Abstract
Objective:
To increase the detection of MuSK-Abs using a CBA and test their pathogenicity.
Methods:
Sera from 69 MuSK-RIA–positive patients with myasthenia gravis (MG) (Definite MuSK-MG), 169 patients negative for MuSK-RIA and AChR-RIA (seronegative MG, SNMG), 35 healthy individuals (healthy controls, HCs), and 16 NMDA receptor-Ab–positive (NMDAR-Ab) disease controls were tested for binding to MuSK on a CBA using different secondary antibodies.
Results:
Initially, in addition to 18% of SNMG sera, 11% of HC and 19% of NMDAR-Ab sera showed positive binding to MuSK-transfected cells; this low specificity was due to anti-IgG(H+L) detection of IgM bound nonspecifically to MuSK. Using an IgG Fc gamma-specific secondary antibody, MuSK-Abs were detected by CBA in 68/69 (99%) of Definite MuSK-MG, 0/35 HCs, 0/16 NMDAR-Ab, and 14/169 (8%) of SNMG sera, providing increased sensitivity with high specificity. The RIA-negative, CBA-positive MuSK-IgG sera, but not IgM-MuSK–binding sera, reduced agrin-induced AChR clustering in C2C12 myotubes, qualitatively similar to RIA-positive MuSK-Abs.
Conclusions:
An IgG-specific MuSK-CBA can reliably detect IgG MuSK-Abs and increase sensitivity. In the MuSK-CBA, IgG specificity is essential. The positive sera demonstrated pathogenic potential in the in vitro AChR-clustering assay, although less effective than Definite MuSK-MG sera, and the patients had less severe clinical disease. Use of IgG-specific secondary antibodies may improve the results of other antibody tests.
Classification of evidence:
This study provides Class III evidence that an IgG-specific MuSK-CBA identifies patients with MG.
Several methods are available for the detection of antigen-specific antibodies (Abs) in the serum or CSF of patients with autoantibody-mediated CNS and peripheral nervous system diseases.1 In the case of suspected autoimmune myasthenia gravis (MG), sera are routinely tested by radioimmunoprecipitation assays (RIAs) for Abs to AChR or MuSK. However, indirect immunofluorescence on live cells transiently transfected with AChRs and clustered by rapsyn, as they are at the neuromuscular junction, increases the detection of AChR-IgG2–5; CBAs have been found to be sensitive and specific for many antibodies, e.g., for aquaporin-4 (AQP-4) antibodies in patients with neuromyelitis optica.6 Here, to see whether a CBA might increase sensitivity for MuSK-Abs, we established a specific MuSK-Ab assay, tested previously MuSK-Ab–negative patients, and evaluated the pathogenic potential of the antibodies detected.
METHODS
Primary research question.
Can a cell-based assay reliably enhance the detection of MuSK antibodies?
Classification of evidence.
This study provides Class III evidence that an IgG-specific MuSK-CBA identifies patients with MG. The optimized MuSK-CBA had a sensitivity of 99% (95% confidence interval [CI] 92.2–100) and a specificity of 100% (95% CI 93.0–100).
Standard protocol approvals, registrations, and patient consents.
The use of patient sera was approved by the Oxfordshire Research Ethics Committee A (07 Q160X/28).
Patient sera.
All sera had been stored at −20°C sera. During use, aliquots were kept at 4°C to avoid repeated freeze/thaw cycles. Archived sera from MuSK-IgG–positive RIA-definite MG (Definite MuSK-MG; n = 69), MuSK- and AChR-RIA–negative MG (seronegative MG [SNMG]; n = 169), NMDA receptor-Ab–positive (NMDAR-Ab; n = 16), and aquaporin-4-Ab–positive (AQP-4-Ab; n = 10) patients and healthy controls (HCs; n = 35) were studied. Definite MuSK-MG sera were from Italy (Pisa). The SNMG sera were from cohorts (Italy [Rome] = 16, Norway = 50, United Kingdom = 32, and South Africa = 33; Germany = 5, Turkey = 7, Japan = 3, South Korea = 12, and Philippines = 11). All patients had been seen by MG specialists who provided brief clinical features, and supportive features of (1) neurophysiology (evidence of decrement on repetitive nerve stimulation [decrement of fourth CMAP amplitude greater than 10% of baseline value]) and/or neuromuscular jitter on single-fiber EMG, (2) treatment response to cholinesterase inhibitors, and (3) treatment response to immunotherapy in 132/169 (78%) cases.
Tissue culture.
Human embryonic kidney-293 (HEK, derived from the European Collection of Authenticated Cell Cultures) cells were grown in Dulbecco Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum (FCS) (Sigma-Aldrich, St. Louis, MO) and 1% each of Penicillin, Streptomycin, and Amphotericin B (PSA, Invitrogen, Carlsbad, CA) at 37°C in an atmosphere of 5% CO2. C2C12 myoblasts were grown in growth medium (DMEM supplemented with 15% FCS and 1% PSA) and differentiated for 5–6 days in differentiation medium (DMEM supplemented with 2% FCS and 1% PSA) at 37°C in an atmosphere of 8% CO2.
Cell-based assays.
HEK-293 cells were detached using trypsin and centrifuged for 5 minutes at 1,100g. The cells were resuspended, counted, and plated at a density of 2 × 105 cells/well on poly-l-lysine–coated 13-mm glass coverslips in 6-well cell culture plates. For the MuSK-CBA, 3 μg of MuSK-EGFP complementary DNA was transfected into HEK cells. For the clustered AChR (clustered-AChR) assay, cells were transiently cotransfected, using polyethylenimine, with plasmids encoding the 4 subunits of human adult AChR and rapsyn-EFGP (with a total of 3 μg of α:β:δ:ε:rapsyn at 2:1:1:1:1, respectively).2 For the LRP4 CBA, LRP4, covalently linked to the transmembrane and cytosolic domains of CASPR2 (to preserve transmembrane positioning) and low-density lipoprotein receptor–related protein-associated protein 1 pcDNA3.1 were cotransfected with a 5:1 ratio (6 μg). In each case, the medium was changed 16 hours posttransfection.
Twenty-four hours later, coverslips were transferred to a 24-well cell culture plate and incubated with human sera diluted 1:20 in assay buffer (DMEM, 20 mM HEPES, 1% bovine serum albumin) for 1 hour at room temperature (RT). After washing and fixing in 3% formaldehyde, coverslips were washed and incubated with one of the following secondary Abs in assay buffer as appropriate: goat anti-human IgG Alexa Fluor 568 (Invitrogen, binds both heavy and light chains), goat anti-human IgG Fc(γ) (Thermo Fisher Scientific, Waltham, MA), goat anti-human IgM Alexa Fluor 594 (Invitrogen), or IgG subclass–specific anti-human IgG1, IgG2, IgG3, or IgG4 (Sigma-Aldrich). For the IgG subclass and Fc(γ) assays, the secondary Abs were not fluorescently labeled, and a third layer of goat anti-mouse Alexa Fluor 568 was used. After final washing, the coverslips were mounted on mounting media (DakoCytomation, Cambridge, UK) with 1% DAPI (4′,6′-diamidino-2-phenylindole dichloride). Slides were read the following day with an Axion Zeiss–inverted fluorescent microscope and all photographs taken under identical conditions with a MacProbe v4.3 digital image system.
Cell-based assay scoring.
Scoring of clustered AChR, LRP4, and MuSK-CBAs was performed by 2 masked observers as previously described.2 Binding of the red fluorescent–labeled secondary antibodies was scored based on the degree of cell surface fluorescence and colocalization with EFGP-labeled AChRs or MuSK. Complementary DNAs used for the LRP4 CBA did not contain EGFP, and only the cell surface staining was scored. Nonspecific binding was excluded when sera positive for 1 antigen were negative for the 2 other antigens. If sera bound 2 antigens, negativity on the third antigen was used to exclude nonspecific binding to HEK cells. If sera bound 3 antigens, nonspecific binding was assessed using colocalization and preadsorption studies (see below). Mean end-point titers between anti-human IgG Fc(γ) and anti-human IgG(H+L) were determined by identifying the highest dilution at which the serum resulted in a score of 1.
Colocalization.
To confirm specific binding to extracellular MuSK, following incubation with human sera, the coverslips were washed and incubated with commercial polyclonal goat anti-MuSK-Ab AF562 (R&D Systems, Minneapolis, MN) for 1 hour at RT. After washing and fixing in 3% formaldehyde, the coverslips were washed and incubated with goat anti-human IgG Alexa Fluor 488 (green, Invitrogen) and rabbit anti-goat IgG Alexa Fluor 568 (red, Invitrogen). Coverslips were then washed and prepared for analysis as previously described.
Preadsorption.
For preadsorption studies, the highest positive SNMG sera (1:10 dilution) were adsorbed sequentially thrice against 9 × 106 live untransfected HEK cells or MuSK-transfected HEK cells in solution for 1 hour at RT. The adsorbed sample (equivalent to 1:20 preadsorption) was tested by MuSK-CBA to confirm adsorption.
Agrin production and AChR-clustering assays.
MuSK-Abs inhibit agrin-induced AChR clustering, as previously described.7,8 For the production of agrin, a T175 flask of HEK-293 cells was transfected with neural agrin (originally donated by the late Dr. Werner Hoch). After 24 hours, the culture medium was changed, and after 48 hours, the conditioned medium was centrifuged at 1,200g for 10 minutes at RT, aliquoted, and stored at −20°C. To demonstrate inhibition of AChR clustering by the samples, the sera were heat inactivated at 55°C for 30 minutes, dialyzed, and filter sterilized before use. C2C12 myotubes were incubated with patient sera (1:10) for 30 minutes followed by 1:1,000 agrin in differentiation medium for 16 hours. Samples were masked before application. AChR clusters were labeled using Alexa Fluor 594-conjugated α-bungarotoxin (Invitrogen) diluted 1:1,000 in differentiation media for 60 minutes at 37°C, 8% CO2. Myotubes were then washed in differentiation media, fixed in 3% formaldehyde, and washed and stored in phosphate-buffered saline at 4°C. Twenty fields containing myotubes were selected with bright field and red fluorescent images were taken using SimplePCI (Digital Pixel) software. Images were analyzed blind for AChR cluster number using a macro with ImageJ software.
Statistics.
Graphs and statistical analysis were performed with GraphPad Prism version 6.0A and MedCalc 16.4.3. End-point titers were compared using the Wilcoxon matched-pairs signed-rank test. Correlation coefficients were assessed using the Spearman correlation coefficient and an R value. Clinical data were compared using a 2-tailed Fisher exact test and the Mann-Whitney U test.
RESULTS
CBA with anti-human IgG(H+L).
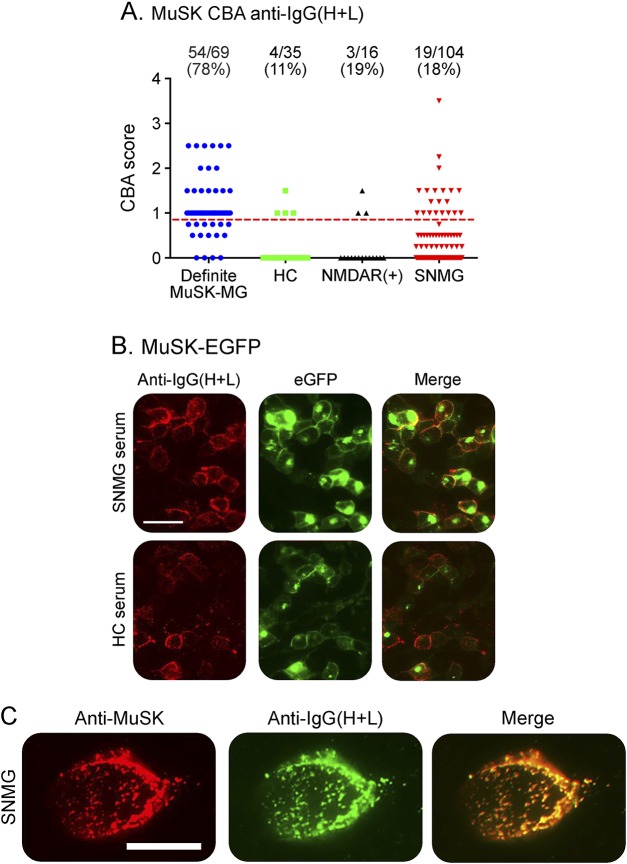
To assess the sensitivity of the MuSK-CBA, we first used a goat anti-human IgG(H+L) Alexa Fluor 568 (Invitrogen) as a secondary antibody to detect binding of IgG antibodies. Surprisingly, with this secondary antibody, only 54/69 Definite MuSK-MG (RIA-positive) patients bound detectably (sensitivity 78.3%). Moreover, the specificity of the assays was poor with 4/35 (11%) HCs and 3/16 NMDAR-Ab sera (19%) demonstrating positive binding (specificity 86.3%). With this assay, MuSK-Abs were also detected in 19/104 SNMG sera (18%), but because of the poor specificity, these initial results were unreliable (figure 1A). Of interest, even with the apparently “false-positive” results, a positive HC serum binding colocalized with MuSK-GFP surface expression (figure 1B), suggesting binding of the patient immunoglobulins to MuSK.
Figure 1. CBA with anti-IgG(H+L).
(A) Scatter plots of results from patients and controls. (B) Representative CBA images from SNMG and HC sera. Scale bar = 50 μM. (C) Colocalization of commercial MuSK-AF562 antibody (red) with anti-IgG(H+L) (green) in an SNMG serum. Scale bar = 20 μM. CBA = cell-based assay; HC = healthy control; MG = myasthenia gravis; SNMG = seronegative MG.
To determine if this was also the case with SNMG sera, a sample that scored 3–4 on the MuSK-CBA was selected for further study. Binding of this serum immunoglobulin colocalized with surface labeling of MuSK using a commercial MuSK-Ab, as expected for an antibody to MuSK (figure 1C). Moreover, this reactivity was adsorbed by incubation with MuSK-transfected HEK cells (but not with untransfected HEK cells) confirming that the binding was specific for MuSK (data not shown).
Subclasses and isotype.
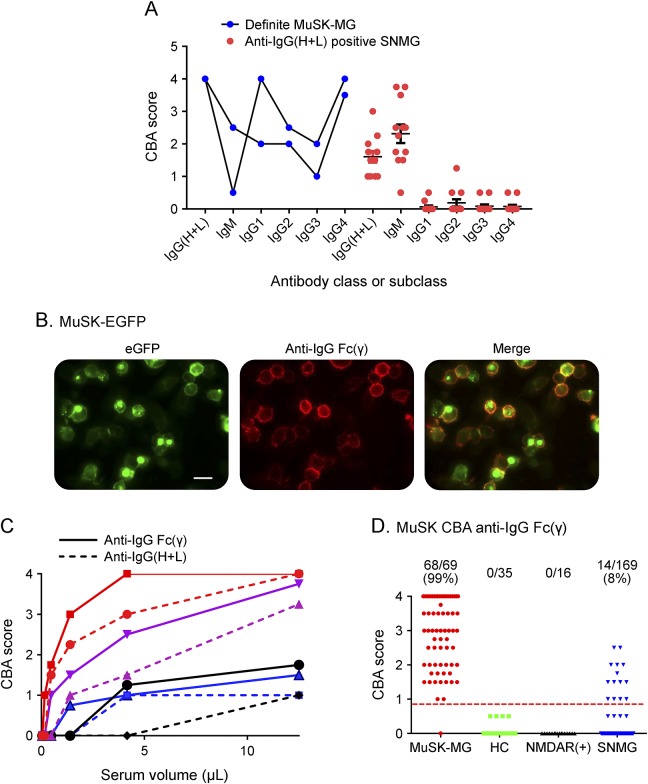
The high proportion and pathogenic potential of MuSK-IgG4 Abs are a recognized feature in MuSK-MG.9 We used subclass and IgM-specific secondary antibodies2,8 to study 12 SNMG sera (of sufficient remaining volume) positive for MuSK-Abs by anti-human IgG(H+L), comparing with 2 Definite MuSK-MG sera. The latter were mainly of IgG class with all subclasses variably represented at the low dilution (1:20) tested (figure 2A). By contrast, 11/12 SNMG sera positive with anti-IgG(H+L) showed strong evidence of IgM binding to MuSK but no IgG antibodies (figure 2A), and only 1 showed low IgG2 subclass binding. Moreover, the CBA scores for IgM binding to MuSK correlated broadly with the results obtained with anti-human IgG(H+L) (R = 0.5, p < 0.0001; data not shown). These results suggested that much of the MuSK-CBA reactivity identified with anti-human IgG(H+L) was due to detection of IgM and not IgG antibodies binding to MuSK.
Figure 2. Improving the CBA with anti-IgG Fc(γ).
(A) Antibodies in 2 Definite MuSK-MG sera were mainly IgG subclasses, particularly IgG1 and IgG4. By contrast, 12 SNMG sera detected with anti-IgG(H+L) were also detected with anti-IgM secondary antibody but not with anti-IgG subclass secondary antibodies. (B) Representative CBA images of a Definite MuSK-MG serum detected with anti-IgG Fc(γ). Scale bar = 50 μM. (C) End-point titrations in 4 Definite MuSK-MG sera were higher with IgG Fc(γ) vs IgG(H+L). (D) Scatter plots of results from the SNMG patients and 51 disease and healthy controls. CBA = cell-based assay; MG = myasthenia gravis; SNMG = seronegative MG.
Optimization of anti-IgG Fc(γ).
The data above confirm previous observations that anti-human IgG(H+L) binds not only to IgG heavy chains but also cross-reacts with different Igs via the light chains which are not Ig class specific.10 To improve the CBA for the detection of IgG-MuSK antibodies, we replaced the anti-human IgG(H+L) secondary antibody with unlabeled goat anti-human IgG Fc(γ) and detected its binding to human antibodies with a tertiary rabbit anti-goat IgG(H+L) Alexa Fluor 568 antibody.
A representative CBA image of a Definite MuSK-MG serum is shown in figure 2B. Four representative Definite MuSK-MG sera demonstrated higher visual scores, with higher end-point dilution titers, with anti-IgG Fc(γ) compared with anti-IgG(H+L) (p = 0.0005 Wilcoxon matched-pairs signed-rank test) (figure 2C). With this improved IgG-specific method, we found IgG MuSK-Abs in 68/69 (sensitivity 99% [95% CI 92.2–100]) of the Definite MuSK-MG patients (the one negative sample [MuSK-RIA titer 0.91 nM] bound nonspecifically to HEK cells by CBA and because of limited volume for further examination was designated negative). Moreover, the 35 HC sera and 16 NMDAR-antibody–positive sera were negative for MuSK-IgG, demonstrating 100% specificity. We subsequently tested 10 AQP4-antibody–positive sera that were also negative. Finally, 14/169 (8%) SNMG, including 6 of the 19 that were positive with anti-IgG(H+L), were positive for IgG-specific MuSK-Abs (figure 2D).
In addition, 18/169 (11%) sera were positive for clustered AChR-Abs, 1/169 for LRP4-Abs, and 1/169 for both clustered AChR-Abs and LRP4-Abs. A flowchart summarizing all results of CBA testing with anti-IgG(H+L) or anti-IgG Fc(γ) is shown in figure e-1 at Neurology.org/nn.
Functional relevance of IgG and IgM-MuSK antibodies.
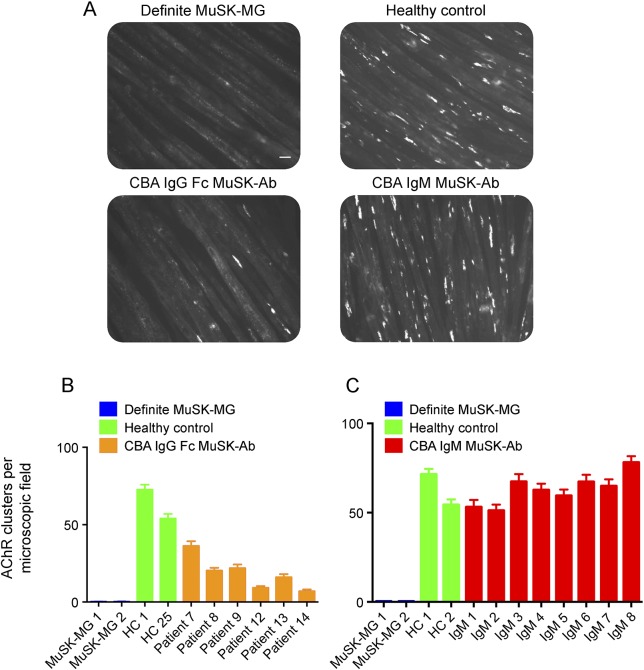
MuSK-MG sera inhibit the agrin/MuSK/LRP4/DOK7 pathway (figure 3A).7,8,11 Six SNMG sera with IgG-specific MuSK-Abs (figure 3B), but not 8 with only IgM nonspecific MuSK-Abs (figure 3C), reduced the number of agrin-induced AChR clusters on C2C12 myotubes, suggesting that only the IgG-specific Abs had pathogenic potential. Nevertheless, they were clearly less effective than the 2 Definite MuSK-MG samples tested in parallel that abolished AChR clusters (figure 3, A–C, figure e-1).
Figure 3. Functional effects of MuSK-IgG and IgM on agrin-LRP4-MuSK–clustering pathway.
(A) Representative images of myotubes, fluorescent labeled for AChR clusters in the presence of different sera. Definite MuSK-MG and CBA MuSK-IgG–positive sera reduced the number of AChR clusters, but CBA MuSK-IgM sera did not reduce clusters. Scale bar = 50 μM. (B) Mean results from 2 experiments of CBA MuSK-IgG and control sera on AChR clusters. (C) Mean results of 2 experiments with CBA MuSK-IgM–positive and control sera. Values shown are mean + SEM. HC = healthy control; MG = myasthenia gravis.
Comparison of MuSK-CBA and RIA phenotypes.
The country of origin and individual clinical data of MuSK-Ab CBA–positive (RIA-negative) patients are summarized in table 1. Patients were predominantly female (3.3:1) and presented at a median age of 25 years (range 16–79 years). At presentation to the neurologists, 8 patients had disease confined to the ocular muscles (median duration of follow-up 2 years [range 1–4.5 years]), and Myasthenia Gravis Foundation of America (MGFA) grades were ≤2 in 11/13 (85%) patients. Patients were treated with therapies including acetylcholinesterase inhibitors (AChEIs) only, or combinations with prednisone, azathioprine, cyclophosphamide, or rituximab. Postintervention status suggested a favorable response to immunotherapy in all patients, and remission was achieved in 3/11 (27%) patients.
Table 1.
Clinical data from 13 patients only positive on IgG-specific MuSK-CBA
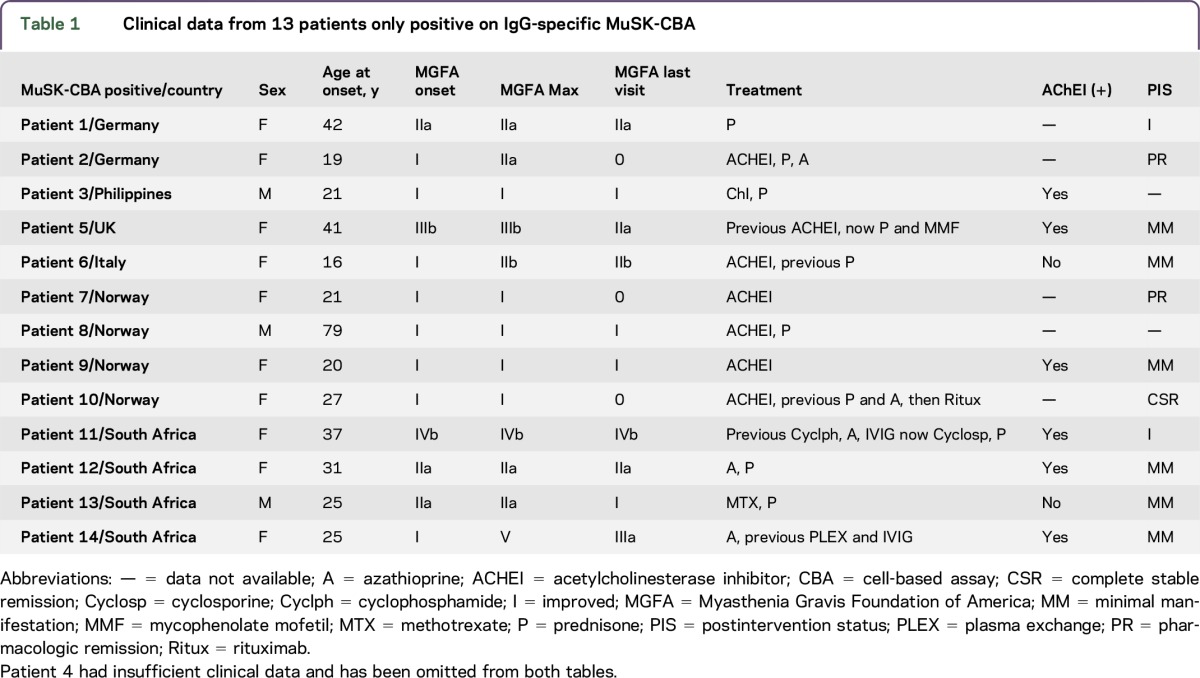
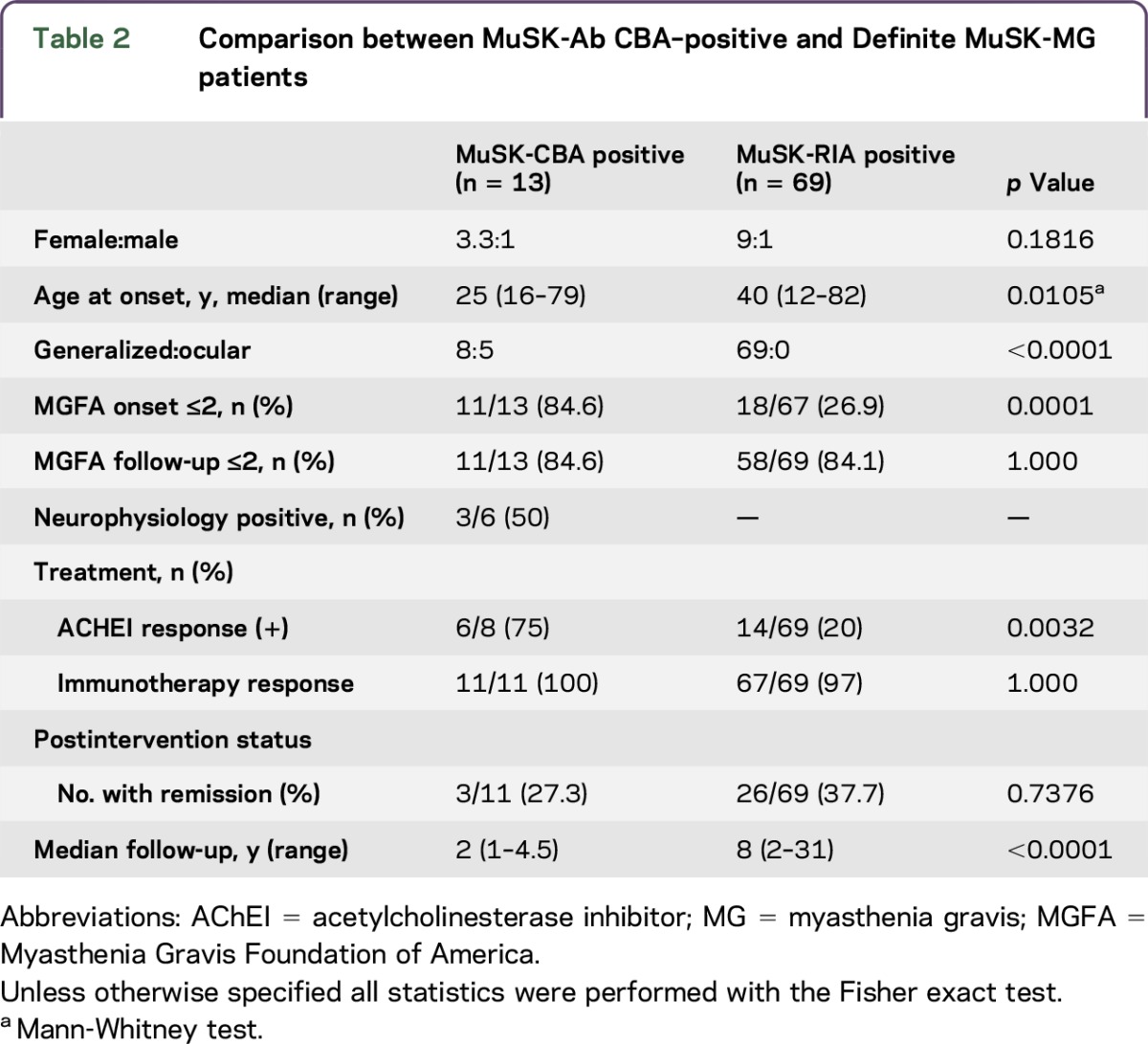
Table 2 compares the clinical features of MuSK-Ab CBA positive with Definite MuSK-MG patients. A female preponderance was noted in both groups (3.3:1 vs 9:1; p = 0.18), but the median age at onset was later in Definite MuSK-MG patients (40 years [range 12–82 years] compared with 25 years [range 16–79 years]; p = 0.01). Generalized MG was reported in all 69 Definite MuSK-MG patients, compared with 8/13 of MuSK-CBA–positive patients (p < 0.0001), with only 18/67 (27%) MGFA ≤2 at onset (p = 0.0001). Neurophysiology was only positive in 3/6 examined MuSK-CBA patients. Reflecting the relatively mild phenotypes, ACHEI was effective in 75% of MuSK-CBA patients, compared with only 20% of Definite MuSK-MG patients, but it is important that immunotherapies appeared equally effective in both groups, and final disease status did not differ, although follow-up times were clearly different.
Table 2.
Comparison between MuSK-Ab CBA–positive and Definite MuSK-MG patients
DISCUSSION
Cell-based assays are now commercially available for the detection of some CNS antibodies but have not yet been developed widely for the diagnosis of MG. Antibodies binding to clustered AChRs on CBAs have improved the diagnosis of a proportion of previously AChR-Ab–negative patients, and CBAs for LRP4 antibodies are being used by several research laboratories,2,4,12 many using anti-human IgG(H+L) to detect the antibodies Here, we found it necessary to optimize a CBA for MuSK-Abs to avoid detecting bound IgM which gave poor disease specificity. Using an unlabeled secondary antibody specific for the Fc(γ) chain of IgG, and applying a tertiary fluorescent antibody for detection, we increased MuSK-Ab detection in Definite MuSK-MG patients, while avoiding the detection of the nonspecific IgM bound to MuSK, which had initially occurred in 11% and 19% of healthy and disease control samples, respectively. Overall, the IgG-specific MuSK-CBA detected antibodies in 8% of previously negative MG samples, and the clinical relevance of these MuSK-IgG antibodies was demonstrated by inhibition of the agrin-LRP4-MuSK–clustering pathway, which did not occur in the presence of the samples that only contained IgM-MuSK reactivity. Clustered AChR antibodies were detected in another 11% of patients, but only 1 additional patient had LRP4 antibodies detected by the same IgG-specific approach.
The patients with MuSK-CBA antibodies were mainly females but younger than the Definite MuSK-MG group (positive by RIA) and had milder disease. After a median follow-up of 2 years, within which the risk of secondary generalization is highest,13 ocular MG still compromised 39% of these patients. All patients treated responded to immunotherapy, but a beneficial response was also seen in 75% of patients treated with AChEIs, either alone or in combination with other therapies. By contrast, 80% of the Definite MuSK-MG cohort responded poorly or with cholinergic side effects in keeping with previous reports.14 MuSK antibodies by RIA are often associated with a particularly severe bulbar form of MG,15 but it is well recognized that not all patients have severe disease, and it appears that the MuSK-CBA–positive patients are within the mild spectrum.
The IgM bound to MuSK in SNMG sera was specific for MuSK by colocalization and preadsorption experiments. However, the MuSK-IgM sera tested did not inhibit the formation of agrin-induced clusters. Although this does not exclude the possibility that MuSK-IgM may act on MuSK function in vivo, the lack of disease specificity argues against it. IgM is normally in the form of pentamers with up to ten binding sites. One possible explanation for IgM detection in these assays could be the nonantigen-specific binding of IgM pentamers to certain types of protein expressed at high levels on cells. Since similar problems have been found with myelin oligodendroglial antibodies,10 it may be due to the extracellular immunoglobulin-like domains and/or glycosylation state of these particular proteins and suggests that caution should be used regarding the use of anti-IgG(H+L) for the detection of IgG binding to similar antigens. Indeed, the Oxford laboratory now screens all diagnostic sera with anti-IgG(H+L) and then confirms IgG with the IgG-specific secondary and fluorescence-labeled tertiary antibody. This approach both improves specificity and the use of a tertiary layer increases a little the fluorescent signal obtained.
A large multinational study reported the detection of MuSK-Abs in 13% (83/633) of seronegative patients with MG using a CBA.12 Further testing of 25 sera (positive by MuSK-CBA) showed that all contained MuSK-IgM, but of these, only 2/25 (8%) also contained MuSK-IgG Abs, very similar to our results; that study may also have benefitted using a more IgG-specific secondary antibody. MuSK-Abs have also been reported in SNMG cohorts from Sri Lanka (3/10; 10%) and China (3/8; 38%), but in both these previous studies, an anti-human IgG(H+L) antibody was used.16,17 The results, therefore, may need to be reassessed.
This study has limitations. First, archived sera stored at −20°C may have degraded over time and with prior freeze-thaw cycles, and many samples were obtained after immunotherapies. Second, because of limited volumes of sera, experiments to determine the functional relevance of MuSK-IgG antibodies by CBA were prioritized over the determination of IgG subclass. It would be interesting in future studies to determine if these antibodies are predominantly of the IgG4 subclass and capable of inhibiting the coimmunoprecipitation of MuSK and LRP4 as shown for Definite MuSK-MG samples.8 Ultimately, only auditing the results of routine diagnostic service testing with the improved assay will demonstrate unequivocally the clinical utility of this IgG-specific MuSK-CBA.
With characteristic clinical features, a diagnosis of SNMG may be relatively straightforward despite reduced neurophysiologic sensitivity, as for instance in ocular MG. By contrast, clinically “ambiguous” cases with unsupportive neurophysiology may be due to MG or an entirely different pathology. In these instances, the absence or detection of an antibody can have important implications for the diagnostic process and management. We suggest that in addition to the established clustered AChR and LRP4 CBAs, which are beginning to be used more widely, RIA-negative MG sera should be tested for IgG-specific antibodies by MuSK-CBA. Confirmation of positive results with an IgG-specific test, as described here, may also improve the sensitivity and specificity of other antibody assays.
ACKNOWLEDGMENT
The authors thank Dr. Jonathan Cheung for developing the ImageJ macro used for automated counting of AChR clusters. They also thank Dr. Arthur Melms, Dr. Feza Deymeer, Dr. Motomura Masa, Dr. Sung Jung-Joon, and Dr. Raymond Rosales for providing some of the SNMG sera that were used in this study.
GLOSSARY
- AChEI
acetylcholinesterase inhibitor
- CBA
cell-based assay
- CI
confidence interval
- DMEM
Dulbecco Modified Eagle Medium
- FCS
fetal calf serum
- HC
healthy control
- HEK
human embryonic kidney
- MG
myasthenia gravis
- MGFA
Myasthenia Gravis Foundation of America
- PSA
Penicillin, Streptomycin, and Amphotericin
- RT
room temperature
- SNMG
seronegative MG
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
Saif Huda: drafting/revising the manuscript, study concept and design, acquisition of data, statistical analysis, and interpretation of data. Patrick Waters: drafting/revising manuscript, study concept and design, and interpretation of data. Mark Woodhall: drafting/revising the manuscript, study concept and design, and acquisition of data. Maria Isabel Leite, Leslie Jacobson, Anna De Rosa, Michelangelo Maestri, Roberta Ricciardi, Jeannine M. Heckmann, Angelina Maniaol, Amelia Evoli, Judy Cossins, and David Hilton-Jones: drafting/revising the manuscript and acquisition of data. Angela Vincent: drafting/revising the manuscript, study concept and design, analysis and interpretation of data, and contribution of vital reagents/tools/patients and study supervision. All authors provided final approval of the version to be published and took responsibility for the conduct of the research.
STUDY FUNDING
S.H. was supported by a Watney Trust/MGA/NIHR Oxford Biomedical Research Centre Fellowship. Additional support was provided by the NIHR Oxford Biomedical Research Centre (A.V.), the Medical Research Council (J.C.), and the Muscular Dystrophy Campaign (J.C.).
DISCLOSURE
S. Huda served on the scientific advisory board for Medefield. P. Waters received honoraria from Biogen Idec Japan, Euroimmun AG, and Mereo Biopharma; holds a patent for and receives royalties from assays for the detection of antibodies to LGI1, CASPR2, Contactin2 and GABAAR; his laboratory runs routine diagnostic assays, one of which is for GABAAR antibodies; and received research support from Euroimmun AG. M. Woodhall reports no disclosures. M.I. Leite received travel funding and speaker honoraria from Biogen Idec; received a travel grant from Novartis; served on the editorial board for Neuromuscular Disorders; and received support from NHS National Specialised Commissioning Group for Neuromyelitis Optica UK and NIHR Oxford Biomedical Research Centre. L. Jacobson, A. De Rosa, M. Maestri, and R. Ricciardi report no disclosures. J.M. Heckmann received research support from South Africa Medical Research Council and AFM Telethon. A. Maniaol received research support from The Norwegian Association of Muscle Diseases. A. Evoli served as a scientific award jury member for Grifols. J. Cossins reports no disclosures. D. Hilton-Jones served on the editorial board for Neuromuscular Disorders, Practical Neurology and receives royalties from Churchill Livingstone and Cambridge University Press. A. Vincent received travel funding and speaker honoraria from Bial Commercial; served as an associate editor for Brain, editorial board member for Neurology®; holds a patent with Oxford University for MuSK antibodies and for LGI1/CASPR2 antibodies; received royalties from Blackwell Publishing, Mac Keith Press, and Elsevier; consulted for Athena Diagnostics; and has received research support from Athena Diagnostics, Euroimmun AG, and NIHR. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Waters P, Pettinghill P, Lang B. Detection methods for neural antibodies. In: Pittock S, Vincent A, editors. Autoimmune Neurology. Amsterdam: Elsevier; 2016:147–163. [DOI] [PubMed] [Google Scholar]
- 2.Leite MI, Jacob S, Viegas S, et al. IgG1 antibodies to acetylcholine receptors in “seronegative” myasthenia gravis. Brain 2008;13:1940–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacob S, Viegas S, Leite MI, et al. Presence and pathogenic relevance of antibodies to clustered acetylcholine receptor in ocular and generalised myasthenia gravis. Arch Neurol 2012;69:994–1001. [DOI] [PubMed] [Google Scholar]
- 4.Devic P, Petiot P, Simonet T, et al. Antibodies to clustered acetylcholine receptor: expanding the phenotype. Eur J Neurol 2014;21:130–134. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez Cruz PM, Al-Hajjar M, Huda S, et al. Clinical features and diagnostic usefulness of antibodies to clustered acetylcholine receptors in the diagnosis of seronegative myasthenia gravis. JAMA Neurol 2015;72:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waters P, Riendl M, Saiz A, et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry 2015;87:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 2001;7:365–368. [DOI] [PubMed] [Google Scholar]
- 8.Koneczny I, Cossins J, Waters P, Beeson D, Vincent A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1-3 can disperse preformed agrin-independent AChR clusters. PLoS One 2013;8:e80695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klooster R, Plomp JJ, Huijbers MG, et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain 2012;135:1081–1101. [DOI] [PubMed] [Google Scholar]
- 10.Waters P, Woodhall M, O'Connor KC, et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm 2015;2:e89 doi: 10.1212/NXI.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huijbers MG, Zhang W, Klooster R, et al. MuSK IgG4 antibodies cause myasthenia gravis by inhibiting binding between MuSK and LRP4. Proc Natl Acad Sci USA 2013;110:20783–20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsonis AI, Zisimopoulou P, Lazaridis K, et al. MuSK autoantibodies in myasthenia gravis detected by cell based assay—a multinational study. J Neuroimmunol 2015;284:10–17. [DOI] [PubMed] [Google Scholar]
- 13.Bever CT, Aquino AV, Penn AS, Lovelace RE, Rowland LP. Prognosis of ocular myasthenia. Ann Neurol 1983;14:516–519. [DOI] [PubMed] [Google Scholar]
- 14.Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve 2011;44:36–40. [DOI] [PubMed] [Google Scholar]
- 15.Evoli A, Tonali PA, Monaco ML, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 2003;126:2304–2311. [DOI] [PubMed] [Google Scholar]
- 16.Chang T, Leite MI, Senanayake S, et al. Clinical and serological study of myasthenia gravis using both radioimmunoprecipitation and cell-based assays in a South Asian population. J Neurol Sci 2014;343:82–87. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Maxwell S, Leite MI, et al. Non-radioactive serological diagnosis of myasthenia gravis and clinical features of patients from Tianjin, China. J Neurol Sci 2011;301:71–76. [DOI] [PubMed] [Google Scholar]