Abstract
A 1.5-year-old dog was evaluated for abnormal mentation, collapse, and weight loss. Radiographs and ultrasonographs revealed soft tissue masses in the mid abdomen. Ultrasound-guided fine-needle aspirates provided a diagnosis of malignant epithelial or round cell neoplasia. Histopathologic and immunohistochemical findings on the tumors were consistent with a primitive neuroblastoma.
Abstract
Résumé — Neuroblastome chez un jeune chien. Un jeune chien de 18 mois a été examiné pour des problèmes d’inconscience, de collapsus et de perte de poids. Plusieurs masses furent observées dans l’abdomen lors des examens échographique et radiographique. Un diagnostic cytologique de tumeur maligne de type épithélial ou à cellules rondes fut posé à la suite de biopsies réalisées à l’aiguille fine échoguidée. L’histopathologie et l’immunohistochimie confirmèrent un diagnostic de neuroblastome primitif.
(Traduit par les auteurs)
A 1.5-year-old, spayed female, English setter was referred to the Veterinary Teaching Hospital (VTH) at the Atlantic Veterinary College with abnormal mentation, collapse, anorexia, and weight loss. Nineteen days prior to presentation, the owners had introduced a new dog into the home. At that time, the patient became anorexic and lethargic, but a physical examination and complete blood (cell) count (CBC) were unremarkable. The dog had received routine vaccinations against distemper, parvovirus, parainfluenza virus, adenovirus type 2, and rabies 9 mo prior to presentation. There was no history of trauma or travel outside Atlantic Canada. Initially, the clinical signs were presumed to be due to disruption in the household by the new dog. Amitriptyline (Elavil; Merck Frosst, Pointe-Claire, Quebec), 1.5 mg/kg bodyweight (BW), PO, q24h was prescribed to relieve anxiety; the new dog was removed from the household.
One week prior to referral, the dog had shown no improvement and had experienced an episode of collapse at home without loss of consciousness. At that time, the referring veterinarian reexamined the dog and found that it was now showing signs of disorientation and dehydration. No significant abnormalities were seen on a serum biochemical profile. An adrenocorticotropic hormone (ACTH) stimulation test revealed a baseline cortisol concentration of 40 nmol/L (reference range: 14 to 180 nmol/L) and a 2-hour post-ACTH cortisol concentration of 166 nmol/L (reference range: 231 to 571 nmol/L). Fluids (Lactated Ringer’s Injection; Baxter, Toronto, Ontario) and 0.5 mg/kg BW of dexamethasone sodium phosphate (Dexamethasone 5; Vetoquinol, Lavaltrie, Quebec) were administered, IV. The patient showed some improvement and was discharged from the referring veterinarian’s hospital on prednisone therapy (type unspecified), 0.5 mg/kg BW, PO, q24h. By 3 d prior to referral, the dog had deteriorated neurologically, with head pressing, circling to either the left or right, and persistent anorexia and weight loss.
Upon presentation at the VTH, the dog was emaciated (body condition score of 1/5), 5% to 7% dehydrated, and with a rectal temperature of 40.0°C. The heart rate was 100 beats per minute, with strong and regular pulses. The mucous membranes were tacky but pink in color, with a capillary refill time of 2 s. The peripheral lymph nodes were of normal size. The cranial and spinal nerve reflexes were normal. The dog displayed abnormal mentation with head pressing, pacing, circling to the left or right, and, occasionally, falling to the floor. A large, firm mass was palpated on the midline of the cranial abdomen. The mass was approximately 10 cm in diameter and nonpainful.
A CBC revealed a mild normocytic, normochromic, nonregenerative anemia (hematocrit 0.31 L/L; reference range: 0.37 to 0.55 L/L). A serum biochemical profile showed no significant findings, except for a moderate decrease in urea concentration (1.7 mmol/L; reference range: 3.0 to 10.5 L/L). Urinalysis, fasting bile acid, and ammonia concentrations were within normal limits. Intravenous fluids (Lactated Ringer’s Injection; Baxter) were administered at 148 mL/kg BW/d for 6 h and, thereafter, at 82 mL/kg BW/d to maintain hydration. Abdominal radiographs revealed a soft tissue opacity in the central abdomen that displaced the intestines ventrally. An abdominal ultrasonograph revealed several mass structures with mixed echogenicity within the liver parenchyma. Either a single, multilobulated soft tissue mass, approximately 10 cm diameter, or multiple soft tissue masses clumped together were seen in the midabdomen. A thrombus or mass was observed in the caudal vena cava, cranial to the renal veins.
Based on the history, physical examination, initial blood analysis, urinalysis, and abdominal diagnostic imaging, the problems identified were the following: anorexia, dehydration, fever, abnormal mentation, abdominal mass, multiple hepatic masses, and caudal vena caval thrombosis. The anorexia was likely secondary to the underlying disease process or processes. The dehydration was likely due to inadequate fluid intake associated with anorexia. Differential diagnoses for the fever included neoplasia, a hypothalamic lesion, infectious disease, and immune-mediated disease. The differential diagnoses for abnormal mentation included intracranial neoplasia (either primary or metastatic), intracranial infectious disease, granulomatous meningo-encephalitis, intracranial infectious disease, cerebral vascular infarction, and hepatic encephalopathy. The latter was considered unlikely as the serum bile acids and ammonia concentrations were normal. The abdominal mass may have represented neoplasia of the liver, pancreas, intra-abdominal lymph nodes, adrenal gland, or intestines (either primary or metastatic); abcess; or granuloma. The differential diagnoses for the multiple hepatic masses included metastatic neoplasia and regenerative nodules. The differential diagnoses for the caudal vena caval thrombosis included neoplastic invasion of the vena cava; thrombosis secondary to compression of the vena cava from the adjacent mass; or an underlying prothrombotic condition, such as disseminated intra-vascular coagulation.
Ultrasound-guided fine needle aspirations of the liver and soft tissue masses were performed and revealed a population of round to oval cells, both singly and in large clusters. Individual cells were predominantly round cells, while the cells in clusters were more typically epithelioid and showed a mild degree of anisocytosis and anisokaryosis with a high nuclear to cytoplasmic ratio. The cytoplasm was basophilic with occasionally very fine pink stippling. Nuclei were round to oval with a reticular chromatin pattern and 1 to 4 small nucleoli (Figure 1). A cytologic diagnosis of malignant neoplasia, either epithelial or round cell, was made. There was no change in the patient’s neurological status in response to fluid therapy. Due to the poor long-term prognosis, the patient was euthanized with an overdose of 176 mg/kg BW pentobarbital (Euthanyl; Bimeda-MTC, Cambridge, Ontario), IV.
Figure 1.
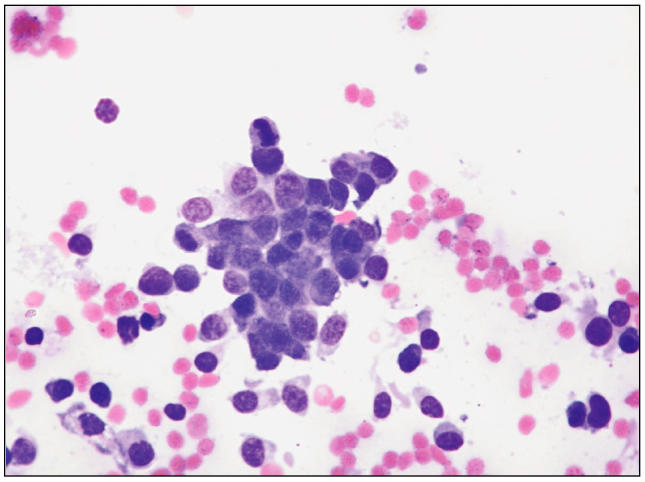
Photomicrograph of fine-needle aspirate of abdominal mass showing a cluster of epithelioid cells with individual round cells. Modified Wright-Giemsa. 400×.
At necropsy, the dog was in very poor body condition. A 12-cm diameter, pale grey-white, multilobulated mass was present in the abdominal cavity cranial and adherent to the left kidney, leaving the renal capsule intact. The mass had partially occluded the caudal vena cava and completely obliterated the left adrenal gland, which could not be identified. The right adrenal gland had several metastatic tumor nodules, less than 0.5-mm diameter. Several 0.5- to 4.0-cm diameter nodular masses were present in the left kidney and liver. In addition, a dark, oval, friable 1- to 2-cm mass was noted in the cranioventral brain stem, obliterating the pituitary gland and extending into the hypothalamus. Based on gross postmortem examination, the differential diagnoses for an adrenal tumor included primary adrenal tumors, such as adrenal cortical carcinoma, pheochromocytoma, ganglioneuroma, and primitive neuroendocrine tumors (PNET), and secondary tumors with metastases to the adrenal gland, such as malignant lymphoma, rhabdomyosarcoma, undifferentiated carcinomas, and other metastatic tumors.
On microscopic examination, the masses within the various organs (including a 2-mm diameter tumor in the lung, which was not observed on gross examination) were of similar morphology. They were irregularly dissected by thick fibrous trabeculae and scant fibrous stroma into variably well defined cords, nests, and packets. Occasional perivascular pseudorosettes were observed; however, Homer-Wright type rosettes were not found. The neoplastic cells were round to polygonal with small amounts of poorly delineated acidophilic cytoplasm and round to ovoid single nuclei with granular chromatin and occasional small single nucleoli. Up to 3 mitotic figures were observed in 10 random high power fields (Figure 2). Thrombi containing aggregates of these neoplastic cells were present within some of the blood vessels of the abdominal mass.
Figure 2.

Photomicrograph of abdominal mass showing small round to slightly spindle shaped cells with hyperchromatic nuclei. Hematoxylin and eosin. Bar = 50 μm.
Based on histopathologic findings, a tentative diagnosis of neuroblastoma, arising in adrenal medulla and with metastasis to liver and pituitary, invasion of the adrenal veins, and partial occlusion of the caudal vena cava, was made. On immunohistochemical staining, tumor cells stained positively for synaptophysin and were negative for neuron specific enolase (NSE), neurofilament protein, and chromogranin A and B. On special histochemical staining with Fontana-Mason, Schmorl’s, modified Giemsa, Luxol fast blue, and Glees and Marsland, the tumor was negative. Since neuroblastomas are usually positive for neuroendocrine cell markers, such as chromogranin A, NSE, and synaptophysin, the lack of staining for NSE and chromogranin A supported a diagnosis of primitive neuroblastoma with more variable immunohistochemical staining (1,2).
Neuroblastomas are one of the most frequently diagnosed neoplasms of children and infants (3), although they are rare in domestic animals. In the dog, they have been reported in several anatomical sites, usually in dogs <3 y of age (1,2,4–8).
In this case, characterization of the tumor was challenging. An adrenal tumor was suspected, as the left adrenal gland could not be identified and the mass was located in this area. Given the young age of the dog, the clinical signs, and the subnormal results of an ACTH stimulation test, a medullary tumor was initially considered more likely than a functional cortical neoplasia. The severe neurological signs were likely related to compression of the cranioventral brain stem by the mass. The primary mass was also assumed to have been partially obliterating the adrenal cortex, which was reflected by the subnormal results of the ACTH stimulation test.
The adrenal medulla is a complex organ derived from neuroectoderm of the neural crest and is populated by neuroendocrine cells. These cells consist of chromaffin cells and sympathetic ganglion cells with glial cell support. Neoplasms deriving from neuroectodermal cells are classified according to the degree of differentiation of the transformed cells. Pheochromocytoma, which is a chromaffin cell neoplasm and the most common adrenal medullary tumor (9,10), was a major rule out in this case. However, other tumors that arise from the neuroectoderm, such as neuroblastoma, which presumably derive from surviving primitive sympathetic ganglion cells (embryonal remnants), and ganglioneuroma, in which mature sympathetic ganglion cells and neurofibrils predominate (9,10), also had to be considered.
Adrenal tumors can be challenging to diagnose antemortem and are more commonly incidental findings at necropsy (10,11). This dog was presented to the referring veterinarian with vague clinical signs of anorexia and lethargy, which were attributed to the disruption in the household by a new dog. Later, the dog developed clinical signs that were likely related to space-occupying effects of the adrenal mass and its metastases.
In human medicine, it is recognized that neuroblastomas, like pheochromocytomas, may produce catecholamines (12). Therefore, clinical diagnosis of a medullary adrenal tumor could perhaps have been aided by measuring plasma or urinary catecholamine concentrations, or their metabolites, such as metanephrine, normetanephrine, and vanillyl mandelic acid. However, use of these tests in veterinary medicine is limited due to handling difficulties, the expense involved, and insufficient information about reference ranges in dogs and other domestic species (13,14). Moreover, based on histopathological examination, there were no lesions of systemic hypertension, such as arteriolar sclerosis, medial hyperplasia of blood vessels, or glomerulosclerosis, to support possible catecholamine secretion by the tumor.
If an adrenal medullary tumor is suspected, further differentiation can pose a problem. Pheochromocytomas and neuroblastomas share many general characteristics; both tumors can be discovered incidentally or can result in similar vague clinical signs related to space-occupying effects or release of catecholamines. Both can attain considerable size and invade adjacent structures. Neuroblastomas, however, usually occur in young dogs (2,10) and are rapidly growing, while pheochromocytomas occur more frequently in middle-aged to older dogs (10) and generally grow less rapidly (11).
Pheochromocytomas and neuroblastomas are also difficult to differentiate on the basis of routine cytologic examination or hematoxylin and eosin-stained sections. Identification often requires special immunohistochemical and electron microscopic investigations. Histologically, neuroblastomas are very infiltrative with prominent blood vessels and are characterized by small round neoplastic cells arranged around a tangle of neuronal processes to form pseudorosettes; these are some of the distinct microscopic features of neuroblastomas not seen in pheochromocytomas (10). Neuroblastomas should also be discriminated from other “small round cell tumors,” such as rhabdomyosarcoma, lymphoma, PNET, and Ewing’s sarcoma (8). Immunohistochemical and genetic markers are employed routinely in humans to differentiate these conditions (3).
In humans and dogs, both primitive and differentiated neuroectodermal tumors, including pheochromocytomas, show variable positive staining for synaptophysin, chromogranin A, neurofilament, and neuron specific enolase. Therefore, although this diagnostic modality may assist in differentiating cortical (which are positive for cytokeratin [16] and lack staining for the medullary markers) from medullary neoplasia, it is less helpful for differentiating the variable tumors derived from the medulla. However, in humans, although the frequency of positive staining is similar for pheochromocytomas and neuroblastomas, pattern and intensity was found to differ for several markers, such as chromogranin and synaptophysin (17,18).
Cytological evaluation of adrenal medullary tumors can also be challenging. On initial evaluation of a fineneedle aspiration, this mass was difficult to characterize as an epithelial or a round cell tumor. Individual cells were similar in appearance to round cells, while cell clusters were typically epithelioid, similar to those in reported cases of adrenal medullary neoplasia, in which exfoliated neoplastic cells are difficult to differentiate cytomorphologically from lymphoid cells and which has been misdiagnosed as lymphoma (4,19).
In human neuroblastomas, metastases are frequent and appear early and widely. In addition to local invasion and lymph node spread, neuroblastomas also have a tendency to spread through the blood stream to involve the liver, lungs, and bones (3). In the reported cases of peripheral neuroblastomas in dogs (1,2,4–8), metastases involved the regional lymph nodes, mesentery, spleen, liver, cerebellum, nasopharynx (8), and vertebral canal (2). To the authors’ knowledge, this is the first reported case in a dog with metastasis to the pituitary gland.
In summary, peripheral neuroblastomas are difficult neoplasms to diagnose clinically, cytologically, and at postmortem. In this case, as the left adrenal gland could not be identified and the mass was located in this area, an adrenal tumor was suspected. Given the young age and the ACTH results, a medullary tumor rather than a functional cortical neoplasia was considered likely; a more complete histopathologic evaluation and immunohistochemical staining were necessary to establish a diagnosis of peripheral neuroblastoma.
Acknowledgments
The authors thank Dr. Charles C. Capen for performing the immunohistochemical staining for neuron specific enolase, neurofilament protein, and chromogranin A, as well as for his insightful comments on the histopathology. CVJ
References
- 1.Matsushima S, Maruyama T, Torii M. Peripheral neuroblastoma in a young beagle dog. Toxicol Pathol. 1998;26:806–809. doi: 10.1177/019262339802600614. [DOI] [PubMed] [Google Scholar]
- 2.Forrest LJ, Galbreath EJ, Dubielzig RR, MacEwen EG. Peripheral neuroblastoma in a dog. Vet Radiol Ultrasound. 1997;38:457–460. doi: 10.1111/j.1740-8261.1997.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 3.Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. 6th ed. Philadelphia: WB Saunders, 1999:485–487.
- 4.Payne-Johnson CE, Brockman DJ. Neuroblastoma in the dog. J Small Anim Pract. 1992;33:395–398. [Google Scholar]
- 5.Helman RG, Adams LG, Hall CL, Read WK. Metastatic neuroblastoma in a dog. Vet Pathol. 1980;17:769–773. doi: 10.1177/030098588001700613. [DOI] [PubMed] [Google Scholar]
- 6.Kelly DF. Neuroblastoma in the dog. J Pathol. 1975;116:209–212. doi: 10.1002/path.1711160404. [DOI] [PubMed] [Google Scholar]
- 7.Louden C, Patterson JS, Sandusky GE. Peripheral neuroblastomas in two dogs. J Vet Diagn Invest. 1992;4:476–480. doi: 10.1177/104063879200400425. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki M, Uchida K, Taniguchi K, Yamaguchi R, Tateyama S. Peripheral neuroblastoma in a young Labrador Retriever. J Vet Med Sci. 2003;65:271–274. doi: 10.1292/jvms.65.271. [DOI] [PubMed] [Google Scholar]
- 9.Capen CC. The endocrine glands. In: Jubb KVF, Kennedy PC, Palmer N, eds. Pathology of Domestic Animals. 4th ed. Vol 3. Toronto: Academic Pr, 1993:267–347.
- 10.Capen CC. Tumors of the adrenal gland. In: Meuten DJ, ed. Tumors in Domestic Animals. 4th ed. Iowa State Univ Press, 2002: 629–638.
- 11.Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction, 3rd ed. Philadelphia: WB Saunders, 2004: 440–463.
- 12.Schilling FH, Spix C, Berthold F, et al. Neuroblastoma screening at one year of age. N Engl J Med. 2002;346:1047–1053. doi: 10.1056/NEJMoa012277. [DOI] [PubMed] [Google Scholar]
- 13.Twedt DC, Wheeler SL. Pheochromocytoma in the dog. Vet Clin North Am Small Anim Pract. 1984;14:767–782. doi: 10.1016/s0195-5616(84)50080-1. [DOI] [PubMed] [Google Scholar]
- 14.Von Dehn BJ, Nelson RW, Feldman EC, Griffey SM. Pheochromocytoma and hyperadrenocorticism in dogs: Six cases (1982–1992) J Am Vet Med Assoc. 1995;207:322–324. [PubMed] [Google Scholar]
- 15.Bouyad H, Feeney DA, Caywood DD, Hayden DW. Pheochromocytoma in dogs: 13 cases (1980–1985) J Am Vet Med Assoc. 1987;191:1610–1615. [PubMed] [Google Scholar]
- 16.Chetty R, Pillay P, Jaichand V. Cytokeratin expression in adrenal phaeochromocytomas and extra-adrenal paragangliomas. J Clin Pathol. 1998;51:477–478. doi: 10.1136/jcp.51.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franquemont DW, Mills SE, Lack EE. Immunohistochemical detection of neuroblastomatous foci in composite adrenal pheochromocytoma- neuroblastoma. Am J Clin Pathol. 1994;102:163–170. doi: 10.1093/ajcp/102.2.163. [DOI] [PubMed] [Google Scholar]
- 18.Tischler AS. Divergent differentiation in neuroendocrine tumors of the adrenal gland. Semin Diagn Pathol. 2000;17:120–126. [PubMed] [Google Scholar]
- 19.Gilson SD, Withrow SJ, Wheeler SL, Twedt DC. Pheochromocytoma in 50 dogs. J Vet Intern Med. 1994;8:228–232. doi: 10.1111/j.1939-1676.1994.tb03222.x. [DOI] [PubMed] [Google Scholar]


