. [DOI] [PubMed] [Google Scholar]
- 8.Warnke C, Leussink VI, Goebels N, et al. Cladribine as a therapeutic option in multiple sclerosis. Clin Immunol 2012;142:68–75. [DOI] [PubMed] [Google Scholar]
- 9.Ruck T, Bittner S, Wiendl H, Meuth SG. Alemtuzumab in multiple sclerosis: mechanism of action and beyond. Int J Mol Sci 2015;16:16414–16439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coles AJ, Wing M, Smith S, et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 1999;354:1691–1695. [DOI] [PubMed] [Google Scholar]
- 11.Mitosek-Szewczyk K, Tabarkiewicz J, Wilczynska B, et al. Impact of cladribine therapy on changes in circulating dendritic cell subsets, T cells and B cells in patients with multiple sclerosis. J Neurol Sci 2013;332:35–40. [DOI] [PubMed] [Google Scholar]
- 12.Available at: ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2011/02/WC500102304.pdf. Accessed March 30, 2017.
- 13.Leist TP, Comi G, Cree BA, et al. ; Oral cladribine for early MS (ORACLE MS) Study Group. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol 2014;13:257–267. [DOI] [PubMed] [Google Scholar]
- 14.Pakpoor J, Disanto G, Altmann DR, et al. No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine. Neurol Neuroimmunol Neuroinflamm 2015;2:e158 doi: 10.1212/NXI.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014;83:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beutler B, Sipe JC, Romine JS, et al. The treatment of chronic progressive multiple sclerosis with cladribine. Proc Natl Acad Sci USA 1996;93:1716–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korsen M, Bragado Alonso S, Peix L, Bröker BM, Dressel A. Cladribine exposure results in a sustained modulation of the cytokine response in human peripheral blood mononuclear cells. PLoS One 2015;10:e0129182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman MS, Kaplan JM, Markovic-Plese S. Insights into the mechanisms of the therapeutic efficacy of alemtuzumab in multiple sclerosis. J Clin Cell Immunol 2013;4:1000152. [PMC free article] [PubMed] [Google Scholar]
- 19.Jones JL, Thompson SA, Loh P, et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc Natl Acad Sci USA 2013;110:20200–20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson SA, Jones JL, Cox AL, et al. B-cell reconstitution and BAFF after alemtuzumab (CAMPATH-1H) treatment of multiple sclerosis. J Clin Immunol 2010;30:99–105. [DOI] [PubMed] [Google Scholar]
- 21.Rieckmann P, Comi G, Cook S, et al. Effects of cladribine tablets on peripheral lymphocyte subtypes implicated in multiple sclerosis immunopathogenesis: surface marker analysis for a subset of patients from the 96-week, phase III, double-blind, placebo controlled CLARITY study. Mult Scler J 2009;15(suppl 9):S248–S249. [Google Scholar]
- 22.Hartung HP, Arnold DL, Cohen J, et al. ; CARE-MS I Investigators. Lymphocyte subset dynamics following alemtuzumab treatment in the CARE-MS I study. Mult Scler J 2012;18(suppl 4):427–428. [Google Scholar]
- 23.Martin R, Sospedra M, Rosito M, et al. Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur J Immunol 2016;46:2078–2090. [DOI] [PubMed] [Google Scholar]
- 24.Baker D, Marta M, Pryce G, et al. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine 2017;16:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kousin-Ezewu O, Azzopardi L, Parker RA, et al. Accelerated lymphocyte recovery after alemtuzumab does not predict multiple sclerosis activity. Neurology 2014;82:2158–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkins HL, Bowman M, Allan D, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet 2016;388:576–585. [DOI] [PubMed] [Google Scholar]
- 27.van Oosten BW, Lai M, Hodgkinson S, et al. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial. Neurology 1997;49:351–357. [DOI] [PubMed] [Google Scholar]
- 28.von Kutzleben S, Pryce G, Giovannoni G, et al. Depletion of CD52-positive cells inhibits the development of CNS autoimmune disease, but deletes an immune-tolerance promoting CD8 T cell population: implications for secondary autoimmunity of alemtuzumab in multiple sclerosis. Immunology 2017;150:444–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salou M, Nicol B, Garcia A, Laplaud DA. Involvement of CD8(+) T cells in multiple sclerosis. Front Immunol 2015;6:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez-Gonzalez C, Allen-Philbey K, Mathews J, et al. Treating multiple sclerosis with generic cladribine. Mult Scler J 2016;22(suppl 3):S604–S605. [Google Scholar]
- 31.Palanichamy A, Jahn S, Nickles D, et al. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J Immunol 2014;193:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378:1779–1787. [DOI] [PubMed] [Google Scholar]
- 33.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol 2008;20:632–638. [DOI] [PubMed] [Google Scholar]
- 34.Kinnunen T, Chamberlain N, Morbach H, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood 2013;121:1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol 2012;30:69–94. [DOI] [PubMed] [Google Scholar]
- 36.Willis M, Pearson O, Illes Z, et al. An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2017;4:e320 doi: 10.1212/NXI.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Y, Turner MJ, Shields J, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology 2009;128:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim SH, Kim W, Li XF, et al. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol 2011;68:1412–1420. [DOI] [PubMed] [Google Scholar]
- 39.Trouvin AP, Jacquot S, Grigioni S, et al. Usefulness of monitoring of B cell depletion in rituximab-treated rheumatoid arthritis patients in order to predict clinical relapse: a prospective observational study. Clin Exp Immunol 2015;180:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]